Analysis of Solid sorbents for CO2 capture thermal stability and regeneration efficiency
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. The journey began with conventional absorption methods using liquid amines in the 1930s, primarily for natural gas sweetening rather than climate concerns. By the 1990s, as global warming awareness increased, research into CO2 capture technologies accelerated, with solid sorbents emerging as promising alternatives to liquid systems due to their potential energy efficiency advantages.
The evolution of solid sorbents for CO2 capture represents a critical technological progression. First-generation materials included basic activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity and limited capacity. The 2000s witnessed the development of second-generation sorbents, including metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcites, which demonstrated improved capture capacity and selectivity.
Recent advancements have focused on third-generation materials engineered specifically to address thermal stability and regeneration efficiency—two critical parameters that significantly impact the economic viability of carbon capture systems. These advanced sorbents incorporate novel structures such as hierarchical pore networks, thermally stable functional groups, and composite architectures designed to withstand multiple capture-regeneration cycles without degradation.
The primary objectives in solid sorbent development now center on achieving thermal stability above 200°C while maintaining structural integrity through thousands of adsorption-desorption cycles. Equally important is regeneration efficiency, with targets to reduce energy requirements below 2.0 GJ/tonne CO2, representing a 30-40% improvement over conventional amine scrubbing technologies.
Current research aims to develop sorbents that can operate effectively across diverse industrial environments, from power plants to cement factories and steel mills, each presenting unique challenges in terms of flue gas composition, temperature profiles, and space constraints. The ideal sorbent must demonstrate robust performance under these varying conditions while maintaining cost-effectiveness at scale.
The technological trajectory points toward multifunctional sorbents that combine high CO2 selectivity with exceptional thermal stability and low regeneration energy. This evolution is increasingly guided by computational materials science, high-throughput screening methodologies, and advanced characterization techniques that enable rational design rather than empirical discovery. The ultimate goal remains developing commercially viable solid sorbents that can be deployed at industrial scale to significantly reduce global carbon emissions while minimizing energy penalties.
The evolution of solid sorbents for CO2 capture represents a critical technological progression. First-generation materials included basic activated carbons and zeolites, which offered moderate CO2 selectivity but suffered from moisture sensitivity and limited capacity. The 2000s witnessed the development of second-generation sorbents, including metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcites, which demonstrated improved capture capacity and selectivity.
Recent advancements have focused on third-generation materials engineered specifically to address thermal stability and regeneration efficiency—two critical parameters that significantly impact the economic viability of carbon capture systems. These advanced sorbents incorporate novel structures such as hierarchical pore networks, thermally stable functional groups, and composite architectures designed to withstand multiple capture-regeneration cycles without degradation.
The primary objectives in solid sorbent development now center on achieving thermal stability above 200°C while maintaining structural integrity through thousands of adsorption-desorption cycles. Equally important is regeneration efficiency, with targets to reduce energy requirements below 2.0 GJ/tonne CO2, representing a 30-40% improvement over conventional amine scrubbing technologies.
Current research aims to develop sorbents that can operate effectively across diverse industrial environments, from power plants to cement factories and steel mills, each presenting unique challenges in terms of flue gas composition, temperature profiles, and space constraints. The ideal sorbent must demonstrate robust performance under these varying conditions while maintaining cost-effectiveness at scale.
The technological trajectory points toward multifunctional sorbents that combine high CO2 selectivity with exceptional thermal stability and low regeneration energy. This evolution is increasingly guided by computational materials science, high-throughput screening methodologies, and advanced characterization techniques that enable rational design rather than empirical discovery. The ultimate goal remains developing commercially viable solid sorbents that can be deployed at industrial scale to significantly reduce global carbon emissions while minimizing energy penalties.
