Advancing Regulatory Standards for PEMF Therapy in Medical Devices
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Evolution
Pulsed Electromagnetic Field (PEMF) therapy has undergone significant evolution since its inception in the mid-20th century. Initially developed for bone healing, PEMF therapy has expanded its applications across various medical fields. The therapy's evolution can be traced through several key phases, each marked by technological advancements and broadening clinical applications.
In the 1950s, scientists first observed the piezoelectric effect in bones, leading to the development of PEMF devices for bone healing. The 1970s saw the FDA approval of PEMF devices for non-union fractures, marking a significant milestone in the therapy's acceptance within mainstream medicine. This period also witnessed the refinement of PEMF technology, with improvements in device portability and treatment protocols.
The 1980s and 1990s brought about a expansion in PEMF research, exploring its potential in treating soft tissue injuries, pain management, and neurological disorders. This era saw the development of more sophisticated PEMF devices, capable of delivering precise electromagnetic pulses at varying frequencies and intensities. Concurrently, researchers began investigating the cellular mechanisms underlying PEMF therapy, enhancing our understanding of its therapeutic effects.
The turn of the millennium heralded a new phase in PEMF therapy evolution, characterized by miniaturization and increased accessibility. Advances in electronics and materials science enabled the creation of smaller, more user-friendly PEMF devices suitable for home use. This democratization of PEMF technology expanded its reach beyond clinical settings, allowing for more frequent and convenient treatments.
Recent years have witnessed a surge in PEMF therapy applications, extending to areas such as mental health, sports medicine, and anti-aging treatments. The integration of PEMF technology with other therapeutic modalities, such as photobiomodulation and biofeedback systems, has opened new avenues for holistic patient care. Additionally, the advent of smart, connected PEMF devices has enabled real-time monitoring and personalization of treatments, enhancing efficacy and patient compliance.
As PEMF therapy continues to evolve, current research focuses on optimizing treatment parameters for specific conditions and exploring novel applications in regenerative medicine and neurodegenerative disorders. The development of targeted PEMF therapies, designed to affect specific cellular pathways or tissue types, represents a promising frontier in this field. Furthermore, ongoing efforts to standardize PEMF protocols and establish comprehensive clinical guidelines underscore the therapy's maturation as a recognized medical intervention.
The evolution of PEMF therapy reflects a broader trend in medical technology towards non-invasive, personalized treatments. As regulatory standards advance to keep pace with these developments, the future of PEMF therapy holds the potential for even greater integration into mainstream medical practice, promising improved patient outcomes across a wide spectrum of health conditions.
In the 1950s, scientists first observed the piezoelectric effect in bones, leading to the development of PEMF devices for bone healing. The 1970s saw the FDA approval of PEMF devices for non-union fractures, marking a significant milestone in the therapy's acceptance within mainstream medicine. This period also witnessed the refinement of PEMF technology, with improvements in device portability and treatment protocols.
The 1980s and 1990s brought about a expansion in PEMF research, exploring its potential in treating soft tissue injuries, pain management, and neurological disorders. This era saw the development of more sophisticated PEMF devices, capable of delivering precise electromagnetic pulses at varying frequencies and intensities. Concurrently, researchers began investigating the cellular mechanisms underlying PEMF therapy, enhancing our understanding of its therapeutic effects.
The turn of the millennium heralded a new phase in PEMF therapy evolution, characterized by miniaturization and increased accessibility. Advances in electronics and materials science enabled the creation of smaller, more user-friendly PEMF devices suitable for home use. This democratization of PEMF technology expanded its reach beyond clinical settings, allowing for more frequent and convenient treatments.
Recent years have witnessed a surge in PEMF therapy applications, extending to areas such as mental health, sports medicine, and anti-aging treatments. The integration of PEMF technology with other therapeutic modalities, such as photobiomodulation and biofeedback systems, has opened new avenues for holistic patient care. Additionally, the advent of smart, connected PEMF devices has enabled real-time monitoring and personalization of treatments, enhancing efficacy and patient compliance.
As PEMF therapy continues to evolve, current research focuses on optimizing treatment parameters for specific conditions and exploring novel applications in regenerative medicine and neurodegenerative disorders. The development of targeted PEMF therapies, designed to affect specific cellular pathways or tissue types, represents a promising frontier in this field. Furthermore, ongoing efforts to standardize PEMF protocols and establish comprehensive clinical guidelines underscore the therapy's maturation as a recognized medical intervention.
The evolution of PEMF therapy reflects a broader trend in medical technology towards non-invasive, personalized treatments. As regulatory standards advance to keep pace with these developments, the future of PEMF therapy holds the potential for even greater integration into mainstream medical practice, promising improved patient outcomes across a wide spectrum of health conditions.
