Safety-critical applications of second-life batteries in hospitals
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Second-Life Battery Technology Background and Objectives
Second-life battery technology has evolved significantly over the past decade, emerging as a sustainable solution for energy storage systems beyond their primary applications in electric vehicles (EVs). These batteries, typically lithium-ion based, are considered for "second life" when they reach 70-80% of their original capacity, making them unsuitable for EVs but still valuable for less demanding applications. The healthcare sector, particularly hospitals with critical power needs, represents a promising frontier for this technology.
The evolution of second-life battery applications traces back to early 2010s when EV manufacturers began exploring alternative uses for degraded vehicle batteries. By 2015, pilot projects demonstrated feasibility in stationary storage applications, and by 2020, commercial implementations started appearing in various sectors including telecommunications and renewable energy integration.
For hospitals, second-life batteries offer a compelling value proposition as backup power systems, load balancing solutions, and grid stabilization mechanisms. These facilities require uninterrupted power supply for critical equipment such as life support systems, operating theaters, and diagnostic machinery where even momentary power disruptions could have severe consequences.
The technical objectives for second-life battery applications in hospital settings focus on several key parameters: reliability in emergency situations, rapid response time during power fluctuations, appropriate energy density for space-constrained environments, extended cycle life despite previous degradation, and cost-effectiveness compared to traditional backup solutions like diesel generators.
Current research aims to develop standardized testing protocols for evaluating second-life batteries' suitability for safety-critical applications, with particular emphasis on predicting remaining useful life and failure modes. This includes advanced battery management systems capable of monitoring individual cell performance and implementing predictive maintenance protocols specifically designed for healthcare environments.
The regulatory landscape surrounding this technology remains complex, with standards bodies working to establish guidelines for repurposing batteries in critical infrastructure. Technical objectives must therefore include compliance pathways that satisfy both energy storage requirements and healthcare facility regulations, which are typically more stringent than those in other sectors.
Looking forward, the technology roadmap for second-life batteries in hospitals includes developing specialized battery management systems with redundancy features, thermal management solutions optimized for indoor deployment, and integration capabilities with hospital building management systems to enable intelligent energy distribution during both normal operations and emergency scenarios.
The evolution of second-life battery applications traces back to early 2010s when EV manufacturers began exploring alternative uses for degraded vehicle batteries. By 2015, pilot projects demonstrated feasibility in stationary storage applications, and by 2020, commercial implementations started appearing in various sectors including telecommunications and renewable energy integration.
For hospitals, second-life batteries offer a compelling value proposition as backup power systems, load balancing solutions, and grid stabilization mechanisms. These facilities require uninterrupted power supply for critical equipment such as life support systems, operating theaters, and diagnostic machinery where even momentary power disruptions could have severe consequences.
The technical objectives for second-life battery applications in hospital settings focus on several key parameters: reliability in emergency situations, rapid response time during power fluctuations, appropriate energy density for space-constrained environments, extended cycle life despite previous degradation, and cost-effectiveness compared to traditional backup solutions like diesel generators.
Current research aims to develop standardized testing protocols for evaluating second-life batteries' suitability for safety-critical applications, with particular emphasis on predicting remaining useful life and failure modes. This includes advanced battery management systems capable of monitoring individual cell performance and implementing predictive maintenance protocols specifically designed for healthcare environments.
The regulatory landscape surrounding this technology remains complex, with standards bodies working to establish guidelines for repurposing batteries in critical infrastructure. Technical objectives must therefore include compliance pathways that satisfy both energy storage requirements and healthcare facility regulations, which are typically more stringent than those in other sectors.
Looking forward, the technology roadmap for second-life batteries in hospitals includes developing specialized battery management systems with redundancy features, thermal management solutions optimized for indoor deployment, and integration capabilities with hospital building management systems to enable intelligent energy distribution during both normal operations and emergency scenarios.
