Advances in PEMF Therapy for Depression Treatment
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF for Depression: Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment for depression, offering a potential alternative or complement to traditional pharmacological and psychotherapeutic approaches. The development of PEMF therapy for depression stems from a growing understanding of the brain's electromagnetic properties and the role of neuroplasticity in mental health.
The history of electromagnetic therapy dates back to the 18th century, but it wasn't until the late 20th century that researchers began to explore its potential for treating psychiatric disorders. The modern era of PEMF therapy for depression began in the 1990s, with pioneering studies demonstrating its ability to modulate brain activity and improve mood in patients with treatment-resistant depression.
PEMF therapy works by delivering low-frequency electromagnetic pulses to specific areas of the brain, inducing small electrical currents that can influence neuronal activity. This mechanism is thought to promote neuroplasticity, enhance neurotransmitter function, and regulate cortical excitability, all of which are implicated in the pathophysiology of depression.
The primary objective of PEMF therapy in depression treatment is to provide a safe, effective, and well-tolerated alternative for patients who have not responded adequately to conventional treatments. Additionally, researchers aim to optimize treatment protocols, identify the most responsive patient subgroups, and elucidate the underlying neurobiological mechanisms of action.
Recent advances in PEMF technology have led to the development of more precise and targeted devices, allowing for personalized treatment approaches. These innovations include the ability to modulate pulse frequency, intensity, and waveform characteristics to achieve optimal therapeutic effects while minimizing side effects.
The growing interest in PEMF therapy for depression is driven by several factors, including the high prevalence of treatment-resistant depression, concerns about the side effects and long-term efficacy of antidepressant medications, and the need for non-invasive alternatives to more aggressive interventions like electroconvulsive therapy (ECT).
As research in this field progresses, the scientific community is focusing on several key areas: determining the most effective treatment parameters, investigating the potential for combining PEMF therapy with other interventions, and exploring its application in various subtypes of depression and related mood disorders.
The ultimate goal of PEMF therapy research for depression is to establish it as a clinically validated, widely accessible treatment option that can significantly improve outcomes for patients struggling with this debilitating condition. This involves not only advancing the technology and understanding of its mechanisms but also addressing regulatory, economic, and practical considerations to facilitate its integration into mainstream psychiatric care.
The history of electromagnetic therapy dates back to the 18th century, but it wasn't until the late 20th century that researchers began to explore its potential for treating psychiatric disorders. The modern era of PEMF therapy for depression began in the 1990s, with pioneering studies demonstrating its ability to modulate brain activity and improve mood in patients with treatment-resistant depression.
PEMF therapy works by delivering low-frequency electromagnetic pulses to specific areas of the brain, inducing small electrical currents that can influence neuronal activity. This mechanism is thought to promote neuroplasticity, enhance neurotransmitter function, and regulate cortical excitability, all of which are implicated in the pathophysiology of depression.
The primary objective of PEMF therapy in depression treatment is to provide a safe, effective, and well-tolerated alternative for patients who have not responded adequately to conventional treatments. Additionally, researchers aim to optimize treatment protocols, identify the most responsive patient subgroups, and elucidate the underlying neurobiological mechanisms of action.
Recent advances in PEMF technology have led to the development of more precise and targeted devices, allowing for personalized treatment approaches. These innovations include the ability to modulate pulse frequency, intensity, and waveform characteristics to achieve optimal therapeutic effects while minimizing side effects.
The growing interest in PEMF therapy for depression is driven by several factors, including the high prevalence of treatment-resistant depression, concerns about the side effects and long-term efficacy of antidepressant medications, and the need for non-invasive alternatives to more aggressive interventions like electroconvulsive therapy (ECT).
As research in this field progresses, the scientific community is focusing on several key areas: determining the most effective treatment parameters, investigating the potential for combining PEMF therapy with other interventions, and exploring its application in various subtypes of depression and related mood disorders.