Market Demand Analysis
The market demand for PEMF (Pulsed Electromagnetic Field) therapy in medical devices has been steadily growing, driven by increasing awareness of its potential benefits and a shift towards non-invasive treatment options. This technology has gained traction in various medical applications, including pain management, bone healing, and tissue regeneration.
The global PEMF therapy devices market is experiencing significant expansion, with projections indicating continued growth in the coming years. This growth is attributed to the rising prevalence of chronic diseases, an aging population, and the increasing adoption of alternative therapies. The market is particularly strong in regions with advanced healthcare systems, such as North America and Europe, where there is a higher acceptance of innovative medical technologies.
One of the key drivers of market demand is the growing body of clinical evidence supporting the efficacy of PEMF therapy. As more research demonstrates positive outcomes in various medical conditions, healthcare providers and patients are becoming more receptive to incorporating PEMF devices into treatment protocols. This increased acceptance is leading to a higher adoption rate in both clinical settings and home-use applications.
The market is also benefiting from technological advancements in PEMF devices. Manufacturers are developing more sophisticated, user-friendly, and portable devices, making PEMF therapy more accessible to a broader range of users. These innovations are expanding the potential applications of PEMF therapy and contributing to market growth.
However, the market faces challenges related to regulatory standards and approval processes. The lack of standardized protocols and varying regulatory requirements across different regions can hinder market expansion. This underscores the need for advancing regulatory standards to ensure safety, efficacy, and consistency in PEMF therapy devices.
Despite these challenges, the market shows promising trends. There is a growing interest in integrating PEMF therapy with other treatment modalities, creating opportunities for combination therapies. Additionally, the increasing focus on personalized medicine is driving demand for customizable PEMF devices that can be tailored to individual patient needs.
The COVID-19 pandemic has also influenced market dynamics, with a heightened interest in non-invasive, at-home treatment options. This has led to an increased demand for portable PEMF devices that can be used outside of clinical settings, further expanding the market potential.
As the field of PEMF therapy continues to evolve, there is a clear need for standardized regulatory frameworks to support market growth and ensure patient safety. Advancing regulatory standards will not only facilitate market expansion but also enhance the credibility and acceptance of PEMF therapy in mainstream medical practice.
The global PEMF therapy devices market is experiencing significant expansion, with projections indicating continued growth in the coming years. This growth is attributed to the rising prevalence of chronic diseases, an aging population, and the increasing adoption of alternative therapies. The market is particularly strong in regions with advanced healthcare systems, such as North America and Europe, where there is a higher acceptance of innovative medical technologies.
One of the key drivers of market demand is the growing body of clinical evidence supporting the efficacy of PEMF therapy. As more research demonstrates positive outcomes in various medical conditions, healthcare providers and patients are becoming more receptive to incorporating PEMF devices into treatment protocols. This increased acceptance is leading to a higher adoption rate in both clinical settings and home-use applications.
The market is also benefiting from technological advancements in PEMF devices. Manufacturers are developing more sophisticated, user-friendly, and portable devices, making PEMF therapy more accessible to a broader range of users. These innovations are expanding the potential applications of PEMF therapy and contributing to market growth.
However, the market faces challenges related to regulatory standards and approval processes. The lack of standardized protocols and varying regulatory requirements across different regions can hinder market expansion. This underscores the need for advancing regulatory standards to ensure safety, efficacy, and consistency in PEMF therapy devices.
Despite these challenges, the market shows promising trends. There is a growing interest in integrating PEMF therapy with other treatment modalities, creating opportunities for combination therapies. Additionally, the increasing focus on personalized medicine is driving demand for customizable PEMF devices that can be tailored to individual patient needs.
The COVID-19 pandemic has also influenced market dynamics, with a heightened interest in non-invasive, at-home treatment options. This has led to an increased demand for portable PEMF devices that can be used outside of clinical settings, further expanding the market potential.
As the field of PEMF therapy continues to evolve, there is a clear need for standardized regulatory frameworks to support market growth and ensure patient safety. Advancing regulatory standards will not only facilitate market expansion but also enhance the credibility and acceptance of PEMF therapy in mainstream medical practice.
Regulatory Challenges
The regulatory landscape for PEMF (Pulsed Electromagnetic Field) therapy in medical devices presents significant challenges for manufacturers, healthcare providers, and regulatory bodies. One of the primary obstacles is the lack of standardized guidelines specific to PEMF technology across different regulatory jurisdictions. This inconsistency creates uncertainty for device manufacturers and can lead to delays in product approval and market entry.
In the United States, the Food and Drug Administration (FDA) classifies PEMF devices under Class II or Class III, depending on their intended use and potential risks. However, the classification criteria and requirements for PEMF devices are not always clear-cut, leading to confusion among manufacturers about the appropriate regulatory pathway to pursue. This ambiguity can result in prolonged review processes and increased costs for companies seeking market approval.