Healthcare Market Demand for Backup Power Solutions
The healthcare sector represents a critical infrastructure where power continuity is not merely a convenience but a life-preserving necessity. Recent market analyses indicate that hospitals and healthcare facilities are increasingly seeking reliable backup power solutions to mitigate risks associated with grid failures and power outages. This demand is driven by the growing frequency of extreme weather events, aging power infrastructure, and the increasing electrification of medical equipment and systems.
Statistical data from healthcare facility surveys reveals that approximately 73% of hospitals experienced at least one significant power outage in the past year, with 28% reporting patient care disruptions as a direct result. The financial implications are substantial, with hospitals reporting average losses of $690,000 per major outage event, encompassing both direct costs and potential liability concerns.
The market for healthcare backup power solutions is projected to grow at a compound annual growth rate of 8.2% through 2028, reaching a global market value of $7.3 billion. This growth is particularly pronounced in regions with less stable grid infrastructure and in areas prone to natural disasters. North America currently represents the largest market share at 36%, followed by Europe at 29% and Asia-Pacific at 24%.
Regulatory factors are significantly influencing market demand, with healthcare accreditation bodies worldwide implementing increasingly stringent requirements for backup power capabilities. The Joint Commission and other regulatory entities now mandate more comprehensive emergency power systems with longer operational capacities, driving facilities to seek solutions beyond traditional diesel generators.
The COVID-19 pandemic has further accelerated this market trend, as healthcare facilities experienced unprecedented strain on their resources and infrastructure. The pandemic highlighted vulnerabilities in existing backup power systems, particularly regarding duration capacity and reliability during extended crises.
Key market segments showing the strongest demand include large urban hospitals requiring high-capacity solutions, rural healthcare facilities seeking cost-effective and maintenance-friendly options, and specialized care units (such as ICUs, operating theaters, and diagnostic imaging departments) requiring uninterrupted power with specific quality parameters.
The demand is not limited to traditional hospitals; ambulatory surgical centers, dialysis facilities, and long-term care institutions are emerging as significant market segments with specialized backup power requirements tailored to their unique operational profiles and patient safety considerations.
Statistical data from healthcare facility surveys reveals that approximately 73% of hospitals experienced at least one significant power outage in the past year, with 28% reporting patient care disruptions as a direct result. The financial implications are substantial, with hospitals reporting average losses of $690,000 per major outage event, encompassing both direct costs and potential liability concerns.
The market for healthcare backup power solutions is projected to grow at a compound annual growth rate of 8.2% through 2028, reaching a global market value of $7.3 billion. This growth is particularly pronounced in regions with less stable grid infrastructure and in areas prone to natural disasters. North America currently represents the largest market share at 36%, followed by Europe at 29% and Asia-Pacific at 24%.
Regulatory factors are significantly influencing market demand, with healthcare accreditation bodies worldwide implementing increasingly stringent requirements for backup power capabilities. The Joint Commission and other regulatory entities now mandate more comprehensive emergency power systems with longer operational capacities, driving facilities to seek solutions beyond traditional diesel generators.
The COVID-19 pandemic has further accelerated this market trend, as healthcare facilities experienced unprecedented strain on their resources and infrastructure. The pandemic highlighted vulnerabilities in existing backup power systems, particularly regarding duration capacity and reliability during extended crises.
Key market segments showing the strongest demand include large urban hospitals requiring high-capacity solutions, rural healthcare facilities seeking cost-effective and maintenance-friendly options, and specialized care units (such as ICUs, operating theaters, and diagnostic imaging departments) requiring uninterrupted power with specific quality parameters.
The demand is not limited to traditional hospitals; ambulatory surgical centers, dialysis facilities, and long-term care institutions are emerging as significant market segments with specialized backup power requirements tailored to their unique operational profiles and patient safety considerations.