The ultimate goal of PEMF therapy research for depression is to establish it as a clinically validated, widely accessible treatment option that can significantly improve outcomes for patients struggling with this debilitating condition. This involves not only advancing the technology and understanding of its mechanisms but also addressing regulatory, economic, and practical considerations to facilitate its integration into mainstream psychiatric care.
Market Analysis: PEMF in Mental Health
The market for PEMF (Pulsed Electromagnetic Field) therapy in mental health treatment, particularly for depression, has been experiencing significant growth in recent years. This expansion is driven by the increasing prevalence of mental health disorders worldwide and the growing acceptance of alternative and complementary therapies in mainstream healthcare.
The global mental health market, which includes treatments for depression, is projected to reach substantial value in the coming years. Within this broader market, PEMF therapy is carving out a niche as a non-invasive and drug-free alternative for managing depression symptoms. The adoption of PEMF therapy for mental health applications is still in its early stages, but it shows promising potential for rapid growth.
Several factors are contributing to the increasing demand for PEMF therapy in depression treatment. Firstly, there is a growing awareness among patients and healthcare providers about the limitations and side effects of traditional pharmacological treatments for depression. This has led to a search for alternative therapies that can offer relief with fewer adverse effects.
Secondly, the non-invasive nature of PEMF therapy makes it an attractive option for patients who are hesitant about more invasive treatments or medication regimens. The ability to use PEMF devices at home also aligns with the trend towards personalized and convenient healthcare solutions.
The market for PEMF devices specifically designed for mental health applications is still relatively small but is expected to grow rapidly. Major players in the medical device industry are starting to invest in research and development of PEMF technologies for mental health, recognizing the potential for significant market expansion.
Geographically, North America currently leads the market for PEMF therapy in mental health applications, followed by Europe. This is largely due to higher awareness, greater healthcare spending, and more favorable regulatory environments in these regions. However, Asia-Pacific is expected to show the fastest growth in the coming years, driven by increasing mental health awareness and improving healthcare infrastructure.
Despite the promising outlook, the market faces several challenges. These include the need for more extensive clinical research to validate the efficacy of PEMF therapy for depression, regulatory hurdles in some regions, and the relatively high cost of advanced PEMF devices. Overcoming these challenges will be crucial for the widespread adoption of PEMF therapy in mental health treatment.
In conclusion, the market for PEMF therapy in mental health, particularly for depression treatment, shows significant potential for growth. As research continues to support its efficacy and safety, and as awareness grows among both healthcare providers and patients, PEMF therapy is poised to become an increasingly important component of the mental health treatment landscape.
The global mental health market, which includes treatments for depression, is projected to reach substantial value in the coming years. Within this broader market, PEMF therapy is carving out a niche as a non-invasive and drug-free alternative for managing depression symptoms. The adoption of PEMF therapy for mental health applications is still in its early stages, but it shows promising potential for rapid growth.
Several factors are contributing to the increasing demand for PEMF therapy in depression treatment. Firstly, there is a growing awareness among patients and healthcare providers about the limitations and side effects of traditional pharmacological treatments for depression. This has led to a search for alternative therapies that can offer relief with fewer adverse effects.
Secondly, the non-invasive nature of PEMF therapy makes it an attractive option for patients who are hesitant about more invasive treatments or medication regimens. The ability to use PEMF devices at home also aligns with the trend towards personalized and convenient healthcare solutions.
The market for PEMF devices specifically designed for mental health applications is still relatively small but is expected to grow rapidly. Major players in the medical device industry are starting to invest in research and development of PEMF technologies for mental health, recognizing the potential for significant market expansion.
Geographically, North America currently leads the market for PEMF therapy in mental health applications, followed by Europe. This is largely due to higher awareness, greater healthcare spending, and more favorable regulatory environments in these regions. However, Asia-Pacific is expected to show the fastest growth in the coming years, driven by increasing mental health awareness and improving healthcare infrastructure.