The European Union's Medical Device Regulation (MDR) presents another set of challenges for PEMF therapy devices. The MDR's emphasis on clinical evidence and post-market surveillance has raised the bar for manufacturers, requiring more comprehensive data on device safety and efficacy. This increased scrutiny, while beneficial for patient safety, has created additional hurdles for companies developing PEMF technologies, particularly in terms of conducting clinical trials and gathering long-term data.
In emerging markets, the regulatory framework for PEMF devices is often less developed, leading to potential safety concerns and market fragmentation. The lack of harmonized standards across these regions can make it difficult for manufacturers to expand their global reach and can limit patient access to potentially beneficial PEMF therapies.
Another significant challenge is the rapid pace of technological advancement in the field of PEMF therapy. As new applications and device configurations emerge, regulatory bodies struggle to keep up with the evolving landscape. This lag can result in outdated regulations that do not adequately address the unique characteristics and potential risks of novel PEMF technologies.
The issue of electromagnetic compatibility (EMC) and potential interference with other medical devices adds another layer of complexity to the regulatory process. Ensuring that PEMF devices do not adversely affect the functioning of other critical medical equipment requires rigorous testing and validation, which can be time-consuming and costly for manufacturers.
Addressing these regulatory challenges requires a multi-faceted approach. Collaboration between industry stakeholders, regulatory bodies, and scientific experts is essential to develop more specific and harmonized guidelines for PEMF devices. Efforts to streamline the regulatory process, such as the FDA's Breakthrough Devices Program, could help accelerate the approval of innovative PEMF technologies that address unmet medical needs.
In the United States, the Food and Drug Administration (FDA) classifies PEMF devices under Class II or Class III, depending on their intended use and potential risks. However, the classification criteria and requirements for PEMF devices are not always clear-cut, leading to confusion among manufacturers about the appropriate regulatory pathway to pursue. This ambiguity can result in prolonged review processes and increased costs for companies seeking market approval.
The European Union's Medical Device Regulation (MDR) presents another set of challenges for PEMF therapy devices. The MDR's emphasis on clinical evidence and post-market surveillance has raised the bar for manufacturers, requiring more comprehensive data on device safety and efficacy. This increased scrutiny, while beneficial for patient safety, has created additional hurdles for companies developing PEMF technologies, particularly in terms of conducting clinical trials and gathering long-term data.
In emerging markets, the regulatory framework for PEMF devices is often less developed, leading to potential safety concerns and market fragmentation. The lack of harmonized standards across these regions can make it difficult for manufacturers to expand their global reach and can limit patient access to potentially beneficial PEMF therapies.
Another significant challenge is the rapid pace of technological advancement in the field of PEMF therapy. As new applications and device configurations emerge, regulatory bodies struggle to keep up with the evolving landscape. This lag can result in outdated regulations that do not adequately address the unique characteristics and potential risks of novel PEMF technologies.
The issue of electromagnetic compatibility (EMC) and potential interference with other medical devices adds another layer of complexity to the regulatory process. Ensuring that PEMF devices do not adversely affect the functioning of other critical medical equipment requires rigorous testing and validation, which can be time-consuming and costly for manufacturers.
Addressing these regulatory challenges requires a multi-faceted approach. Collaboration between industry stakeholders, regulatory bodies, and scientific experts is essential to develop more specific and harmonized guidelines for PEMF devices. Efforts to streamline the regulatory process, such as the FDA's Breakthrough Devices Program, could help accelerate the approval of innovative PEMF technologies that address unmet medical needs.
Current PEMF Standards
01 FDA Regulation and Approval Process
PEMF therapy devices are regulated by the FDA as medical devices. Manufacturers must follow specific guidelines for safety and efficacy testing, clinical trials, and documentation submission. The approval process may involve premarket notification (510(k)) or premarket approval (PMA) depending on the device classification and intended use.- FDA regulation and clearance for PEMF devices: PEMF therapy devices are regulated by the FDA as medical devices. Manufacturers must obtain FDA clearance or approval before marketing these devices in the US. The regulatory process involves demonstrating safety and efficacy through clinical trials and compliance with quality system regulations.
- International standards for electromagnetic field exposure: PEMF therapy devices must comply with international standards for electromagnetic field exposure limits. These standards, set by organizations like ICNIRP and IEEE, ensure that the devices do not emit harmful levels of electromagnetic radiation. Manufacturers must conduct testing to demonstrate compliance with these standards.
- Quality management systems for PEMF device manufacturing: Manufacturers of PEMF therapy devices are required to implement and maintain quality management systems that comply with ISO 13485 or similar standards. These systems ensure consistent production quality, traceability, and post-market surveillance of devices to maintain regulatory compliance and patient safety.