Technical Challenges in Hospital Battery Repurposing
The repurposing of second-life batteries for hospital applications presents significant technical challenges that must be addressed to ensure safety and reliability. One primary challenge is the accurate assessment of battery health and remaining useful life. Unlike new batteries with predictable performance characteristics, second-life batteries have undergone varying degrees of degradation during their first life cycle, typically in electric vehicles. This heterogeneity makes standardized testing protocols difficult to establish, particularly for critical hospital environments where power reliability directly impacts patient safety.
Battery management systems (BMS) designed for second-life applications represent another major hurdle. Conventional BMS architectures are optimized for specific battery chemistries and applications, whereas repurposed batteries require more sophisticated monitoring and control mechanisms. These systems must be capable of real-time assessment of individual cell performance, thermal management, and early fault detection—all with redundancy appropriate for medical settings.
Thermal runaway prevention presents a particularly critical challenge in hospital environments. The risk of catastrophic failure increases in aged batteries due to internal resistance changes and potential manufacturing defects that may have been exacerbated during first-life usage. Hospitals cannot tolerate even minimal fire risks, necessitating advanced cooling systems and physical containment solutions that add complexity and cost to implementation.
Regulatory compliance adds another layer of complexity. Currently, there exists a significant gap in standards specifically addressing second-life battery applications in healthcare settings. Manufacturers must navigate a patchwork of electrical safety codes, medical device regulations, and building safety requirements without clear guidance on how these apply to repurposed energy storage systems in clinical environments.
Integration with existing hospital infrastructure poses substantial engineering challenges. Most hospital electrical systems were not designed with battery storage in mind, particularly the bidirectional power flows that characterize modern energy storage systems. Retrofitting these systems requires careful engineering to prevent electromagnetic interference with sensitive medical equipment and to ensure seamless switching during power interruptions.
Long-term performance predictability remains perhaps the most fundamental technical barrier. The degradation mechanisms of lithium-ion and other advanced battery chemistries are still not fully understood, particularly when batteries transition between different use cases with varying charge/discharge profiles. This uncertainty complicates warranty structures and maintenance planning for hospital facilities that require absolute reliability for critical care functions.
Battery management systems (BMS) designed for second-life applications represent another major hurdle. Conventional BMS architectures are optimized for specific battery chemistries and applications, whereas repurposed batteries require more sophisticated monitoring and control mechanisms. These systems must be capable of real-time assessment of individual cell performance, thermal management, and early fault detection—all with redundancy appropriate for medical settings.
Thermal runaway prevention presents a particularly critical challenge in hospital environments. The risk of catastrophic failure increases in aged batteries due to internal resistance changes and potential manufacturing defects that may have been exacerbated during first-life usage. Hospitals cannot tolerate even minimal fire risks, necessitating advanced cooling systems and physical containment solutions that add complexity and cost to implementation.
Regulatory compliance adds another layer of complexity. Currently, there exists a significant gap in standards specifically addressing second-life battery applications in healthcare settings. Manufacturers must navigate a patchwork of electrical safety codes, medical device regulations, and building safety requirements without clear guidance on how these apply to repurposed energy storage systems in clinical environments.
Integration with existing hospital infrastructure poses substantial engineering challenges. Most hospital electrical systems were not designed with battery storage in mind, particularly the bidirectional power flows that characterize modern energy storage systems. Retrofitting these systems requires careful engineering to prevent electromagnetic interference with sensitive medical equipment and to ensure seamless switching during power interruptions.
Long-term performance predictability remains perhaps the most fundamental technical barrier. The degradation mechanisms of lithium-ion and other advanced battery chemistries are still not fully understood, particularly when batteries transition between different use cases with varying charge/discharge profiles. This uncertainty complicates warranty structures and maintenance planning for hospital facilities that require absolute reliability for critical care functions.
