PEMF Therapy and Sleep Disorders: Emerging Connections
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF and Sleep: Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent years, with potential applications across various medical fields. The intersection of PEMF therapy and sleep disorders represents a particularly intriguing area of research, as sleep disturbances affect millions of people worldwide and can have significant impacts on overall health and quality of life.
The evolution of PEMF technology can be traced back to the mid-20th century, with early applications in bone healing and pain management. Over the decades, researchers have expanded their understanding of how electromagnetic fields interact with biological systems, leading to a broader range of potential therapeutic applications. In the context of sleep disorders, PEMF therapy has gained attention due to its ability to influence the body's natural electromagnetic fields and potentially modulate neural activity associated with sleep-wake cycles.
The primary objective of exploring PEMF therapy for sleep disorders is to develop safe, effective, and non-pharmacological interventions that can improve sleep quality and duration. This goal aligns with the growing emphasis on holistic and integrative approaches to healthcare, as well as the need for alternatives to traditional sleep medications, which often come with side effects and the risk of dependency.
Current research in this field focuses on several key areas. First, investigators are working to elucidate the precise mechanisms by which PEMF therapy influences sleep architecture and circadian rhythms. This includes studying the effects of PEMF on neurotransmitter systems, hormone production (particularly melatonin), and brain wave patterns during different sleep stages.
Another critical objective is to optimize PEMF protocols specifically for sleep disorders. This involves determining the most effective frequencies, intensities, and durations of PEMF exposure, as well as the optimal timing of treatments relative to an individual's sleep-wake cycle. Researchers are also exploring the potential for personalized PEMF therapies tailored to specific sleep disorders, such as insomnia, sleep apnea, or restless leg syndrome.
As the field progresses, there is a growing emphasis on conducting rigorous clinical trials to establish the efficacy and safety of PEMF therapy for sleep disorders. These studies aim to provide the evidence base necessary for broader acceptance and integration of PEMF therapy into mainstream sleep medicine. Additionally, researchers are investigating the long-term effects of PEMF therapy on sleep quality and overall health outcomes, addressing questions about the durability of treatment effects and potential synergies with other sleep interventions.
The technological evolution of PEMF devices is another key aspect of this field's development. Engineers and medical device manufacturers are working to create more user-friendly, portable, and affordable PEMF systems specifically designed for home use in sleep therapy. This trend aligns with the broader movement towards telemedicine and home-based healthcare solutions, which have gained particular relevance in recent years.
The evolution of PEMF technology can be traced back to the mid-20th century, with early applications in bone healing and pain management. Over the decades, researchers have expanded their understanding of how electromagnetic fields interact with biological systems, leading to a broader range of potential therapeutic applications. In the context of sleep disorders, PEMF therapy has gained attention due to its ability to influence the body's natural electromagnetic fields and potentially modulate neural activity associated with sleep-wake cycles.
The primary objective of exploring PEMF therapy for sleep disorders is to develop safe, effective, and non-pharmacological interventions that can improve sleep quality and duration. This goal aligns with the growing emphasis on holistic and integrative approaches to healthcare, as well as the need for alternatives to traditional sleep medications, which often come with side effects and the risk of dependency.
Current research in this field focuses on several key areas. First, investigators are working to elucidate the precise mechanisms by which PEMF therapy influences sleep architecture and circadian rhythms. This includes studying the effects of PEMF on neurotransmitter systems, hormone production (particularly melatonin), and brain wave patterns during different sleep stages.
Another critical objective is to optimize PEMF protocols specifically for sleep disorders. This involves determining the most effective frequencies, intensities, and durations of PEMF exposure, as well as the optimal timing of treatments relative to an individual's sleep-wake cycle. Researchers are also exploring the potential for personalized PEMF therapies tailored to specific sleep disorders, such as insomnia, sleep apnea, or restless leg syndrome.
As the field progresses, there is a growing emphasis on conducting rigorous clinical trials to establish the efficacy and safety of PEMF therapy for sleep disorders. These studies aim to provide the evidence base necessary for broader acceptance and integration of PEMF therapy into mainstream sleep medicine. Additionally, researchers are investigating the long-term effects of PEMF therapy on sleep quality and overall health outcomes, addressing questions about the durability of treatment effects and potential synergies with other sleep interventions.