- Clinical trial requirements for PEMF therapy: Regulatory bodies often require clinical trials to demonstrate the safety and efficacy of PEMF therapy for specific medical conditions. These trials must adhere to Good Clinical Practice (GCP) guidelines and may involve randomized controlled studies to provide evidence supporting the therapeutic claims of PEMF devices.
- Labeling and marketing regulations for PEMF devices: PEMF therapy devices must comply with labeling and marketing regulations set by regulatory authorities. This includes providing accurate information about the device's intended use, contraindications, and potential risks. Marketing claims must be supported by scientific evidence and adhere to regulatory guidelines to prevent misleading consumers.
02 International Standards and Certifications
PEMF devices must comply with international standards such as IEC 60601 for medical electrical equipment safety. Certifications like CE marking for European markets or other region-specific approvals may be required. These standards ensure consistent quality, safety, and performance across different countries.Expand Specific Solutions03 Electromagnetic Field Exposure Limits
Regulatory bodies set specific limits on electromagnetic field exposure for PEMF devices. These limits are based on scientific research and aim to prevent potential health risks associated with prolonged exposure to electromagnetic fields. Manufacturers must ensure their devices operate within these prescribed limits.Expand Specific Solutions04 Quality Management Systems
PEMF device manufacturers are required to implement and maintain quality management systems, such as ISO 13485 for medical devices. These systems ensure consistent product quality, traceability, and compliance with regulatory requirements throughout the device lifecycle, from design to post-market surveillance.Expand Specific Solutions05 Clinical Evidence and Post-Market Surveillance
Regulatory standards often require manufacturers to provide clinical evidence supporting the safety and efficacy of PEMF therapy devices. Additionally, post-market surveillance programs are necessary to monitor device performance, gather real-world data, and report adverse events to regulatory authorities.Expand Specific Solutions
Key Industry Players
The regulatory landscape for PEMF therapy in medical devices is evolving rapidly, reflecting the industry's growth phase. The market is expanding, driven by increasing applications in pain management, wound healing, and orthopedics. While the technology is maturing, it's not yet fully standardized. Key players like Regenesis Biomedical, Medtronic, and ZOLL Medical are leading innovation, with emerging companies such as SofPulse and Biomagnetic Sciences contributing to market diversification. Academic institutions like the University of Rochester and ETH Zurich are advancing research, potentially influencing future regulatory standards. The competitive landscape is characterized by a mix of established medical device manufacturers and specialized PEMF therapy companies, indicating a dynamic and growing market with potential for further technological advancements and regulatory refinement.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical specializes in PEMF therapy devices for wound healing and pain management. Their proprietary technology utilizes targeted electromagnetic fields to stimulate cellular repair and reduce inflammation. The company has developed a unique pulsed radio frequency energy (PRFE) system that operates at specific frequencies shown to be most effective for tissue regeneration[4]. Regenesis has also implemented advanced treatment tracking software to ensure regulatory compliance and facilitate clinical studies[5]. Their devices feature adjustable treatment parameters to accommodate various medical conditions and patient needs.
Strengths: Focused expertise in PEMF therapy, strong clinical evidence base. Weaknesses: Limited product range compared to larger competitors, potential challenges in scaling production.
SofPulse, Inc.
Technical Solution: SofPulse has developed a portable PEMF therapy system designed for post-surgical pain management and accelerated healing. Their technology utilizes low-frequency, low-intensity electromagnetic fields to reduce inflammation and promote tissue repair. The company's devices feature a proprietary waveform design that maximizes therapeutic effect while minimizing power consumption[6]. SofPulse has implemented smart dosing algorithms that adjust treatment intensity based on the healing stage and patient feedback[7]. Their systems also include built-in compliance monitoring to support regulatory requirements and improve treatment adherence.
Strengths: Innovative portable design, focus on post-surgical applications. Weaknesses: Limited market presence compared to larger medical device companies, potential challenges in expanding to other PEMF applications.