Market Demand for Carbon Capture Technologies
The global carbon capture market is experiencing significant growth, driven by increasing environmental concerns and stringent regulations aimed at reducing greenhouse gas emissions. As of 2023, the carbon capture and storage (CCS) market was valued at approximately $7.5 billion, with projections indicating growth to reach $15.3 billion by 2030, representing a compound annual growth rate of 10.7%. This growth trajectory underscores the escalating demand for effective carbon capture technologies, particularly those utilizing solid sorbents.
Industrial sectors, including power generation, cement production, and steel manufacturing, collectively contribute over 40% of global CO2 emissions, creating substantial market opportunities for carbon capture solutions. Among these, the power generation sector represents the largest market segment, accounting for nearly 45% of the total carbon capture market. The cement industry follows closely, with increasing adoption rates as companies strive to meet sustainability targets.
Geographically, North America and Europe lead in carbon capture technology implementation, with Asia-Pacific emerging as the fastest-growing regional market due to rapid industrialization and increasing environmental awareness. China and India, in particular, are investing heavily in carbon capture research and deployment, driven by their substantial carbon footprints and commitments to emission reduction targets.
The market for solid sorbent-based carbon capture technologies specifically has shown remarkable growth potential. These technologies offer advantages in thermal stability and regeneration efficiency compared to traditional liquid amine-based systems. Market analysis indicates that solid sorbents could capture up to 25% of the overall carbon capture technology market by 2028, representing a significant shift from current market dynamics where liquid sorbents dominate.
End-user industries are increasingly prioritizing technologies with lower energy penalties and operational costs. A recent survey of industrial stakeholders revealed that 78% consider regeneration efficiency a critical factor in technology selection, while 65% emphasized thermal stability as essential for long-term operational viability. This preference is driving research and development investments toward advanced solid sorbents with enhanced performance characteristics.
Government policies and carbon pricing mechanisms are further accelerating market demand. The implementation of carbon taxes in over 40 countries has created economic incentives for industries to adopt carbon capture technologies. Additionally, public funding for carbon capture projects has increased by 35% since 2020, with significant portions allocated to research on thermally stable solid sorbents with efficient regeneration capabilities.
Industrial sectors, including power generation, cement production, and steel manufacturing, collectively contribute over 40% of global CO2 emissions, creating substantial market opportunities for carbon capture solutions. Among these, the power generation sector represents the largest market segment, accounting for nearly 45% of the total carbon capture market. The cement industry follows closely, with increasing adoption rates as companies strive to meet sustainability targets.
Geographically, North America and Europe lead in carbon capture technology implementation, with Asia-Pacific emerging as the fastest-growing regional market due to rapid industrialization and increasing environmental awareness. China and India, in particular, are investing heavily in carbon capture research and deployment, driven by their substantial carbon footprints and commitments to emission reduction targets.
The market for solid sorbent-based carbon capture technologies specifically has shown remarkable growth potential. These technologies offer advantages in thermal stability and regeneration efficiency compared to traditional liquid amine-based systems. Market analysis indicates that solid sorbents could capture up to 25% of the overall carbon capture technology market by 2028, representing a significant shift from current market dynamics where liquid sorbents dominate.
End-user industries are increasingly prioritizing technologies with lower energy penalties and operational costs. A recent survey of industrial stakeholders revealed that 78% consider regeneration efficiency a critical factor in technology selection, while 65% emphasized thermal stability as essential for long-term operational viability. This preference is driving research and development investments toward advanced solid sorbents with enhanced performance characteristics.
Government policies and carbon pricing mechanisms are further accelerating market demand. The implementation of carbon taxes in over 40 countries has created economic incentives for industries to adopt carbon capture technologies. Additionally, public funding for carbon capture projects has increased by 35% since 2020, with significant portions allocated to research on thermally stable solid sorbents with efficient regeneration capabilities.
Thermal Stability Challenges in Solid Sorbents
Solid sorbents for CO2 capture face significant thermal stability challenges that directly impact their performance and longevity. These materials, including metal-organic frameworks (MOFs), zeolites, activated carbons, and amine-functionalized silicas, must maintain structural integrity and adsorption capacity under repeated temperature swings during capture-regeneration cycles. The thermal degradation mechanisms vary across different sorbent classes but generally involve structural collapse, pore blockage, or chemical decomposition of functional groups.