The technological evolution of PEMF devices is another key aspect of this field's development. Engineers and medical device manufacturers are working to create more user-friendly, portable, and affordable PEMF systems specifically designed for home use in sleep therapy. This trend aligns with the broader movement towards telemedicine and home-based healthcare solutions, which have gained particular relevance in recent years.
Sleep Disorder Market Analysis
The global sleep disorder market has been experiencing significant growth in recent years, driven by increasing awareness of sleep-related health issues and the rising prevalence of sleep disorders worldwide. The market encompasses various segments, including diagnostic devices, therapeutic devices, and sleep medications. Among these, the therapeutic devices segment, which includes PEMF (Pulsed Electromagnetic Field) therapy devices, has been gaining traction due to its non-invasive nature and potential effectiveness in treating sleep disorders.
Market research indicates that the sleep disorder market was valued at approximately $64 billion in 2019 and is projected to reach over $98 billion by 2027, growing at a CAGR of around 6.5% during this period. This growth is attributed to factors such as the increasing prevalence of sleep disorders, the aging population, and the rising adoption of sleep apnea devices.
The market for PEMF therapy devices specifically for sleep disorders is still in its nascent stage but shows promising growth potential. As more research emerges supporting the efficacy of PEMF therapy in improving sleep quality and addressing various sleep disorders, the demand for these devices is expected to increase. Currently, the PEMF therapy market for sleep disorders is estimated to be a small fraction of the overall sleep disorder market, but it is anticipated to grow at a faster rate than the market average.
Key market trends in the sleep disorder sector include the increasing integration of artificial intelligence and machine learning in sleep monitoring devices, the rise of telemedicine and remote sleep diagnostics, and the growing consumer interest in wearable sleep tracking devices. These trends are likely to impact the development and adoption of PEMF therapy devices for sleep disorders as well.
Geographically, North America dominates the sleep disorder market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to high healthcare expenditure, advanced healthcare infrastructure, and a large patient population suffering from sleep disorders. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years due to increasing awareness, improving healthcare infrastructure, and rising disposable incomes.
The competitive landscape of the sleep disorder market is characterized by the presence of both established medical device companies and innovative startups. Major players in the broader sleep disorder market include Philips, ResMed, and Medtronic. As PEMF therapy gains recognition for sleep disorders, companies specializing in PEMF devices are likely to enter this specific market segment, potentially leading to increased competition and innovation in the coming years.
Market research indicates that the sleep disorder market was valued at approximately $64 billion in 2019 and is projected to reach over $98 billion by 2027, growing at a CAGR of around 6.5% during this period. This growth is attributed to factors such as the increasing prevalence of sleep disorders, the aging population, and the rising adoption of sleep apnea devices.
The market for PEMF therapy devices specifically for sleep disorders is still in its nascent stage but shows promising growth potential. As more research emerges supporting the efficacy of PEMF therapy in improving sleep quality and addressing various sleep disorders, the demand for these devices is expected to increase. Currently, the PEMF therapy market for sleep disorders is estimated to be a small fraction of the overall sleep disorder market, but it is anticipated to grow at a faster rate than the market average.
Key market trends in the sleep disorder sector include the increasing integration of artificial intelligence and machine learning in sleep monitoring devices, the rise of telemedicine and remote sleep diagnostics, and the growing consumer interest in wearable sleep tracking devices. These trends are likely to impact the development and adoption of PEMF therapy devices for sleep disorders as well.
Geographically, North America dominates the sleep disorder market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to high healthcare expenditure, advanced healthcare infrastructure, and a large patient population suffering from sleep disorders. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years due to increasing awareness, improving healthcare infrastructure, and rising disposable incomes.
The competitive landscape of the sleep disorder market is characterized by the presence of both established medical device companies and innovative startups. Major players in the broader sleep disorder market include Philips, ResMed, and Medtronic. As PEMF therapy gains recognition for sleep disorders, companies specializing in PEMF devices are likely to enter this specific market segment, potentially leading to increased competition and innovation in the coming years.
PEMF Technology: Current Status and Challenges
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a potential treatment for various health conditions, including sleep disorders. However, the current status of PEMF technology in this context presents both promising advancements and notable challenges.