Despite the promising outlook, the market faces several challenges. These include the need for more extensive clinical research to validate the efficacy of PEMF therapy for depression, regulatory hurdles in some regions, and the relatively high cost of advanced PEMF devices. Overcoming these challenges will be crucial for the widespread adoption of PEMF therapy in mental health treatment.
In conclusion, the market for PEMF therapy in mental health, particularly for depression treatment, shows significant potential for growth. As research continues to support its efficacy and safety, and as awareness grows among both healthcare providers and patients, PEMF therapy is poised to become an increasingly important component of the mental health treatment landscape.
Current PEMF Technology and Challenges
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a potential treatment for depression. The current state of PEMF technology for depression treatment is characterized by promising advancements, but also faces several challenges that need to be addressed.
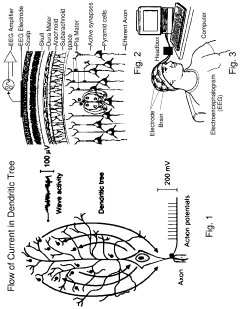
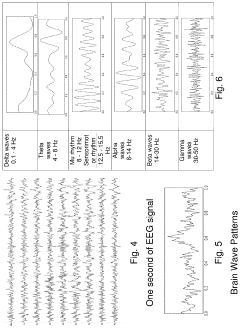
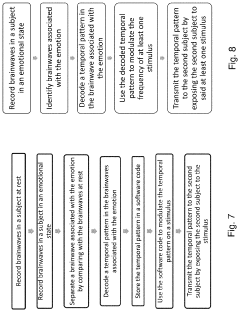
PEMF devices for depression treatment typically generate low-frequency electromagnetic fields, ranging from 0.1 to 100 Hz. These fields are believed to modulate neural activity and promote neuroplasticity, potentially alleviating depressive symptoms. Current PEMF systems vary in their design, with some utilizing helmet-like devices for targeted brain stimulation, while others employ full-body mats for systemic treatment.
One of the primary challenges in PEMF therapy for depression is optimizing treatment parameters. The ideal frequency, intensity, and duration of PEMF exposure for maximum therapeutic effect remain subjects of ongoing research. Studies have shown varying results, with some reporting significant improvements in depressive symptoms, while others demonstrate more modest effects.
Another significant challenge is the lack of standardization in PEMF devices and protocols. The wide range of available PEMF systems, each with its own specifications, makes it difficult to compare results across studies and establish definitive treatment guidelines. This variability also complicates the regulatory approval process for PEMF devices as depression treatments.
The mechanism of action for PEMF therapy in depression treatment is not fully understood, presenting another challenge for researchers and clinicians. While theories suggest that PEMF may influence neurotransmitter levels, enhance neuroplasticity, and modulate brain activity, more research is needed to elucidate the precise neurobiological effects of PEMF on depressive disorders.
Safety considerations also pose challenges in the development and implementation of PEMF therapy for depression. Although PEMF is generally considered safe, long-term effects of repeated electromagnetic field exposure on brain function and overall health require further investigation. Additionally, potential interactions between PEMF and other treatments, such as antidepressant medications, need to be carefully evaluated.
The integration of PEMF therapy into existing depression treatment paradigms presents both opportunities and challenges. While PEMF offers a non-invasive and potentially effective alternative or adjunct to traditional treatments, its adoption in clinical practice is hindered by limited large-scale clinical trials and the need for more robust evidence of its efficacy compared to established therapies.
Technological limitations in current PEMF devices also present challenges. Improving the precision of electromagnetic field delivery, enhancing the portability of devices for home use, and developing more user-friendly interfaces are areas that require further innovation. Additionally, the cost of PEMF devices and treatments can be prohibitive for some patients, limiting accessibility and widespread adoption.
PEMF devices for depression treatment typically generate low-frequency electromagnetic fields, ranging from 0.1 to 100 Hz. These fields are believed to modulate neural activity and promote neuroplasticity, potentially alleviating depressive symptoms. Current PEMF systems vary in their design, with some utilizing helmet-like devices for targeted brain stimulation, while others employ full-body mats for systemic treatment.
