Silicone Rubber: Advancing Medical Implants
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicone Rubber Evolution in Medical Implants
Silicone rubber has undergone a remarkable evolution in the field of medical implants, transforming from a simple material to a sophisticated biocompatible solution. The journey began in the 1960s when silicone rubber was first introduced in medical applications, primarily for breast implants. This initial use sparked extensive research into its properties and potential for broader medical applications.
Throughout the 1970s and 1980s, researchers focused on enhancing the biocompatibility of silicone rubber. This period saw significant improvements in the material's purity and the development of specialized formulations tailored for specific medical uses. The introduction of medical-grade silicone rubber marked a crucial milestone, setting new standards for safety and performance in implantable devices.
The 1990s brought about a revolution in silicone rubber technology for medical implants. Advanced manufacturing techniques, such as liquid silicone rubber injection molding, enabled the production of more complex and precise implant shapes. This technological leap expanded the range of possible applications, from cardiovascular devices to neurological implants.
In the early 2000s, the focus shifted towards enhancing the functional properties of silicone rubber implants. Researchers began exploring ways to incorporate drug-eluting capabilities, allowing implants to deliver medications directly to targeted areas of the body. This innovation opened new avenues for treating chronic conditions and reducing post-operative complications.
The past decade has seen a surge in the development of smart silicone rubber implants. These advanced devices integrate sensors and responsive elements, enabling real-time monitoring of physiological parameters and adaptive functionality. Such innovations have significantly improved patient outcomes and expanded the possibilities for personalized medicine.
Recent advancements have also focused on improving the long-term performance and durability of silicone rubber implants. Researchers have developed new crosslinking techniques and incorporated nanoparticles to enhance mechanical properties and reduce wear. These improvements have extended the lifespan of implants and reduced the need for revision surgeries.
Looking ahead, the evolution of silicone rubber in medical implants is poised to continue at an accelerated pace. Emerging technologies such as 3D printing and tissue engineering are opening new frontiers for customized, patient-specific implants. The integration of silicone rubber with other advanced materials, such as graphene and biodegradable polymers, promises to create a new generation of hybrid implants with unprecedented capabilities.
Throughout the 1970s and 1980s, researchers focused on enhancing the biocompatibility of silicone rubber. This period saw significant improvements in the material's purity and the development of specialized formulations tailored for specific medical uses. The introduction of medical-grade silicone rubber marked a crucial milestone, setting new standards for safety and performance in implantable devices.
The 1990s brought about a revolution in silicone rubber technology for medical implants. Advanced manufacturing techniques, such as liquid silicone rubber injection molding, enabled the production of more complex and precise implant shapes. This technological leap expanded the range of possible applications, from cardiovascular devices to neurological implants.
In the early 2000s, the focus shifted towards enhancing the functional properties of silicone rubber implants. Researchers began exploring ways to incorporate drug-eluting capabilities, allowing implants to deliver medications directly to targeted areas of the body. This innovation opened new avenues for treating chronic conditions and reducing post-operative complications.
The past decade has seen a surge in the development of smart silicone rubber implants. These advanced devices integrate sensors and responsive elements, enabling real-time monitoring of physiological parameters and adaptive functionality. Such innovations have significantly improved patient outcomes and expanded the possibilities for personalized medicine.
Recent advancements have also focused on improving the long-term performance and durability of silicone rubber implants. Researchers have developed new crosslinking techniques and incorporated nanoparticles to enhance mechanical properties and reduce wear. These improvements have extended the lifespan of implants and reduced the need for revision surgeries.
Looking ahead, the evolution of silicone rubber in medical implants is poised to continue at an accelerated pace. Emerging technologies such as 3D printing and tissue engineering are opening new frontiers for customized, patient-specific implants. The integration of silicone rubber with other advanced materials, such as graphene and biodegradable polymers, promises to create a new generation of hybrid implants with unprecedented capabilities.
Market Demand Analysis for Biocompatible Implants
The global market for biocompatible implants is experiencing significant growth, driven by an aging population, increasing prevalence of chronic diseases, and advancements in medical technology. Silicone rubber, with its exceptional biocompatibility and versatile properties, is playing a crucial role in this expanding market.
The demand for silicone-based medical implants is particularly strong in orthopedics, cardiovascular, and plastic surgery sectors. In orthopedics, silicone implants are widely used for joint replacements, especially in fingers and toes, due to their ability to mimic natural joint movement. The cardiovascular sector utilizes silicone in various applications, including heart valves and pacemaker leads, benefiting from the material's durability and resistance to body fluids.