Innovative PEMF Research
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
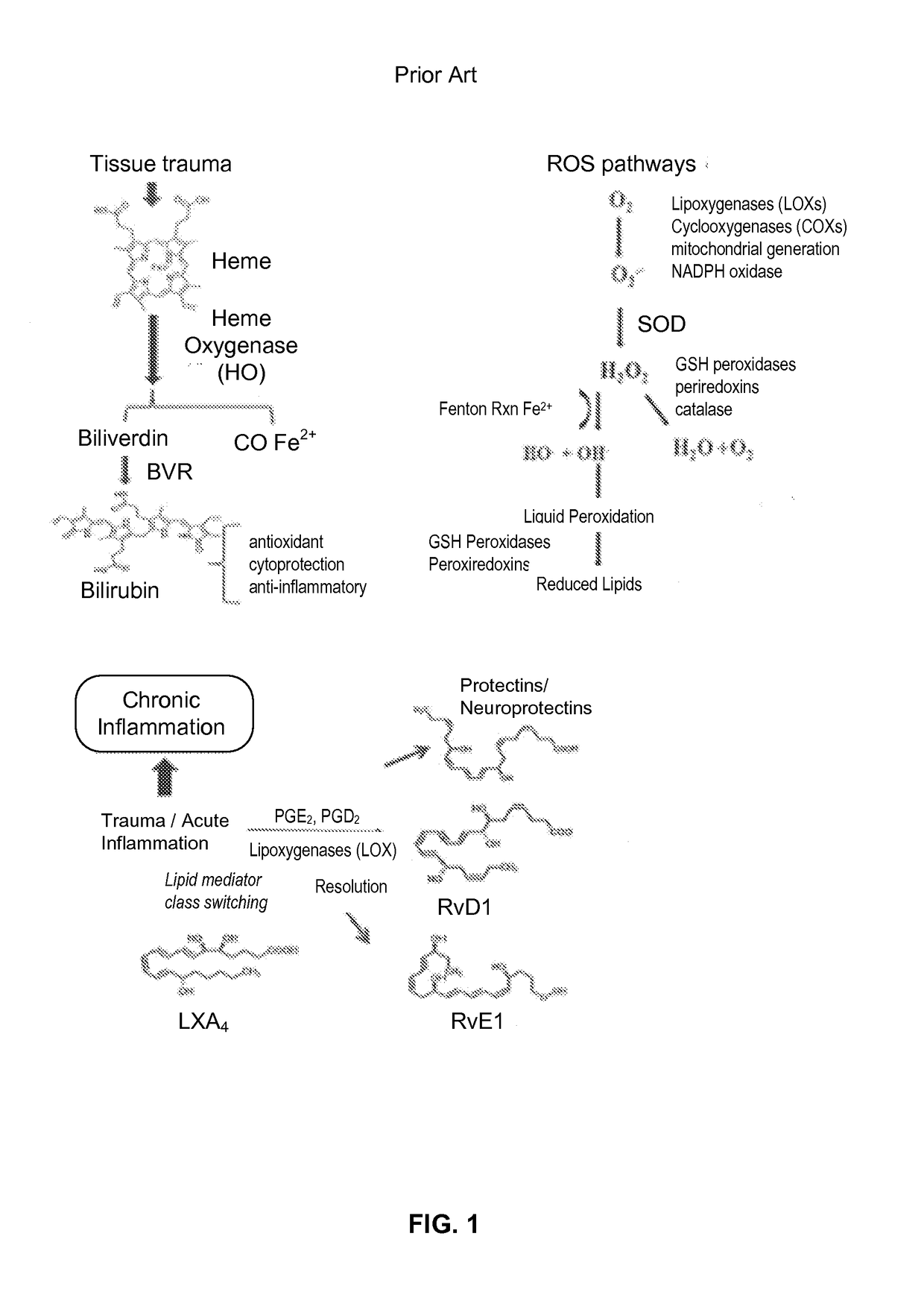
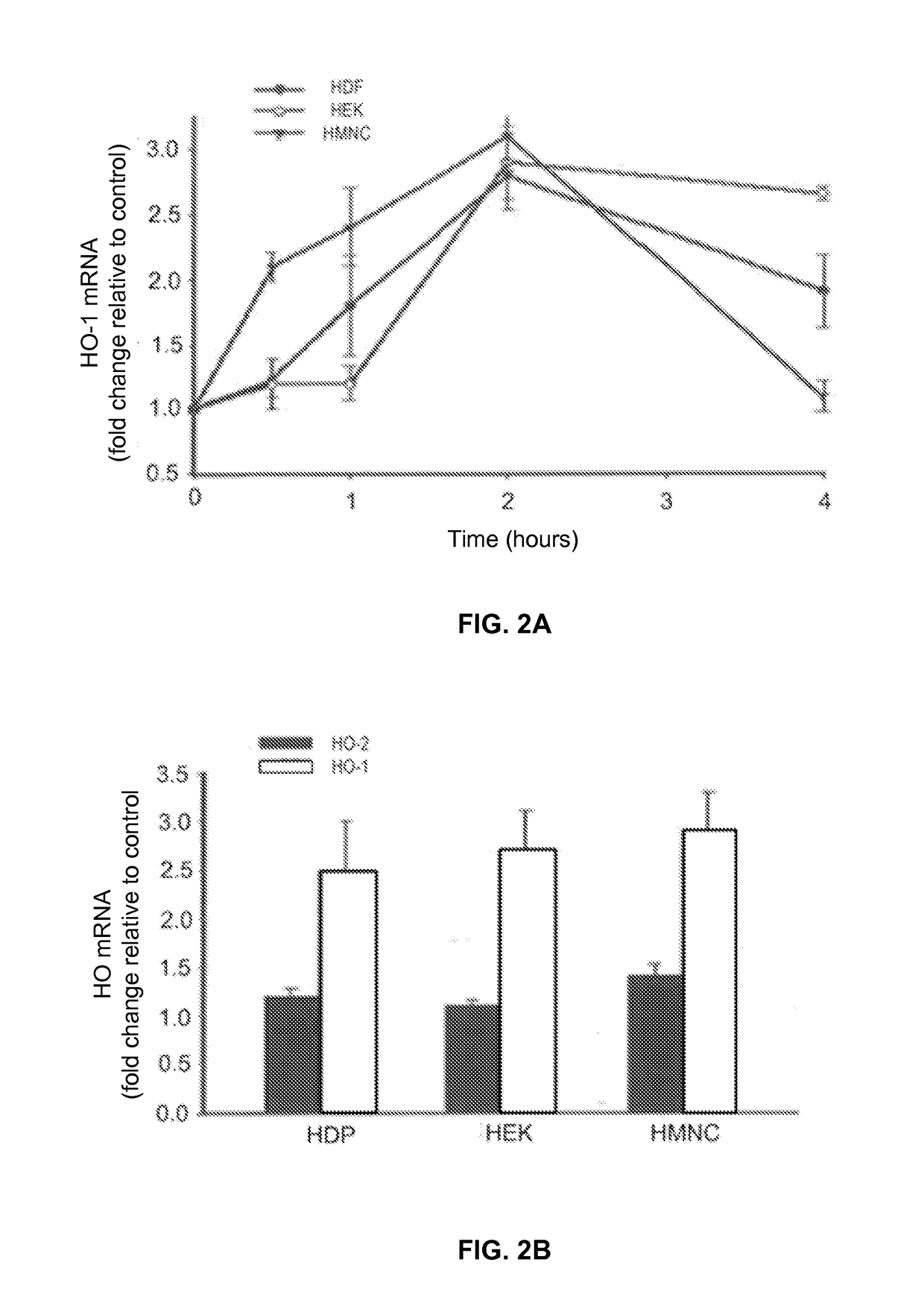
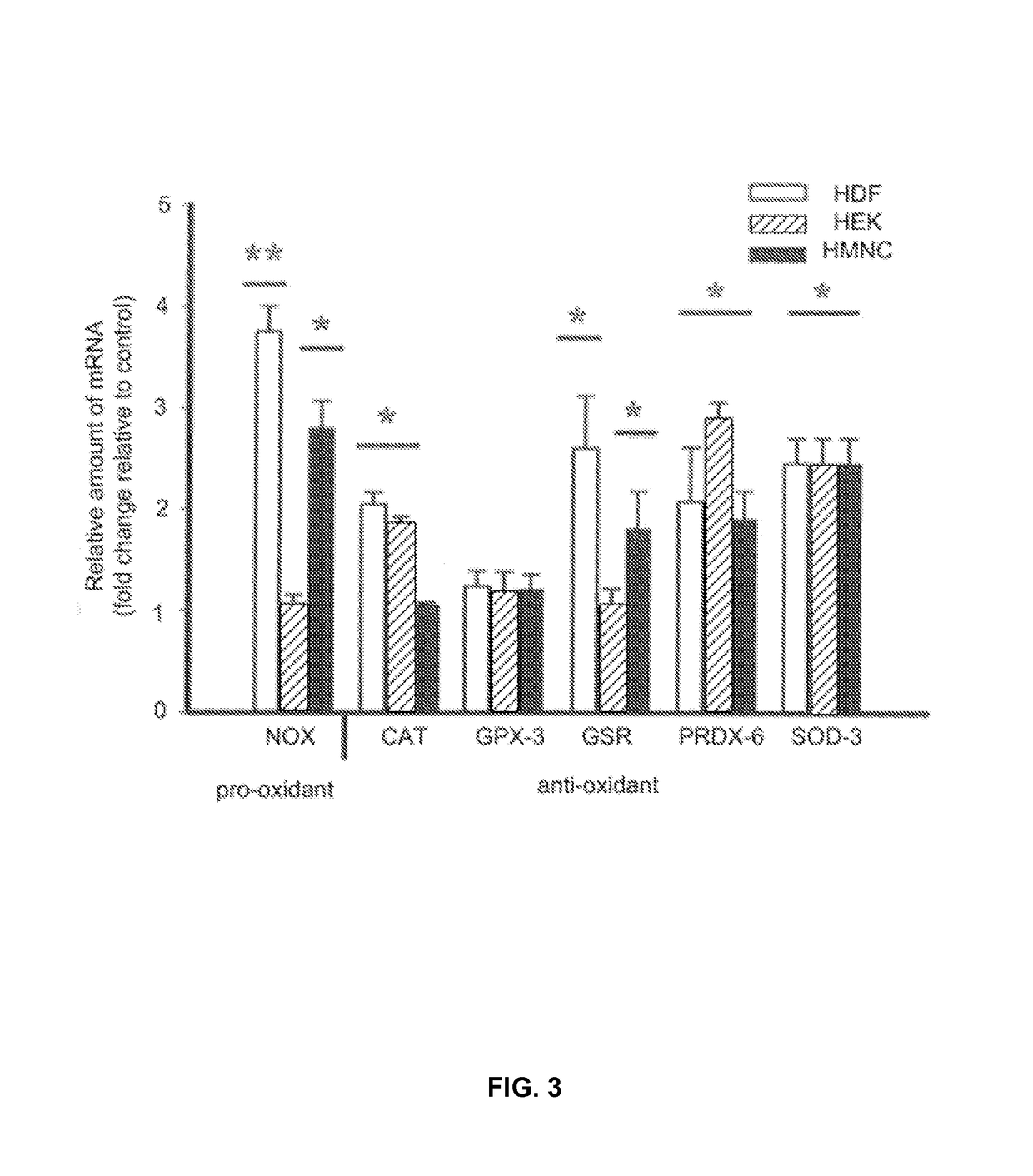
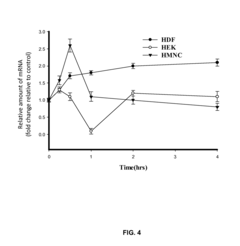
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Pulsed electromagnetic field therapy device
PatentActiveUS11752357B2
Innovation
- A pulsed electromagnetic field therapy device with a parallel resonant circuit that omits a switch between the capacitor and inductor, using an external switch to control current ramping, reducing energy dissipation and allowing for longer decay times and lower operating voltages, thus minimizing interference and safety risks.
Safety and Efficacy Data
The safety and efficacy data for Pulsed Electromagnetic Field (PEMF) therapy in medical devices are crucial components in advancing regulatory standards. Extensive clinical trials and research studies have demonstrated the potential benefits of PEMF therapy across various medical applications, including bone healing, pain management, and tissue regeneration.
Safety data from long-term studies indicate that PEMF therapy, when applied within recommended parameters, poses minimal risk to patients. The non-invasive nature of PEMF devices contributes to their