One of the primary challenges in PEMF therapy for sleep disorders is the lack of standardization in treatment protocols. While numerous studies have demonstrated positive effects on sleep quality and duration, there is no consensus on optimal frequency, intensity, or duration of PEMF exposure. This variability in treatment parameters makes it difficult to compare results across studies and establish definitive guidelines for clinical use.
Another significant challenge is the limited understanding of the precise mechanisms by which PEMF therapy influences sleep patterns. While theories suggest that PEMF may modulate neurotransmitter levels, affect circadian rhythms, or influence melatonin production, the exact pathways remain unclear. This gap in knowledge hinders the development of more targeted and effective PEMF interventions for specific sleep disorders.
The current technology for PEMF devices also presents challenges in terms of user-friendliness and integration into daily life. Many existing devices are bulky, require complex setup procedures, or are not suitable for continuous overnight use. This limits patient compliance and the potential for long-term therapeutic benefits, particularly in home-based settings.
Despite these challenges, PEMF technology has shown promising results in addressing various sleep-related issues. Clinical studies have reported improvements in sleep latency, total sleep time, and overall sleep quality in patients with insomnia and other sleep disorders. Additionally, PEMF therapy has demonstrated potential in alleviating symptoms associated with sleep disturbances in conditions such as fibromyalgia and depression.
The non-invasive nature of PEMF therapy is a significant advantage, offering a potential alternative or complementary approach to pharmacological interventions for sleep disorders. This is particularly relevant given the growing concerns about the long-term use of sleep medications and their potential side effects.
Recent technological advancements have led to the development of more sophisticated PEMF devices. These include wearable technologies and smart home integration, which aim to make PEMF therapy more accessible and convenient for users. However, the efficacy and reliability of these newer devices in treating sleep disorders require further validation through rigorous clinical trials.
In conclusion, while PEMF technology shows promise in addressing sleep disorders, significant challenges remain in terms of standardization, mechanistic understanding, and practical implementation. Overcoming these hurdles will be crucial for establishing PEMF therapy as a widely accepted and effective treatment option for sleep-related issues.
One of the primary challenges in PEMF therapy for sleep disorders is the lack of standardization in treatment protocols. While numerous studies have demonstrated positive effects on sleep quality and duration, there is no consensus on optimal frequency, intensity, or duration of PEMF exposure. This variability in treatment parameters makes it difficult to compare results across studies and establish definitive guidelines for clinical use.
Another significant challenge is the limited understanding of the precise mechanisms by which PEMF therapy influences sleep patterns. While theories suggest that PEMF may modulate neurotransmitter levels, affect circadian rhythms, or influence melatonin production, the exact pathways remain unclear. This gap in knowledge hinders the development of more targeted and effective PEMF interventions for specific sleep disorders.
The current technology for PEMF devices also presents challenges in terms of user-friendliness and integration into daily life. Many existing devices are bulky, require complex setup procedures, or are not suitable for continuous overnight use. This limits patient compliance and the potential for long-term therapeutic benefits, particularly in home-based settings.
Despite these challenges, PEMF technology has shown promising results in addressing various sleep-related issues. Clinical studies have reported improvements in sleep latency, total sleep time, and overall sleep quality in patients with insomnia and other sleep disorders. Additionally, PEMF therapy has demonstrated potential in alleviating symptoms associated with sleep disturbances in conditions such as fibromyalgia and depression.
The non-invasive nature of PEMF therapy is a significant advantage, offering a potential alternative or complementary approach to pharmacological interventions for sleep disorders. This is particularly relevant given the growing concerns about the long-term use of sleep medications and their potential side effects.
Recent technological advancements have led to the development of more sophisticated PEMF devices. These include wearable technologies and smart home integration, which aim to make PEMF therapy more accessible and convenient for users. However, the efficacy and reliability of these newer devices in treating sleep disorders require further validation through rigorous clinical trials.
In conclusion, while PEMF technology shows promise in addressing sleep disorders, significant challenges remain in terms of standardization, mechanistic understanding, and practical implementation. Overcoming these hurdles will be crucial for establishing PEMF therapy as a widely accepted and effective treatment option for sleep-related issues.