Plastic surgery represents another significant market segment for silicone implants, with breast implants being the most prominent application. The global breast implant market is projected to grow steadily, driven by increasing aesthetic consciousness and reconstructive surgeries following breast cancer treatment.
The neurology sector is emerging as a promising area for silicone-based implants, with applications in neural prosthetics and brain-computer interfaces. This segment is expected to witness rapid growth as research in neurotechnology advances.
Geographically, North America and Europe currently dominate the biocompatible implant market, owing to advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is anticipated to exhibit the fastest growth rate, fueled by improving healthcare access, rising disposable incomes, and a growing elderly population.
Key market trends include the development of smart implants incorporating sensors and communication technologies, as well as the integration of drug-eluting capabilities in silicone implants for localized drug delivery. These innovations are expected to further expand the application scope of silicone-based implants.
Regulatory factors play a significant role in shaping market demand. Stringent approval processes by regulatory bodies like the FDA and EMA ensure product safety but can also impact market entry timelines for new implant technologies. Manufacturers investing in clinical trials and quality assurance are better positioned to meet regulatory requirements and gain market share.
The increasing focus on personalized medicine is driving demand for customized implants. Silicone rubber's moldability and the advent of 3D printing technologies are enabling the production of patient-specific implants, opening new market opportunities.
The demand for silicone-based medical implants is particularly strong in orthopedics, cardiovascular, and plastic surgery sectors. In orthopedics, silicone implants are widely used for joint replacements, especially in fingers and toes, due to their ability to mimic natural joint movement. The cardiovascular sector utilizes silicone in various applications, including heart valves and pacemaker leads, benefiting from the material's durability and resistance to body fluids.
Plastic surgery represents another significant market segment for silicone implants, with breast implants being the most prominent application. The global breast implant market is projected to grow steadily, driven by increasing aesthetic consciousness and reconstructive surgeries following breast cancer treatment.
The neurology sector is emerging as a promising area for silicone-based implants, with applications in neural prosthetics and brain-computer interfaces. This segment is expected to witness rapid growth as research in neurotechnology advances.
Geographically, North America and Europe currently dominate the biocompatible implant market, owing to advanced healthcare infrastructure and higher healthcare spending. However, the Asia-Pacific region is anticipated to exhibit the fastest growth rate, fueled by improving healthcare access, rising disposable incomes, and a growing elderly population.
Key market trends include the development of smart implants incorporating sensors and communication technologies, as well as the integration of drug-eluting capabilities in silicone implants for localized drug delivery. These innovations are expected to further expand the application scope of silicone-based implants.
Regulatory factors play a significant role in shaping market demand. Stringent approval processes by regulatory bodies like the FDA and EMA ensure product safety but can also impact market entry timelines for new implant technologies. Manufacturers investing in clinical trials and quality assurance are better positioned to meet regulatory requirements and gain market share.
The increasing focus on personalized medicine is driving demand for customized implants. Silicone rubber's moldability and the advent of 3D printing technologies are enabling the production of patient-specific implants, opening new market opportunities.
Current Challenges in Silicone Rubber Implants
Despite the widespread use of silicone rubber in medical implants, several challenges persist in this field. One of the primary concerns is the potential for silicone implants to trigger foreign body responses in patients. This immune reaction can lead to inflammation, capsular contracture, and in some cases, implant rejection. The long-term biocompatibility of silicone rubber remains a subject of ongoing research and debate within the medical community.
Another significant challenge is the mechanical durability of silicone implants. While silicone rubber offers excellent flexibility and elasticity, prolonged exposure to physiological conditions can lead to material degradation. This degradation may manifest as changes in mechanical properties, surface roughness, or even rupture of the implant. Ensuring the long-term structural integrity of silicone implants, particularly in high-stress applications like joint replacements or cardiovascular devices, remains a critical area of focus.
The potential for silicone leaching is another concern that researchers and manufacturers must address. Over time, small molecules from the silicone rubber matrix may migrate into surrounding tissues or the bloodstream. While the toxicological impact of these leached compounds is generally considered minimal, there is ongoing research to fully understand and mitigate any potential long-term effects on patient health.