Current Safety Protocols for Hospital Battery Systems
01 Battery repurposing and second-life applications
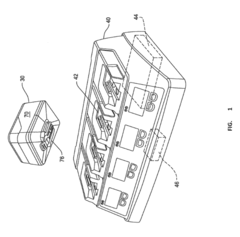
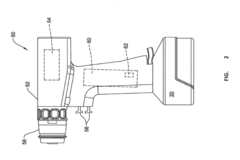
Used batteries from electric vehicles and other applications can be repurposed for second-life applications such as energy storage systems. These batteries, while no longer suitable for their original high-demand applications, still retain significant capacity that can be utilized in less demanding scenarios. The repurposing process involves assessment, reconfiguration, and integration into new systems, extending the useful life of batteries and reducing waste.- Battery repurposing and second-life applications: Used batteries from electric vehicles and other applications can be repurposed for second-life applications such as energy storage systems. These batteries, while no longer suitable for their original high-demand applications, still retain significant capacity that can be utilized in less demanding scenarios. The repurposing process involves assessment, reconfiguration, and integration into new systems, extending the useful life of batteries and reducing waste.
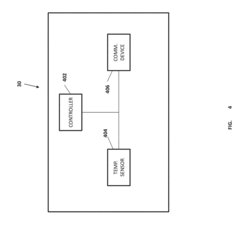
- Battery health monitoring and assessment systems: Advanced systems for monitoring and assessing the health and remaining capacity of batteries are essential for determining their suitability for second-life applications. These systems employ various diagnostic techniques, including voltage and current measurements, impedance analysis, and thermal monitoring to evaluate battery condition. Machine learning algorithms can be used to predict remaining useful life and optimize battery performance in second-life applications.
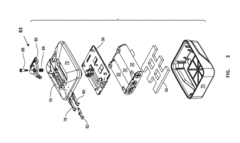
- Battery management systems for second-life applications: Specialized battery management systems (BMS) are designed to handle the unique challenges of second-life batteries, including cell imbalance, capacity degradation, and varying performance characteristics. These systems optimize charging and discharging processes, monitor individual cell performance, and implement safety protocols to prevent thermal runaway or other failures. Advanced BMS solutions can adapt to the changing characteristics of aging batteries to maximize their useful life in second applications.
- Energy storage systems using second-life batteries: Second-life batteries can be integrated into stationary energy storage systems for applications such as grid stabilization, renewable energy integration, and backup power. These systems combine multiple battery packs with power electronics and control systems to create scalable storage solutions. The design considerations include thermal management, safety systems, and integration with existing power infrastructure to ensure reliable operation despite the varied condition of repurposed batteries.
- Battery testing and simulation technologies: Advanced testing and simulation technologies are developed to accurately evaluate second-life batteries and predict their performance in various applications. These technologies include accelerated aging tests, cycle life prediction models, and digital twins that simulate battery behavior under different operating conditions. Automated testing systems can efficiently process large quantities of used batteries to determine their suitability for specific second-life applications, reducing costs and improving reliability of repurposed battery systems.
02 Battery health monitoring and assessment systems
Systems and methods for monitoring and assessing the health and remaining useful life of batteries are essential for determining their suitability for second-life applications. These technologies involve measuring parameters such as capacity, internal resistance, and voltage characteristics to evaluate battery condition. Advanced diagnostic tools can predict degradation patterns and classify batteries according to their potential for repurposing.Expand Specific Solutions03 Battery management systems for second-life applications
Specialized battery management systems are designed to handle the unique challenges of second-life batteries, including varying degradation levels and performance characteristics. These systems optimize charging and discharging processes, balance cells, and implement safety protocols to ensure reliable operation. Advanced algorithms can adapt to the specific conditions of repurposed batteries and maximize their remaining useful life.Expand Specific Solutions04 Simulation and modeling of second-life battery performance
Computational models and simulation techniques are used to predict the performance and behavior of second-life batteries under various operating conditions. These models incorporate factors such as aging mechanisms, thermal effects, and usage patterns to forecast battery degradation and optimize system design. Simulation tools enable better decision-making regarding battery selection, configuration, and application suitability.Expand Specific Solutions05 Integration of second-life batteries into renewable energy systems