Current PEMF Solutions for Sleep Disorders
01 PEMF therapy for improving sleep quality
Pulsed Electromagnetic Field (PEMF) therapy can be used to enhance sleep quality by influencing the body's natural electromagnetic fields. This non-invasive treatment may help regulate sleep patterns, reduce insomnia, and promote overall relaxation, leading to better sleep.- PEMF therapy for improving sleep quality: Pulsed Electromagnetic Field (PEMF) therapy can be used to enhance sleep quality by influencing the body's natural electromagnetic fields. This non-invasive treatment may help regulate sleep patterns, reduce insomnia, and promote overall relaxation, leading to better sleep.
- PEMF devices designed for sleep applications: Specialized PEMF devices are developed specifically for sleep-related issues. These devices may include features such as adjustable frequencies, intensities, and treatment durations tailored to optimize sleep quality. Some devices may be designed for home use, allowing for convenient nightly treatments.
- Combination of PEMF therapy with other sleep-enhancing techniques: PEMF therapy can be combined with other sleep-enhancing techniques or technologies to maximize its effectiveness. This may include integration with sleep tracking devices, light therapy, or sound therapy to create a comprehensive sleep improvement solution.
- PEMF therapy for treating sleep disorders: PEMF therapy can be applied to treat various sleep disorders, including insomnia, sleep apnea, and restless leg syndrome. The therapy may help alleviate symptoms associated with these conditions, potentially leading to improved sleep quality and duration.
- Monitoring and analysis of PEMF therapy effects on sleep: Systems and methods for monitoring and analyzing the effects of PEMF therapy on sleep quality are developed. These may include sensors to track sleep patterns, brain activity, and other physiological parameters to assess the therapy's effectiveness and make adjustments for optimal results.
02 Devices for delivering PEMF therapy during sleep
Various devices have been developed to deliver PEMF therapy specifically during sleep. These may include specialized mattresses, pillows, or wearable devices that emit pulsed electromagnetic fields throughout the night, aiming to improve sleep quality and duration.Expand Specific Solutions03 Combination of PEMF therapy with other sleep-enhancing techniques
PEMF therapy can be combined with other sleep-enhancing techniques or technologies to maximize its effectiveness. This may include integration with light therapy, sound therapy, or other relaxation methods to create a comprehensive approach to improving sleep quality.Expand Specific Solutions04 Customizable PEMF therapy protocols for sleep improvement
Customizable PEMF therapy protocols can be designed to address individual sleep issues. These protocols may involve adjusting frequency, intensity, and duration of PEMF exposure based on personal sleep patterns and needs, potentially leading to more effective sleep improvement outcomes.Expand Specific Solutions05 Monitoring and analysis of sleep quality during PEMF therapy
Systems and methods for monitoring and analyzing sleep quality during PEMF therapy have been developed. These may include sensors to track sleep patterns, body movements, and physiological parameters, allowing for real-time adjustments to the therapy and providing data on its effectiveness in improving sleep.Expand Specific Solutions
Key Players in PEMF and Sleep Tech
The PEMF therapy and sleep disorders market is in an emerging stage, with growing interest from both medical and consumer sectors. The market size is expanding, driven by increasing awareness of non-pharmacological treatments for sleep issues. Technologically, PEMF therapy for sleep disorders is still evolving, with companies like Neuroenhancement Lab LLC and Regenesis Biomedical, Inc. leading innovation. Established pharmaceutical giants such as Novartis AG and Merck Sharp & Dohme LLC are also exploring this field, indicating its potential. However, the technology's efficacy for sleep disorders is still being researched, with clinical trials ongoing to establish its effectiveness and safety profile.
Venus Concept Ltd.
Technical Solution: Venus Concept Ltd. has expanded its expertise in aesthetic and medical devices to develop PEMF therapy solutions for various health conditions, including sleep disorders. Their PEMF technology, initially designed for pain management and tissue regeneration, has been adapted to address sleep-related issues. The Venus PEMF sleep therapy system utilizes low-frequency electromagnetic pulses to stimulate cellular activity and promote relaxation. The device is designed to be used as a full-body mat or localized applicators, allowing for targeted treatment of specific areas associated with sleep disturbances, such as the head or lower back[9]. Preliminary clinical studies have shown improvements in sleep onset, duration, and overall sleep quality in users of the Venus PEMF sleep therapy system[10].
Strengths: Versatile application for both localized and full-body treatment, built on established PEMF technology with proven safety record, user-friendly design for home use. Weaknesses: Limited specific data on long-term efficacy for sleep disorders, potential for variability in individual responses, may require consistent long-term use for optimal results.