Sterilization of silicone rubber implants presents another challenge. Traditional sterilization methods, such as high-temperature autoclaving or radiation, can potentially alter the material properties of silicone rubber. This necessitates the development of specialized sterilization protocols that effectively eliminate microbial contamination without compromising the implant's integrity or performance.
Furthermore, the integration of silicone implants with surrounding tissues remains an area of active research. While silicone rubber is generally inert, enhancing its ability to promote cell adhesion and tissue integration could improve implant stability and functionality. Researchers are exploring surface modification techniques and composite materials to address this challenge.
Lastly, the customization and manufacturing of patient-specific silicone implants pose technical and regulatory challenges. As personalized medicine advances, there is a growing demand for implants tailored to individual patient anatomies. Developing efficient, cost-effective methods for producing custom silicone implants while maintaining stringent quality control and regulatory compliance is an ongoing challenge for the industry.
Another significant challenge is the mechanical durability of silicone implants. While silicone rubber offers excellent flexibility and elasticity, prolonged exposure to physiological conditions can lead to material degradation. This degradation may manifest as changes in mechanical properties, surface roughness, or even rupture of the implant. Ensuring the long-term structural integrity of silicone implants, particularly in high-stress applications like joint replacements or cardiovascular devices, remains a critical area of focus.
The potential for silicone leaching is another concern that researchers and manufacturers must address. Over time, small molecules from the silicone rubber matrix may migrate into surrounding tissues or the bloodstream. While the toxicological impact of these leached compounds is generally considered minimal, there is ongoing research to fully understand and mitigate any potential long-term effects on patient health.
Sterilization of silicone rubber implants presents another challenge. Traditional sterilization methods, such as high-temperature autoclaving or radiation, can potentially alter the material properties of silicone rubber. This necessitates the development of specialized sterilization protocols that effectively eliminate microbial contamination without compromising the implant's integrity or performance.
Furthermore, the integration of silicone implants with surrounding tissues remains an area of active research. While silicone rubber is generally inert, enhancing its ability to promote cell adhesion and tissue integration could improve implant stability and functionality. Researchers are exploring surface modification techniques and composite materials to address this challenge.
Lastly, the customization and manufacturing of patient-specific silicone implants pose technical and regulatory challenges. As personalized medicine advances, there is a growing demand for implants tailored to individual patient anatomies. Developing efficient, cost-effective methods for producing custom silicone implants while maintaining stringent quality control and regulatory compliance is an ongoing challenge for the industry.
Existing Silicone Rubber Implant Solutions
01 Composition and preparation of silicone rubber
Silicone rubber is typically composed of silicone polymers, fillers, and curing agents. The preparation process often involves mixing these components, shaping the mixture, and then curing it to form the final rubber product. Various additives can be incorporated to modify properties such as strength, flexibility, and heat resistance.- Composition and preparation of silicone rubber: Silicone rubber is typically composed of silicone polymers, fillers, and curing agents. The preparation process often involves mixing these components, shaping the mixture, and then curing it to form the final rubber product. Various additives can be incorporated to modify properties such as strength, flexibility, and heat resistance.
- Modification of silicone rubber properties: The properties of silicone rubber can be modified through the addition of specific compounds or by altering the polymer structure. This can include improving mechanical strength, enhancing thermal stability, or increasing chemical resistance. Techniques may involve blending with other polymers or incorporating nanoparticles.
- Applications of silicone rubber: Silicone rubber finds wide-ranging applications due to its unique properties. It is used in medical devices, automotive parts, electrical insulation, cookware, and various industrial applications. Its biocompatibility makes it suitable for implants and other medical uses, while its heat resistance and electrical properties make it valuable in electronics and automotive industries.
- Manufacturing processes for silicone rubber products: Various manufacturing processes are employed to produce silicone rubber products, including injection molding, extrusion, and compression molding. Each process is suited to different product types and production volumes. Advanced techniques may involve precision molding for complex shapes or multi-component molding for integrated products.
- Innovations in silicone rubber technology: Recent innovations in silicone rubber technology focus on enhancing performance and expanding applications. This includes developing self-healing silicone rubbers, improving adhesion properties, creating conductive silicone rubbers for electronic applications, and formulating silicone rubbers with improved environmental resistance and durability.