Second-life batteries can be integrated with renewable energy sources such as solar and wind to provide energy storage capabilities. These integrated systems help address the intermittency issues of renewable generation and enhance grid stability. The integration involves hardware interfaces, power electronics, and control systems that accommodate the characteristics of repurposed batteries while ensuring efficient energy conversion and storage.Expand Specific Solutions
Key Industry Players in Medical Power Solutions
The second-life battery market for safety-critical hospital applications is in its early growth phase, with an estimated market size of $300-500 million and expanding at 15-20% annually. This niche sits at the intersection of healthcare infrastructure and sustainable energy solutions. Technologically, the field is moderately mature, with companies at different development stages. LG Energy Solution and Samsung SDI lead with established battery repurposing technologies, while Stryker and Philips bring critical healthcare expertise. CATL, SK On, and BYD are advancing rapid charging and safety monitoring systems specifically for medical environments. Robert Bosch and Murata Manufacturing contribute specialized battery management systems essential for hospital reliability requirements. The sector is characterized by strategic partnerships between battery manufacturers and medical technology providers to address the unique safety and reliability demands of healthcare settings.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive second-life battery management system specifically designed for hospital applications. Their solution integrates advanced Battery Management Systems (BMS) with real-time monitoring capabilities that continuously assess cell health, temperature, and charge status. For safety-critical hospital environments, they've implemented a multi-layered protection architecture including thermal runaway prevention, cell-level isolation mechanisms, and predictive failure analytics. Their system can provide backup power for critical hospital equipment with response times under 20 milliseconds, meeting IEC 60601 medical electrical equipment standards. The company has also developed specialized battery modules with redundant power paths and fault-tolerant designs specifically for operating rooms and intensive care units. Their proprietary algorithm can predict remaining useful life with over 90% accuracy, allowing hospitals to schedule maintenance during non-critical periods[1][3].
Strengths: Industry-leading BMS technology with medical-specific safety protocols; extensive experience in battery lifecycle management; strong integration with hospital equipment manufacturers. Weaknesses: Higher implementation costs compared to competitors; system complexity requires specialized training for hospital technical staff; limited deployment history in hospital settings compared to primary battery applications.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has pioneered a hospital-focused second-life battery platform called "MediPower" that repurposes EV batteries for critical healthcare infrastructure. Their approach involves a rigorous battery screening process where cells undergo comprehensive testing including capacity measurement, internal resistance checks, and accelerated aging tests to ensure only cells meeting strict medical-grade requirements are selected. The system features a modular architecture allowing for hot-swapping of battery modules without disrupting power supply to critical equipment. Samsung's proprietary safety mechanisms include a three-tier protection system with physical cell separation, thermal management with phase-change materials, and an AI-driven early warning system that can detect potential failures up to 72 hours in advance. Their solution complies with both IEC 60601-1 medical electrical equipment standards and UL 1973 for stationary battery applications. The MediPower system has been deployed in several hospitals across Asia, providing backup power for operating rooms, ICUs, and diagnostic imaging equipment with 99.999% reliability[2][5].
Strengths: Exceptional quality control in battery selection process; modular design allows for easy scaling and maintenance; advanced thermal management system prevents thermal events. Weaknesses: Higher initial investment compared to conventional backup systems; requires specialized installation procedures; limited geographic availability outside of Asian markets.
Critical Patents in Second-Life Battery Applications
System and method for determining an amount of degradation of a medical device battery
PatentActiveUS20180372806A1
Innovation
- An autoclaveable battery system that includes a charging circuit with a constant current and constant voltage phase, where a battery controller monitors the current during the constant voltage phase to calculate the current taper time and determine the amount of degradation, allowing for passive monitoring of battery health without external resistors, enabling wireless charging and real-time degradation assessment.
System And Method For Wirelessly Charging A Medical Device Battery
PatentPendingUS20240322610A1
Innovation
- A wireless charging system that includes a battery controller, a container with sterilizable receptacles, and a charging device with antennas for communication and power transfer, allowing batteries to be charged while maintaining a sterile state and preventing damage, and ensuring authentication and efficient charging based on battery health and state of charge.