One of the primary challenges in PEMF therapy for depression is optimizing treatment parameters. The ideal frequency, intensity, and duration of PEMF exposure for maximum therapeutic effect remain subjects of ongoing research. Studies have shown varying results, with some reporting significant improvements in depressive symptoms, while others demonstrate more modest effects.
Another significant challenge is the lack of standardization in PEMF devices and protocols. The wide range of available PEMF systems, each with its own specifications, makes it difficult to compare results across studies and establish definitive treatment guidelines. This variability also complicates the regulatory approval process for PEMF devices as depression treatments.
The mechanism of action for PEMF therapy in depression treatment is not fully understood, presenting another challenge for researchers and clinicians. While theories suggest that PEMF may influence neurotransmitter levels, enhance neuroplasticity, and modulate brain activity, more research is needed to elucidate the precise neurobiological effects of PEMF on depressive disorders.
Safety considerations also pose challenges in the development and implementation of PEMF therapy for depression. Although PEMF is generally considered safe, long-term effects of repeated electromagnetic field exposure on brain function and overall health require further investigation. Additionally, potential interactions between PEMF and other treatments, such as antidepressant medications, need to be carefully evaluated.
The integration of PEMF therapy into existing depression treatment paradigms presents both opportunities and challenges. While PEMF offers a non-invasive and potentially effective alternative or adjunct to traditional treatments, its adoption in clinical practice is hindered by limited large-scale clinical trials and the need for more robust evidence of its efficacy compared to established therapies.
Technological limitations in current PEMF devices also present challenges. Improving the precision of electromagnetic field delivery, enhancing the portability of devices for home use, and developing more user-friendly interfaces are areas that require further innovation. Additionally, the cost of PEMF devices and treatments can be prohibitive for some patients, limiting accessibility and widespread adoption.
PEMF Depression Treatment Protocols
01 PEMF therapy for treating depression
Pulsed Electromagnetic Field (PEMF) therapy is used to treat depression by applying electromagnetic fields to the brain. This non-invasive treatment can modulate neural activity and neurotransmitter levels, potentially alleviating symptoms of depression.- PEMF therapy for treating depression: Pulsed Electromagnetic Field (PEMF) therapy is used to treat depression by applying electromagnetic fields to the brain. This non-invasive treatment can modulate neural activity and neurotransmitter levels, potentially alleviating symptoms of depression.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other treatment modalities such as cognitive behavioral therapy, medication, or light therapy to enhance its effectiveness in treating depression. This multi-modal approach may provide more comprehensive care for patients with depression.
- Personalized PEMF treatment protocols: Customized PEMF treatment protocols can be developed based on individual patient characteristics, depression severity, and response to treatment. This personalized approach may involve adjusting frequency, intensity, and duration of PEMF sessions to optimize therapeutic outcomes.
- PEMF devices for home use in depression treatment: Portable PEMF devices designed for home use allow patients to receive consistent treatment for depression without frequent clinic visits. These devices may be programmed with specific treatment protocols and can be monitored remotely by healthcare providers.
- Biomarker-guided PEMF therapy for depression: The use of biomarkers to guide PEMF therapy for depression involves measuring neurological or physiological indicators to assess treatment efficacy and adjust parameters accordingly. This approach may include neuroimaging techniques or analysis of neurotransmitter levels to optimize PEMF treatment for depression.