favorable safety profile. However, regulatory bodies emphasize the importance of establishing clear guidelines for device specifications, treatment protocols, and contraindications to ensure consistent safety across different PEMF devices and applications.
Efficacy data show promising results in several therapeutic areas. In orthopedics, PEMF therapy has demonstrated significant improvements in bone healing rates and pain reduction for conditions such as non-union fractures and osteoarthritis. Neurological applications have shown potential in managing chronic pain and improving quality of life for patients with conditions like fibromyalgia and multiple sclerosis.
Regulatory agencies are particularly interested in standardizing efficacy measurements across different PEMF devices and treatment protocols. This includes establishing consistent outcome measures, treatment durations, and follow-up periods to enable meaningful comparisons between studies and devices.
One challenge in advancing regulatory standards is the variability in PEMF device parameters, such as frequency, intensity, and waveform. This diversity makes it difficult to establish universal efficacy benchmarks. Consequently, there is a growing push for more comprehensive, comparative studies that evaluate the efficacy of different PEMF parameters for specific medical conditions.
The accumulation of real-world evidence is becoming increasingly important in complementing traditional clinical trial data. Regulatory bodies are encouraging the implementation of post-market surveillance programs to gather long-term safety and efficacy data from a broader patient population. This approach helps identify rare adverse events and provides insights into the therapy's effectiveness in diverse clinical settings.
As the field of PEMF therapy continues to evolve, regulatory standards are adapting to incorporate emerging technologies and treatment modalities. This includes addressing the integration of PEMF devices with other medical technologies, such as wearable devices and telemedicine platforms, to enhance treatment monitoring and patient compliance.
Safety data from long-term studies indicate that PEMF therapy, when applied within recommended parameters, poses minimal risk to patients. The non-invasive nature of PEMF devices contributes to their favorable safety profile. However, regulatory bodies emphasize the importance of establishing clear guidelines for device specifications, treatment protocols, and contraindications to ensure consistent safety across different PEMF devices and applications.