02 Modification of silicone rubber properties
The properties of silicone rubber can be modified through the addition of specific compounds or by altering the polymer structure. This can include improving thermal stability, enhancing electrical properties, or increasing chemical resistance. Techniques may involve blending with other polymers or incorporating nanoparticles.Expand Specific Solutions03 Applications of silicone rubber
Silicone rubber finds wide-ranging applications due to its unique properties. It is used in medical devices, automotive parts, electrical insulation, cookware, and various industrial applications. Its biocompatibility makes it suitable for implants and other medical uses, while its heat resistance is valuable in automotive and industrial settings.Expand Specific Solutions04 Manufacturing processes for silicone rubber products
Various manufacturing processes are employed to produce silicone rubber products, including injection molding, extrusion, and compression molding. These processes can be optimized for specific product requirements, such as shape complexity, size, and production volume. Advanced techniques may involve multi-component molding or the use of specialized equipment for precision parts.Expand Specific Solutions05 Innovations in silicone rubber technology
Recent innovations in silicone rubber technology include the development of self-healing silicone rubbers, electrically conductive silicone composites, and silicone rubbers with enhanced biodegradability. These advancements expand the potential applications of silicone rubber and address emerging needs in various industries, such as electronics, environmental sustainability, and advanced manufacturing.Expand Specific Solutions
Key Players in Medical Grade Silicone Industry
The silicone rubber market for medical implants is in a growth phase, driven by increasing demand for advanced medical devices and an aging population. The market size is expanding, with projections indicating significant growth in the coming years. Technologically, silicone rubber for medical implants is relatively mature, but continuous innovations are occurring. Key players like Dow Silicones, Wacker Chemie, and Momentive Performance Materials are leading the field with advanced formulations and manufacturing processes. Emerging companies such as Hunan Jingtu Medical Technology and Shandong Wego Newlife Medical Device are also contributing to market dynamics, particularly in the Asian region. The competitive landscape is characterized by a mix of established global corporations and specialized medical device manufacturers, all striving to enhance biocompatibility and performance of silicone-based implants.
Shin-Etsu Chemical Co., Ltd.
Technical Solution: Shin-Etsu Chemical Co., Ltd. has made significant advancements in silicone rubber technology for medical implants through their LIMS (Liquid Injection Molding System) materials. These high-purity silicone rubbers are specifically designed for the production of precision medical components and implants. Shin-Etsu's LIMS materials offer excellent transparency, which is crucial for certain implant applications like intraocular lenses[13]. The company has developed specialized silicone adhesives that maintain their bonding strength even in the presence of bodily fluids, ensuring long-term implant integrity[14]. Shin-Etsu has also introduced self-bleeding silicone rubbers that exude a controlled amount of silicone fluid over time, potentially reducing friction and improving the longevity of implanted devices with moving parts[15].
Strengths: High-precision molding capabilities, transparent formulations, and innovative self-bleeding technology. Weaknesses: May have a more limited presence in certain geographic markets compared to some global competitors.
Saint-Gobain Performance Plastics Corp.
Technical Solution: Saint-Gobain Performance Plastics Corp. has developed a range of high-performance silicone rubber materials for medical implants under their Sil-Pro® brand. These materials are engineered for long-term implantation and offer excellent biocompatibility and biostability. Saint-Gobain's silicone rubbers feature customizable durometers, allowing for precise control of mechanical properties to match specific implant requirements[10]. The company has introduced innovative processing techniques, such as overmolding silicone onto other substrates, enabling the creation of complex multi-material implants[11]. Their silicone formulations also incorporate antimicrobial properties to reduce the risk of implant-associated infections. Saint-Gobain has developed specialized silicone foam materials for soft tissue implants, providing a more natural feel and improved patient comfort[12].
Strengths: Customizable material properties, advanced processing capabilities, and innovative antimicrobial and foam formulations. Weaknesses: May have limited in-house medical device design expertise compared to dedicated medical technology companies.
Innovative Silicone Rubber Formulations
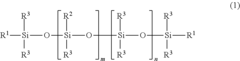
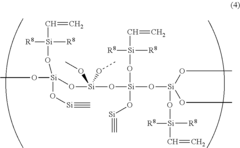
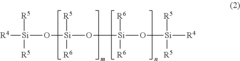
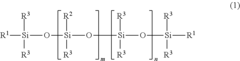
Silicone-rubber-based curable composition, method for producing silicone rubber, silicone rubber, molding, and medical tubing
PatentWO2013042707A1
Innovation
- A curable silicone rubber composition incorporating a combination of vinyl group-containing linear and branched organopolysiloxanes, organohydrogenpolysiloxanes, silica particles, and a silane coupling agent, along with a platinum or platinum compound, is developed to enhance tensile and tear strength, ensuring improved mechanical properties without compromising usability or transparency.