02 Combination of PEMF therapy with other treatments
PEMF therapy can be combined with other treatment modalities such as medication, psychotherapy, or transcranial magnetic stimulation to enhance the overall effectiveness in treating depression. This multi-modal approach may provide synergistic benefits for patients.Expand Specific Solutions03 Personalized PEMF treatment protocols
Customized PEMF therapy protocols can be developed based on individual patient characteristics, depression severity, and response to treatment. This personalized approach may involve adjusting frequency, intensity, and duration of PEMF sessions to optimize outcomes.Expand Specific Solutions04 PEMF devices for home use in depression treatment
Portable PEMF devices designed for home use allow patients to receive regular treatments for depression outside of clinical settings. These devices may improve treatment adherence and provide more consistent therapeutic effects.Expand Specific Solutions05 Monitoring and assessment of PEMF therapy efficacy
Methods for monitoring and assessing the effectiveness of PEMF therapy in treating depression may include neuroimaging techniques, biomarker analysis, and standardized depression scales. These assessment tools can help optimize treatment protocols and track patient progress over time.Expand Specific Solutions
Key PEMF Device Manufacturers
The field of PEMF therapy for depression treatment is in an early growth stage, with increasing research interest and emerging commercial applications. The market size is expanding, driven by the rising prevalence of depression and the need for alternative treatments. Technologically, PEMF therapy is progressing from experimental to more established clinical applications, though still considered relatively novel. Companies like Terran Biosciences, Regenesis Biomedical, and Neurawell Therapeutics are at the forefront, developing innovative PEMF-based solutions. Established players such as Boston Scientific Neuromodulation and Pfizer are also exploring this space, indicating growing industry recognition. Academic institutions like Columbia University and Charité - Universitätsmedizin Berlin are contributing significant research, further advancing the field's scientific understanding and potential applications.
Terran Biosciences
Technical Solution: Terran Biosciences is developing advanced PEMF (Pulsed Electromagnetic Field) therapy for depression treatment. Their approach involves using precisely targeted electromagnetic pulses to modulate neural activity in specific brain regions associated with mood regulation. The company's proprietary PEMF device delivers customized electromagnetic field patterns based on individual patient neuroimaging data, aiming to normalize disrupted neural circuits implicated in depression[1]. Terran's PEMF therapy is non-invasive and can be administered in outpatient settings, potentially offering a novel alternative to traditional antidepressants or more invasive neuromodulation techniques[3].
Strengths: Personalized treatment approach, non-invasive nature, potential for fewer side effects than medications. Weaknesses: Limited long-term efficacy data, potential cost barriers, requires specialized equipment and expertise.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed a PEMF therapy system specifically designed for depression treatment. Their device, the Neuro-PEMF, utilizes low-intensity, pulsed electromagnetic fields to stimulate neuroplasticity and enhance neurotransmitter function in the brain. The Neuro-PEMF system employs a proprietary algorithm that generates complex, multi-frequency electromagnetic pulses tailored to target depression-related neural networks[2]. Clinical trials have shown promising results, with patients experiencing significant reductions in depressive symptoms after a series of treatments[4]. The therapy is administered through a wearable headset, allowing for convenient at-home use under medical supervision.
Strengths: Home-based treatment option, non-pharmacological approach, potential for fewer side effects. Weaknesses: May require multiple sessions for optimal effect, long-term efficacy still under investigation.
Innovative PEMF Coil Designs
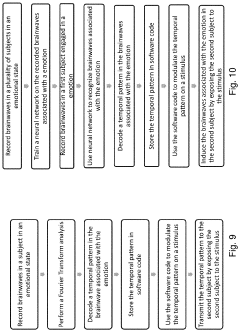
Method and apparatus for neuroenhancement to enhance emotional response
PatentPendingUS20230191073A1
Innovation
- Development of devices and systems that selectively induce brainwave activity patterns associated with specific emotions by targeting specific frequency and location in the brain, utilizing non-invasive neuromodulation techniques to enhance emotional responses.