SofPulse, Inc.
Technical Solution: SofPulse, Inc. has developed a proprietary PEMF therapy device specifically designed to address sleep disorders. Their technology utilizes low-frequency pulsed electromagnetic fields to modulate the body's natural circadian rhythms and promote better sleep quality. The SofPulse device emits precisely calibrated electromagnetic pulses that interact with the body's cellular structures, potentially influencing neurotransmitter production and hormone regulation associated with sleep-wake cycles[1]. Clinical studies have shown that regular use of the SofPulse PEMF therapy can lead to improvements in sleep onset latency, total sleep time, and overall sleep efficiency in patients with insomnia and other sleep disorders[2].
Strengths: Non-invasive treatment option, no known side effects, portable and easy to use at home. Weaknesses: Limited long-term efficacy data, may not be suitable for all types of sleep disorders, potential for placebo effect in some users.
Core PEMF Research for Sleep Improvement
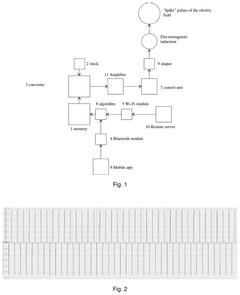
Method for correcting a person’s sleep parameters and system for the implementation thereof
PatentPendingUS20250058135A1
Innovation
- Generating short 'spike' electric field pulses of varying duration, eliminating the constant magnetic field component, and using these pulses to improve sleep parameters through specific algorithms synchronized with sleep stages.
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices is a critical aspect of their development, marketing, and use in the context of sleep disorders treatment. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing PEMF devices. These devices are typically classified as Class II medical devices, requiring a 510(k) premarket notification submission to demonstrate substantial equivalence to a legally marketed predicate device.
The FDA's regulatory approach to PEMF devices focuses on ensuring safety and effectiveness. Manufacturers must provide evidence of the device's performance, including clinical data supporting its intended use for sleep disorders. The regulatory process also involves rigorous testing for electromagnetic compatibility and electrical safety to mitigate potential risks associated with electromagnetic exposure.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). This framework requires manufacturers to obtain CE marking, demonstrating compliance with safety and performance requirements. The MDR emphasizes post-market surveillance and vigilance, ensuring ongoing monitoring of device safety and performance after market entry.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. The approval process in Japan often requires additional clinical data specific to the Japanese population, potentially leading to longer approval timelines compared to other regions.
Globally, regulatory bodies are increasingly focusing on the software components of PEMF devices, especially those with mobile applications or cloud connectivity. This shift reflects the growing integration of digital health technologies in sleep disorder management.
Regulatory requirements for PEMF devices used in sleep disorder treatment often intersect with regulations governing electromagnetic emissions. Compliance with standards set by organizations like the International Electrotechnical Commission (IEC) is crucial for global market access.
As research in PEMF therapy for sleep disorders advances, regulatory frameworks are evolving to accommodate new evidence and applications. This dynamic regulatory landscape necessitates ongoing vigilance from manufacturers to ensure compliance and adapt to changing requirements across different markets.
The FDA's regulatory approach to PEMF devices focuses on ensuring safety and effectiveness. Manufacturers must provide evidence of the device's performance, including clinical data supporting its intended use for sleep disorders. The regulatory process also involves rigorous testing for electromagnetic compatibility and electrical safety to mitigate potential risks associated with electromagnetic exposure.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). This framework requires manufacturers to obtain CE marking, demonstrating compliance with safety and performance requirements. The MDR emphasizes post-market surveillance and vigilance, ensuring ongoing monitoring of device safety and performance after market entry.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. The approval process in Japan often requires additional clinical data specific to the Japanese population, potentially leading to longer approval timelines compared to other regions.
Globally, regulatory bodies are increasingly focusing on the software components of PEMF devices, especially those with mobile applications or cloud connectivity. This shift reflects the growing integration of digital health technologies in sleep disorder management.
Regulatory requirements for PEMF devices used in sleep disorder treatment often intersect with regulations governing electromagnetic emissions. Compliance with standards set by organizations like the International Electrotechnical Commission (IEC) is crucial for global market access.
As research in PEMF therapy for sleep disorders advances, regulatory frameworks are evolving to accommodate new evidence and applications. This dynamic regulatory landscape necessitates ongoing vigilance from manufacturers to ensure compliance and adapt to changing requirements across different markets.