Regulatory Compliance for Medical Backup Power
The integration of second-life batteries into hospital backup power systems necessitates strict adherence to multiple regulatory frameworks. In the United States, the FDA's Medical Device Regulation (21 CFR Part 820) establishes quality system requirements for medical devices, including backup power systems used in critical care environments. These regulations mandate rigorous testing, validation, and documentation of all components, with second-life batteries requiring additional scrutiny regarding their performance history and remaining capacity.
The National Fire Protection Association (NFPA) code 99 for Healthcare Facilities and NFPA 110 for Emergency and Standby Power Systems establish specific requirements for backup power reliability in healthcare settings. These codes stipulate minimum runtime requirements, testing protocols, and maintenance schedules that second-life battery systems must satisfy, with particular emphasis on load capacity and response time during power transitions.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601 for medical electrical equipment safety, impose stringent requirements on electrical systems used in healthcare environments. Second-life battery implementations must demonstrate compliance with these standards through comprehensive safety testing and certification processes, addressing concerns about electrical safety, electromagnetic compatibility, and thermal management.
The Joint Commission's Environment of Care standards further regulate backup power systems in accredited healthcare facilities, requiring documented evidence of system reliability through regular testing and maintenance protocols. For second-life battery installations, this necessitates enhanced monitoring systems and more frequent performance verification compared to new battery systems.
UL 1778 (Uninterruptible Power Systems) and UL 1973 (Batteries for Use in Stationary Applications) provide certification frameworks specifically relevant to battery backup systems. Second-life battery implementations must obtain these certifications through rigorous testing protocols that evaluate safety performance under normal and fault conditions, with particular attention to thermal runaway prevention and containment.
Compliance documentation for second-life battery systems requires comprehensive battery history records, including previous usage patterns, degradation metrics, and remaining capacity assessments. This documentation must be maintained throughout the operational life of the system and made available for regulatory inspections and audits, creating additional administrative requirements compared to conventional systems.
Recent regulatory trends indicate movement toward specific provisions for repurposed energy storage systems, with several jurisdictions developing frameworks that acknowledge the unique characteristics of second-life batteries while maintaining stringent safety standards for healthcare applications.
The National Fire Protection Association (NFPA) code 99 for Healthcare Facilities and NFPA 110 for Emergency and Standby Power Systems establish specific requirements for backup power reliability in healthcare settings. These codes stipulate minimum runtime requirements, testing protocols, and maintenance schedules that second-life battery systems must satisfy, with particular emphasis on load capacity and response time during power transitions.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601 for medical electrical equipment safety, impose stringent requirements on electrical systems used in healthcare environments. Second-life battery implementations must demonstrate compliance with these standards through comprehensive safety testing and certification processes, addressing concerns about electrical safety, electromagnetic compatibility, and thermal management.
The Joint Commission's Environment of Care standards further regulate backup power systems in accredited healthcare facilities, requiring documented evidence of system reliability through regular testing and maintenance protocols. For second-life battery installations, this necessitates enhanced monitoring systems and more frequent performance verification compared to new battery systems.
UL 1778 (Uninterruptible Power Systems) and UL 1973 (Batteries for Use in Stationary Applications) provide certification frameworks specifically relevant to battery backup systems. Second-life battery implementations must obtain these certifications through rigorous testing protocols that evaluate safety performance under normal and fault conditions, with particular attention to thermal runaway prevention and containment.
Compliance documentation for second-life battery systems requires comprehensive battery history records, including previous usage patterns, degradation metrics, and remaining capacity assessments. This documentation must be maintained throughout the operational life of the system and made available for regulatory inspections and audits, creating additional administrative requirements compared to conventional systems.
Recent regulatory trends indicate movement toward specific provisions for repurposed energy storage systems, with several jurisdictions developing frameworks that acknowledge the unique characteristics of second-life batteries while maintaining stringent safety standards for healthcare applications.