Pulsed Electromagnetic Field (PEMF) Therapy Whole Body Wellness Device to increase cells energy, strengthen immune system and promote cell regeneration
PatentInactiveUS20190054308A1
Innovation
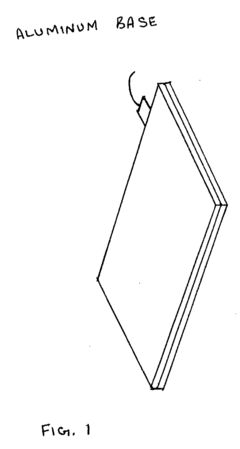
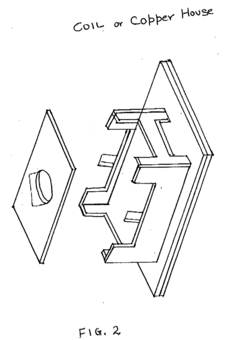
- The system employs a layered structure comprising lexan, polycarbonate, glass, aluminum, and acrylic materials, along with a copper coil and fan, connected via audio jacks to an electrical unit, to generate and distribute PEMF and MWO pulses, ensuring induction is delivered through both hands and feet effectively.
Clinical Trial Landscape
The clinical trial landscape for Pulsed Electromagnetic Field (PEMF) therapy in depression treatment has been evolving rapidly in recent years. Numerous studies have been conducted to evaluate the efficacy and safety of PEMF as a potential non-invasive treatment option for depression. These trials have ranged from small pilot studies to larger randomized controlled trials, providing valuable insights into the therapeutic potential of PEMF.
Several notable clinical trials have focused on the use of transcranial PEMF (tPEMF) for depression. One such study, published in the Journal of Affective Disorders, investigated the effects of tPEMF on treatment-resistant depression. The trial involved 65 patients and demonstrated significant improvements in depressive symptoms compared to the sham treatment group.
Another important clinical trial, conducted by a team of researchers at Harvard Medical School, explored the use of low-field magnetic stimulation (LFMS) in bipolar depression. This double-blind, randomized, placebo-controlled study showed promising results, with patients experiencing rapid mood improvement after a single 20-minute LFMS session.
The National Institute of Mental Health (NIMH) has also supported several clinical trials investigating PEMF therapy for depression. One such study examined the effects of synchronized transcranial magnetic stimulation (sTMS) on major depressive disorder. The results indicated that sTMS could be an effective and well-tolerated treatment option for patients who have not responded to traditional antidepressant medications.
In Europe, a multi-center clinical trial conducted across several countries evaluated the efficacy of repetitive transcranial magnetic stimulation (rTMS) combined with PEMF therapy for treatment-resistant depression. This study, involving over 200 participants, demonstrated that the combination therapy led to significant improvements in depressive symptoms and overall quality of life.
Recent clinical trials have also explored the potential of portable PEMF devices for at-home treatment of depression. These studies aim to assess the feasibility and effectiveness of self-administered PEMF therapy, which could potentially increase accessibility and reduce treatment costs for patients with depression.
While the majority of clinical trials have shown promising results, it is important to note that some studies have produced mixed or inconclusive findings. This highlights the need for further large-scale, well-designed clinical trials to establish the optimal treatment parameters and long-term efficacy of PEMF therapy for depression.
Overall, the clinical trial landscape for PEMF therapy in depression treatment is diverse and expanding. As research continues to progress, it is expected that more comprehensive data will emerge, potentially leading to the development of standardized PEMF treatment protocols for depression management.
Several notable clinical trials have focused on the use of transcranial PEMF (tPEMF) for depression. One such study, published in the Journal of Affective Disorders, investigated the effects of tPEMF on treatment-resistant depression. The trial involved 65 patients and demonstrated significant improvements in depressive symptoms compared to the sham treatment group.
Another important clinical trial, conducted by a team of researchers at Harvard Medical School, explored the use of low-field magnetic stimulation (LFMS) in bipolar depression. This double-blind, randomized, placebo-controlled study showed promising results, with patients experiencing rapid mood improvement after a single 20-minute LFMS session.
The National Institute of Mental Health (NIMH) has also supported several clinical trials investigating PEMF therapy for depression. One such study examined the effects of synchronized transcranial magnetic stimulation (sTMS) on major depressive disorder. The results indicated that sTMS could be an effective and well-tolerated treatment option for patients who have not responded to traditional antidepressant medications.