Silicone rubber-based curable composition, method of producing silicone rubber, silicone rubber, molded body and medical tube
PatentInactiveUS20140242312A1
Innovation
- A silicone rubber-based curable composition comprising a combination of vinyl group-containing organopolysiloxanes, organohydrogen polysiloxanes, silica particles, and a silane coupling agent, with specific molecular structures and ratios to enhance crosslink density and mechanical properties, such as the use of both linear and branch organopolysiloxanes and a silane coupling agent with hydrolysable and vinyl groups.
Regulatory Framework for Medical Implants
The regulatory framework for medical implants involving silicone rubber is complex and multifaceted, reflecting the critical importance of ensuring patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) plays a central role in overseeing the development, testing, and approval of medical implants. The FDA classifies silicone rubber implants as Class III devices, which are subject to the most stringent regulatory controls due to their potential risks and long-term implantation in the human body.
The premarket approval (PMA) process is a key component of the regulatory framework for silicone rubber implants. This process requires manufacturers to submit comprehensive data demonstrating the safety and effectiveness of their devices. This typically includes results from extensive preclinical testing, such as biocompatibility studies, mechanical testing, and long-term implantation studies in animal models. Additionally, clinical trials in human subjects are often necessary to provide evidence of the implant's performance and safety in real-world conditions.
Post-market surveillance is another crucial aspect of the regulatory framework. Manufacturers are required to implement robust systems for monitoring and reporting adverse events related to their implants. This includes maintaining detailed records of complaints, conducting thorough investigations of reported issues, and submitting periodic safety reports to regulatory authorities.
International regulatory bodies, such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), have similar stringent requirements for silicone rubber implants. The EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous standards for medical devices, including implants, with a focus on clinical evidence, post-market surveillance, and traceability.
Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to align regulatory approaches across different countries. This includes developing common guidelines for the evaluation of medical implants and promoting the use of international standards in the regulatory process.
Specific regulations also address the manufacturing processes for silicone rubber implants. Good Manufacturing Practices (GMP) guidelines outline requirements for quality control, documentation, and validation of production processes. These regulations ensure consistency in product quality and help minimize the risk of defects or contamination during manufacturing.
As the field of medical implants continues to evolve, regulatory frameworks are adapting to address emerging technologies and materials. This includes considerations for combination products that incorporate silicone rubber with other materials or active components, as well as the potential use of 3D printing technologies in implant manufacturing. Regulatory agencies are also focusing on the long-term performance and durability of silicone rubber implants, requiring manufacturers to provide data on the expected lifespan of their devices and potential degradation mechanisms.
The premarket approval (PMA) process is a key component of the regulatory framework for silicone rubber implants. This process requires manufacturers to submit comprehensive data demonstrating the safety and effectiveness of their devices. This typically includes results from extensive preclinical testing, such as biocompatibility studies, mechanical testing, and long-term implantation studies in animal models. Additionally, clinical trials in human subjects are often necessary to provide evidence of the implant's performance and safety in real-world conditions.
Post-market surveillance is another crucial aspect of the regulatory framework. Manufacturers are required to implement robust systems for monitoring and reporting adverse events related to their implants. This includes maintaining detailed records of complaints, conducting thorough investigations of reported issues, and submitting periodic safety reports to regulatory authorities.
International regulatory bodies, such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), have similar stringent requirements for silicone rubber implants. The EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous standards for medical devices, including implants, with a focus on clinical evidence, post-market surveillance, and traceability.
Harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to align regulatory approaches across different countries. This includes developing common guidelines for the evaluation of medical implants and promoting the use of international standards in the regulatory process.
Specific regulations also address the manufacturing processes for silicone rubber implants. Good Manufacturing Practices (GMP) guidelines outline requirements for quality control, documentation, and validation of production processes. These regulations ensure consistency in product quality and help minimize the risk of defects or contamination during manufacturing.
As the field of medical implants continues to evolve, regulatory frameworks are adapting to address emerging technologies and materials. This includes considerations for combination products that incorporate silicone rubber with other materials or active components, as well as the potential use of 3D printing technologies in implant manufacturing. Regulatory agencies are also focusing on the long-term performance and durability of silicone rubber implants, requiring manufacturers to provide data on the expected lifespan of their devices and potential degradation mechanisms.