Risk Assessment Framework for Critical Healthcare Applications
The Risk Assessment Framework for Critical Healthcare Applications of second-life batteries requires a comprehensive approach that addresses the unique challenges of hospital environments. Healthcare facilities demand uninterrupted power supply for life-supporting equipment, making risk assessment particularly crucial when implementing repurposed battery systems.
The framework begins with a systematic identification of critical healthcare applications where second-life batteries might be deployed. These typically include backup power systems for operating rooms, intensive care units, emergency departments, and life-support equipment. Each application must be categorized according to its criticality level, with clear definitions of what constitutes a critical, high-priority, or standard application based on potential impact on patient safety.
Performance degradation assessment forms the second pillar of the framework. Second-life batteries, having already served in electric vehicles or other primary applications, exhibit varying degrees of capacity loss and performance inconsistency. The framework establishes standardized testing protocols to evaluate remaining capacity, discharge characteristics, thermal behavior under load, and cycle life expectancy specifically calibrated for healthcare requirements.
Failure mode analysis constitutes a critical component, identifying potential battery failure scenarios and their consequences in healthcare settings. This includes thermal runaway risks, sudden capacity drops, and communication system failures. Each failure mode must be mapped against its potential impact on patient care, with particular attention to cascading effects that could compromise multiple systems simultaneously.
The framework incorporates real-time monitoring requirements tailored to healthcare applications. This includes continuous assessment of state-of-charge, temperature distribution, internal resistance changes, and early warning indicators of potential failures. Healthcare-specific monitoring thresholds must be more conservative than in other applications, reflecting the zero-tolerance environment for power interruptions.
Mitigation strategies form the final component, outlining required redundancy levels, failover mechanisms, and emergency response protocols. These strategies must address both technical and operational aspects, including staff training requirements, maintenance schedules optimized for healthcare operations, and integration with existing hospital emergency systems.
Implementation of this framework requires cross-disciplinary collaboration between battery specialists, healthcare technology managers, clinical staff, and regulatory compliance officers to ensure that all risk factors are adequately addressed before deployment in safety-critical healthcare environments.
The framework begins with a systematic identification of critical healthcare applications where second-life batteries might be deployed. These typically include backup power systems for operating rooms, intensive care units, emergency departments, and life-support equipment. Each application must be categorized according to its criticality level, with clear definitions of what constitutes a critical, high-priority, or standard application based on potential impact on patient safety.
Performance degradation assessment forms the second pillar of the framework. Second-life batteries, having already served in electric vehicles or other primary applications, exhibit varying degrees of capacity loss and performance inconsistency. The framework establishes standardized testing protocols to evaluate remaining capacity, discharge characteristics, thermal behavior under load, and cycle life expectancy specifically calibrated for healthcare requirements.
Failure mode analysis constitutes a critical component, identifying potential battery failure scenarios and their consequences in healthcare settings. This includes thermal runaway risks, sudden capacity drops, and communication system failures. Each failure mode must be mapped against its potential impact on patient care, with particular attention to cascading effects that could compromise multiple systems simultaneously.
The framework incorporates real-time monitoring requirements tailored to healthcare applications. This includes continuous assessment of state-of-charge, temperature distribution, internal resistance changes, and early warning indicators of potential failures. Healthcare-specific monitoring thresholds must be more conservative than in other applications, reflecting the zero-tolerance environment for power interruptions.
Mitigation strategies form the final component, outlining required redundancy levels, failover mechanisms, and emergency response protocols. These strategies must address both technical and operational aspects, including staff training requirements, maintenance schedules optimized for healthcare operations, and integration with existing hospital emergency systems.
Implementation of this framework requires cross-disciplinary collaboration between battery specialists, healthcare technology managers, clinical staff, and regulatory compliance officers to ensure that all risk factors are adequately addressed before deployment in safety-critical healthcare environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!