In Europe, a multi-center clinical trial conducted across several countries evaluated the efficacy of repetitive transcranial magnetic stimulation (rTMS) combined with PEMF therapy for treatment-resistant depression. This study, involving over 200 participants, demonstrated that the combination therapy led to significant improvements in depressive symptoms and overall quality of life.
Recent clinical trials have also explored the potential of portable PEMF devices for at-home treatment of depression. These studies aim to assess the feasibility and effectiveness of self-administered PEMF therapy, which could potentially increase accessibility and reduce treatment costs for patients with depression.
While the majority of clinical trials have shown promising results, it is important to note that some studies have produced mixed or inconclusive findings. This highlights the need for further large-scale, well-designed clinical trials to establish the optimal treatment parameters and long-term efficacy of PEMF therapy for depression.
Overall, the clinical trial landscape for PEMF therapy in depression treatment is diverse and expanding. As research continues to progress, it is expected that more comprehensive data will emerge, potentially leading to the development of standardized PEMF treatment protocols for depression management.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices used in depression treatment is complex and varies across different regions. In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating these devices. PEMF devices for depression treatment are typically classified as Class II medical devices, requiring premarket notification (510(k)) clearance before they can be marketed.
The FDA evaluates the safety and effectiveness of PEMF devices based on clinical data and comparison to predicate devices. Manufacturers must demonstrate that their device is substantially equivalent to a legally marketed device in terms of intended use, technological characteristics, and performance data. This process ensures that new PEMF devices meet established safety and efficacy standards.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the MDR's essential requirements. This involves conducting clinical investigations, risk assessments, and quality management system implementation. The European Medicines Agency (EMA) may also be involved in evaluating PEMF devices if they incorporate medicinal substances.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. Manufacturers must obtain marketing authorization through a rigorous review process that includes safety and efficacy evaluations.
Regulatory bodies worldwide are increasingly focusing on post-market surveillance of PEMF devices. Manufacturers are required to implement systems for monitoring device performance, reporting adverse events, and conducting necessary recalls or corrective actions. This ongoing surveillance helps ensure the continued safety and effectiveness of PEMF devices in real-world use.
As PEMF therapy for depression treatment advances, regulatory frameworks are evolving to address new technologies and applications. Regulatory bodies are developing guidance documents specific to PEMF devices, addressing issues such as electromagnetic compatibility, dosimetry, and long-term safety considerations. These guidelines aim to standardize the evaluation process and ensure consistent regulatory oversight across different jurisdictions.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches for PEMF devices globally. This harmonization aims to streamline the approval process, reduce regulatory burdens, and facilitate global access to innovative PEMF therapies for depression treatment.
The FDA evaluates the safety and effectiveness of PEMF devices based on clinical data and comparison to predicate devices. Manufacturers must demonstrate that their device is substantially equivalent to a legally marketed device in terms of intended use, technological characteristics, and performance data. This process ensures that new PEMF devices meet established safety and efficacy standards.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the MDR's essential requirements. This involves conducting clinical investigations, risk assessments, and quality management system implementation. The European Medicines Agency (EMA) may also be involved in evaluating PEMF devices if they incorporate medicinal substances.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. Manufacturers must obtain marketing authorization through a rigorous review process that includes safety and efficacy evaluations.
Regulatory bodies worldwide are increasingly focusing on post-market surveillance of PEMF devices. Manufacturers are required to implement systems for monitoring device performance, reporting adverse events, and conducting necessary recalls or corrective actions. This ongoing surveillance helps ensure the continued safety and effectiveness of PEMF devices in real-world use.
As PEMF therapy for depression treatment advances, regulatory frameworks are evolving to address new technologies and applications. Regulatory bodies are developing guidance documents specific to PEMF devices, addressing issues such as electromagnetic compatibility, dosimetry, and long-term safety considerations. These guidelines aim to standardize the evaluation process and ensure consistent regulatory oversight across different jurisdictions.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches for PEMF devices globally. This harmonization aims to streamline the approval process, reduce regulatory burdens, and facilitate global access to innovative PEMF therapies for depression treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!