Efficacy data show promising results in several therapeutic areas. In orthopedics, PEMF therapy has demonstrated significant improvements in bone healing rates and pain reduction for conditions such as non-union fractures and osteoarthritis. Neurological applications have shown potential in managing chronic pain and improving quality of life for patients with conditions like fibromyalgia and multiple sclerosis.
Regulatory agencies are particularly interested in standardizing efficacy measurements across different PEMF devices and treatment protocols. This includes establishing consistent outcome measures, treatment durations, and follow-up periods to enable meaningful comparisons between studies and devices.
One challenge in advancing regulatory standards is the variability in PEMF device parameters, such as frequency, intensity, and waveform. This diversity makes it difficult to establish universal efficacy benchmarks. Consequently, there is a growing push for more comprehensive, comparative studies that evaluate the efficacy of different PEMF parameters for specific medical conditions.
The accumulation of real-world evidence is becoming increasingly important in complementing traditional clinical trial data. Regulatory bodies are encouraging the implementation of post-market surveillance programs to gather long-term safety and efficacy data from a broader patient population. This approach helps identify rare adverse events and provides insights into the therapy's effectiveness in diverse clinical settings.
As the field of PEMF therapy continues to evolve, regulatory standards are adapting to incorporate emerging technologies and treatment modalities. This includes addressing the integration of PEMF devices with other medical technologies, such as wearable devices and telemedicine platforms, to enhance treatment monitoring and patient compliance.
Global Harmonization
Global harmonization of regulatory standards for PEMF (Pulsed Electromagnetic Field) therapy in medical devices is a critical step towards ensuring consistent safety, efficacy, and quality across international markets. This process involves aligning various national and regional regulatory frameworks to create a unified approach to the evaluation and approval of PEMF devices.
The International Medical Device Regulators Forum (IMDRF) plays a pivotal role in facilitating this harmonization. By bringing together regulatory authorities from different countries, the IMDRF works to develop guidance documents and promote convergence in medical device regulations. For PEMF therapy, this could involve establishing common definitions, classification criteria, and performance standards.
One key aspect of global harmonization is the development of internationally recognized standards. Organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) are instrumental in creating these standards. For PEMF devices, relevant standards might address electromagnetic compatibility, electrical safety, and specific performance requirements.
The harmonization process also involves aligning clinical evidence requirements across different jurisdictions. This includes standardizing protocols for clinical trials, defining acceptable endpoints, and establishing common criteria for evaluating the risk-benefit profile of PEMF devices. Such alignment can significantly reduce the burden on manufacturers seeking multi-market approvals and accelerate patient access to innovative therapies.
Regulatory cooperation initiatives, such as the Medical Device Single Audit Program (MDSAP), contribute to harmonization by allowing a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. Expanding such programs to include PEMF devices could streamline the regulatory process and reduce redundant inspections.
Challenges in achieving global harmonization include varying cultural and societal perspectives on medical device regulation, differing healthcare systems, and existing regulatory infrastructures. Overcoming these challenges requires sustained dialogue, mutual recognition agreements, and a commitment to regulatory convergence from all stakeholders.
As the field of PEMF therapy continues to evolve, regulatory harmonization efforts must also address emerging technologies and applications. This includes developing flexible frameworks that can accommodate future innovations while maintaining rigorous safety and efficacy standards.
The International Medical Device Regulators Forum (IMDRF) plays a pivotal role in facilitating this harmonization. By bringing together regulatory authorities from different countries, the IMDRF works to develop guidance documents and promote convergence in medical device regulations. For PEMF therapy, this could involve establishing common definitions, classification criteria, and performance standards.
One key aspect of global harmonization is the development of internationally recognized standards. Organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) are instrumental in creating these standards. For PEMF devices, relevant standards might address electromagnetic compatibility, electrical safety, and specific performance requirements.
The harmonization process also involves aligning clinical evidence requirements across different jurisdictions. This includes standardizing protocols for clinical trials, defining acceptable endpoints, and establishing common criteria for evaluating the risk-benefit profile of PEMF devices. Such alignment can significantly reduce the burden on manufacturers seeking multi-market approvals and accelerate patient access to innovative therapies.
Regulatory cooperation initiatives, such as the Medical Device Single Audit Program (MDSAP), contribute to harmonization by allowing a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. Expanding such programs to include PEMF devices could streamline the regulatory process and reduce redundant inspections.
Challenges in achieving global harmonization include varying cultural and societal perspectives on medical device regulation, differing healthcare systems, and existing regulatory infrastructures. Overcoming these challenges requires sustained dialogue, mutual recognition agreements, and a commitment to regulatory convergence from all stakeholders.
As the field of PEMF therapy continues to evolve, regulatory harmonization efforts must also address emerging technologies and applications. This includes developing flexible frameworks that can accommodate future innovations while maintaining rigorous safety and efficacy standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!