Biocompatibility Testing Methods
Biocompatibility testing methods are crucial for evaluating the safety and efficacy of silicone rubber medical implants. These methods assess the material's interaction with living tissues and biological systems, ensuring that the implants do not cause adverse reactions or harm to the patient. The testing process typically involves a series of in vitro and in vivo studies, following established guidelines and standards set by regulatory bodies such as the FDA and ISO.
One of the primary biocompatibility tests for silicone rubber implants is cytotoxicity testing. This in vitro assay evaluates the potential of the material to cause cell death or inhibit cell growth. Researchers expose cultured cells to extracts of the silicone rubber or directly to the material surface, then assess cell viability and proliferation using various techniques such as MTT assay or live/dead staining.
Sensitization and irritation tests are conducted to determine if the silicone rubber implant can cause allergic reactions or local tissue irritation. These tests often involve animal models, such as guinea pigs or rabbits, where the material is applied to the skin or implanted subcutaneously. Researchers then observe and score any inflammatory responses or signs of sensitization over a specified period.
Hemocompatibility testing is essential for silicone rubber implants that come into contact with blood. This involves evaluating the material's potential to cause hemolysis, thrombosis, or activate the complement system. In vitro tests using human blood samples and specialized equipment like hemolysis analyzers are employed to assess these parameters.
Genotoxicity and carcinogenicity tests are conducted to ensure that the silicone rubber implant does not cause genetic mutations or promote tumor formation. These tests include in vitro assays such as the Ames test and in vivo studies using animal models to evaluate long-term effects.
Implantation tests are performed to assess the local tissue response to the silicone rubber implant over extended periods. This involves surgically implanting the material into animal models, typically rats or rabbits, and evaluating the surrounding tissue response at various time points. Histological analysis and immunohistochemistry techniques are used to examine the tissue-implant interface and identify any signs of inflammation, fibrosis, or other adverse reactions.
Systemic toxicity tests evaluate the potential of silicone rubber implants to cause adverse effects on organ systems throughout the body. These tests often involve administering extracts of the material to animal models and monitoring various physiological parameters, blood chemistry, and organ function over time.
To ensure comprehensive evaluation, researchers also conduct degradation studies to assess the stability of silicone rubber implants under physiological conditions. This involves exposing the material to simulated body fluids and analyzing changes in its physical and chemical properties over time, including potential leaching of compounds that could affect biocompatibility.
One of the primary biocompatibility tests for silicone rubber implants is cytotoxicity testing. This in vitro assay evaluates the potential of the material to cause cell death or inhibit cell growth. Researchers expose cultured cells to extracts of the silicone rubber or directly to the material surface, then assess cell viability and proliferation using various techniques such as MTT assay or live/dead staining.
Sensitization and irritation tests are conducted to determine if the silicone rubber implant can cause allergic reactions or local tissue irritation. These tests often involve animal models, such as guinea pigs or rabbits, where the material is applied to the skin or implanted subcutaneously. Researchers then observe and score any inflammatory responses or signs of sensitization over a specified period.
Hemocompatibility testing is essential for silicone rubber implants that come into contact with blood. This involves evaluating the material's potential to cause hemolysis, thrombosis, or activate the complement system. In vitro tests using human blood samples and specialized equipment like hemolysis analyzers are employed to assess these parameters.
Genotoxicity and carcinogenicity tests are conducted to ensure that the silicone rubber implant does not cause genetic mutations or promote tumor formation. These tests include in vitro assays such as the Ames test and in vivo studies using animal models to evaluate long-term effects.
Implantation tests are performed to assess the local tissue response to the silicone rubber implant over extended periods. This involves surgically implanting the material into animal models, typically rats or rabbits, and evaluating the surrounding tissue response at various time points. Histological analysis and immunohistochemistry techniques are used to examine the tissue-implant interface and identify any signs of inflammation, fibrosis, or other adverse reactions.
Systemic toxicity tests evaluate the potential of silicone rubber implants to cause adverse effects on organ systems throughout the body. These tests often involve administering extracts of the material to animal models and monitoring various physiological parameters, blood chemistry, and organ function over time.
To ensure comprehensive evaluation, researchers also conduct degradation studies to assess the stability of silicone rubber implants under physiological conditions. This involves exposing the material to simulated body fluids and analyzing changes in its physical and chemical properties over time, including potential leaching of compounds that could affect biocompatibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!