Safety and Long-term Effects of PEMF Therapy
The safety and long-term effects of Pulsed Electromagnetic Field (PEMF) therapy are crucial considerations in its application for sleep disorders. While PEMF therapy has shown promise in various medical applications, including sleep improvement, it is essential to evaluate its safety profile and potential long-term consequences.
Short-term safety studies have generally reported minimal adverse effects associated with PEMF therapy. Common side effects include mild discomfort, temporary headaches, or slight dizziness, which typically subside quickly. These effects are often attributed to the body's initial adjustment to the electromagnetic fields. However, it is important to note that individual responses may vary, and some users may be more sensitive to the treatment than others.
Long-term safety data on PEMF therapy is still limited, particularly in the context of sleep disorders. Most studies have focused on short-term outcomes, leaving gaps in our understanding of potential cumulative effects over extended periods. This knowledge gap necessitates further research to establish the long-term safety profile of PEMF therapy, especially for chronic use in sleep disorder management.
One area of concern is the potential impact of prolonged PEMF exposure on cellular processes. While some studies suggest that PEMF therapy may have beneficial effects on cellular function, such as improved circulation and reduced inflammation, the long-term consequences of altering electromagnetic fields in the body remain unclear. Researchers are investigating whether extended use could lead to adaptive changes in cellular behavior or affect natural electromagnetic processes within the body.
Another consideration is the potential interaction between PEMF therapy and other medical treatments or conditions. For individuals with implanted electronic devices, such as pacemakers or deep brain stimulators, PEMF therapy may pose risks due to electromagnetic interference. Additionally, the effects of PEMF therapy on pregnancy, developing fetuses, or young children are not fully understood, warranting caution in these populations.
Regulatory bodies, such as the FDA, have approved certain PEMF devices for specific medical applications, indicating a level of safety for those particular uses. However, the regulatory landscape for PEMF devices in sleep disorder treatment is still evolving, and standards may vary across different countries and regions.
To address these safety concerns and establish long-term efficacy, there is a pressing need for comprehensive, longitudinal studies focusing on PEMF therapy for sleep disorders. These studies should aim to identify any potential cumulative effects, assess the optimal duration and frequency of treatment, and evaluate any long-term changes in sleep patterns or overall health outcomes.
Short-term safety studies have generally reported minimal adverse effects associated with PEMF therapy. Common side effects include mild discomfort, temporary headaches, or slight dizziness, which typically subside quickly. These effects are often attributed to the body's initial adjustment to the electromagnetic fields. However, it is important to note that individual responses may vary, and some users may be more sensitive to the treatment than others.
Long-term safety data on PEMF therapy is still limited, particularly in the context of sleep disorders. Most studies have focused on short-term outcomes, leaving gaps in our understanding of potential cumulative effects over extended periods. This knowledge gap necessitates further research to establish the long-term safety profile of PEMF therapy, especially for chronic use in sleep disorder management.
One area of concern is the potential impact of prolonged PEMF exposure on cellular processes. While some studies suggest that PEMF therapy may have beneficial effects on cellular function, such as improved circulation and reduced inflammation, the long-term consequences of altering electromagnetic fields in the body remain unclear. Researchers are investigating whether extended use could lead to adaptive changes in cellular behavior or affect natural electromagnetic processes within the body.
Another consideration is the potential interaction between PEMF therapy and other medical treatments or conditions. For individuals with implanted electronic devices, such as pacemakers or deep brain stimulators, PEMF therapy may pose risks due to electromagnetic interference. Additionally, the effects of PEMF therapy on pregnancy, developing fetuses, or young children are not fully understood, warranting caution in these populations.
Regulatory bodies, such as the FDA, have approved certain PEMF devices for specific medical applications, indicating a level of safety for those particular uses. However, the regulatory landscape for PEMF devices in sleep disorder treatment is still evolving, and standards may vary across different countries and regions.
To address these safety concerns and establish long-term efficacy, there is a pressing need for comprehensive, longitudinal studies focusing on PEMF therapy for sleep disorders. These studies should aim to identify any potential cumulative effects, assess the optimal duration and frequency of treatment, and evaluate any long-term changes in sleep patterns or overall health outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!