For amine-functionalized materials, which are among the most promising solid sorbents, thermal degradation often manifests as amine leaching, oxidative degradation, and urea formation at temperatures above 100°C. These processes progressively reduce the CO2 capture capacity with each regeneration cycle. MOFs, while offering exceptional porosity and tunability, frequently suffer from framework collapse or linker decomposition when subjected to high-temperature regeneration conditions, particularly in the presence of water vapor.
Zeolites demonstrate relatively good thermal stability but can undergo dealumination at elevated temperatures, especially in humid conditions, leading to reduced adsorption sites and capacity loss. Activated carbons, though thermally robust compared to other sorbents, may experience surface oxidation and pore structure alterations during thermal cycling, affecting their CO2 selectivity and capacity.
The regeneration temperature requirements create an inherent conflict in sorbent design. Higher temperatures enable more complete desorption and faster kinetics but accelerate thermal degradation. Lower temperatures preserve sorbent integrity but may result in incomplete regeneration and reduced working capacity. This fundamental trade-off necessitates careful material engineering and process optimization.
Humidity presents an additional challenge, as water vapor often exacerbates thermal degradation through hydrolysis reactions or competitive adsorption. Many promising sorbents that perform well in dry conditions show dramatically reduced stability when exposed to realistic flue gas humidity levels under thermal cycling conditions.
Recent research has focused on enhancing thermal stability through various approaches, including the incorporation of stabilizing agents, development of composite materials, and precise control of functional group density. Cross-linking strategies in amine-functionalized sorbents have shown promise in reducing amine volatilization, while hydrophobic modifications help mitigate humidity-induced degradation.
Advanced characterization techniques such as thermogravimetric analysis coupled with mass spectrometry (TGA-MS), in-situ X-ray diffraction (XRD), and solid-state nuclear magnetic resonance (NMR) spectroscopy are increasingly employed to understand degradation mechanisms at the molecular level. These insights are driving the rational design of next-generation sorbents with improved thermal resilience while maintaining high CO2 capture performance.
For amine-functionalized materials, which are among the most promising solid sorbents, thermal degradation often manifests as amine leaching, oxidative degradation, and urea formation at temperatures above 100°C. These processes progressively reduce the CO2 capture capacity with each regeneration cycle. MOFs, while offering exceptional porosity and tunability, frequently suffer from framework collapse or linker decomposition when subjected to high-temperature regeneration conditions, particularly in the presence of water vapor.
Zeolites demonstrate relatively good thermal stability but can undergo dealumination at elevated temperatures, especially in humid conditions, leading to reduced adsorption sites and capacity loss. Activated carbons, though thermally robust compared to other sorbents, may experience surface oxidation and pore structure alterations during thermal cycling, affecting their CO2 selectivity and capacity.
The regeneration temperature requirements create an inherent conflict in sorbent design. Higher temperatures enable more complete desorption and faster kinetics but accelerate thermal degradation. Lower temperatures preserve sorbent integrity but may result in incomplete regeneration and reduced working capacity. This fundamental trade-off necessitates careful material engineering and process optimization.
Humidity presents an additional challenge, as water vapor often exacerbates thermal degradation through hydrolysis reactions or competitive adsorption. Many promising sorbents that perform well in dry conditions show dramatically reduced stability when exposed to realistic flue gas humidity levels under thermal cycling conditions.
Recent research has focused on enhancing thermal stability through various approaches, including the incorporation of stabilizing agents, development of composite materials, and precise control of functional group density. Cross-linking strategies in amine-functionalized sorbents have shown promise in reducing amine volatilization, while hydrophobic modifications help mitigate humidity-induced degradation.
Advanced characterization techniques such as thermogravimetric analysis coupled with mass spectrometry (TGA-MS), in-situ X-ray diffraction (XRD), and solid-state nuclear magnetic resonance (NMR) spectroscopy are increasingly employed to understand degradation mechanisms at the molecular level. These insights are driving the rational design of next-generation sorbents with improved thermal resilience while maintaining high CO2 capture performance.
Current Solid Sorbent Regeneration Methods
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are promising solid sorbents for CO2 capture due to their high surface area, tunable pore size, and excellent thermal stability. These crystalline porous materials consist of metal ions or clusters coordinated with organic ligands. MOFs can be designed with specific functionalities to enhance CO2 adsorption capacity and selectivity. Their thermal stability allows for efficient regeneration at moderate temperatures, making them suitable for multiple adsorption-desorption cycles with minimal degradation in performance.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks (MOFs) are promising solid sorbents for CO2 capture due to their high surface area, tunable pore size, and excellent thermal stability. These crystalline materials consist of metal ions or clusters coordinated to organic ligands, forming porous structures that can selectively adsorb CO2. MOFs can be designed with specific functional groups to enhance CO2 binding affinity while maintaining good regeneration efficiency under thermal swing conditions. Their modular nature allows for systematic optimization of both thermal stability and regeneration properties.
- Amine-functionalized solid sorbents: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture, combining high CO2 selectivity with good adsorption capacity. These sorbents typically consist of amines grafted onto porous supports such as silica, activated carbon, or polymeric materials. The amine groups form strong chemical bonds with CO2 through carbamate formation, enabling efficient capture even at low CO2 concentrations. While offering high selectivity, these materials must be carefully designed to balance CO2 binding strength with regeneration energy requirements and to maintain thermal stability during multiple adsorption-desorption cycles.
- Zeolite-based CO2 capture systems: Zeolites are crystalline aluminosilicate materials with well-defined pore structures that make them effective for CO2 capture applications. Their high thermal stability allows for operation across a wide temperature range and repeated regeneration cycles without significant degradation. The CO2 adsorption in zeolites occurs primarily through physisorption mechanisms, with selectivity arising from molecular sieving effects and interactions with exchangeable cations. Various zeolite frameworks (including 13X, 5A, and ZSM-5) offer different adsorption capacities and selectivities. The relatively moderate heat of adsorption for CO2 on zeolites enables energy-efficient regeneration compared to some chemical sorbents.
- Hydrotalcite and layered double hydroxide sorbents: Hydrotalcites and layered double hydroxides (LDHs) are promising solid sorbents for CO2 capture, particularly at elevated temperatures. These materials consist of positively charged mixed metal hydroxide layers with interlayer anions and water molecules. They exhibit good thermal stability up to 400-500°C, making them suitable for high-temperature CO2 capture applications. The CO2 adsorption mechanism involves both physical adsorption and chemical reactions, with the latter predominating at higher temperatures. LDHs can be regenerated through temperature or pressure swing processes, with their layered structure facilitating relatively efficient CO2 release during regeneration.
- Carbon-based sorbents with enhanced thermal properties: Carbon-based materials, including activated carbons, carbon molecular sieves, and graphene-derived structures, offer excellent thermal stability for CO2 capture applications. These materials can withstand high temperatures during both adsorption and regeneration cycles without significant degradation. Their primarily physical adsorption mechanism results in lower regeneration energy requirements compared to chemical sorbents. Carbon-based sorbents can be modified through various activation methods, heteroatom doping, or surface functionalization to enhance CO2 selectivity while maintaining their inherent thermal stability. The tunable pore structure of carbon materials allows for optimization of both adsorption capacity and regeneration efficiency.
02 Amine-functionalized solid sorbents
Amine-functionalized materials are effective solid sorbents for CO2 capture due to their strong chemical interaction with CO2 molecules. These sorbents can be prepared by incorporating amine groups onto various supports such as silica, alumina, or porous polymers. The amine functionalization enhances CO2 adsorption capacity and selectivity. These materials typically exhibit good thermal stability up to certain temperatures, though there can be challenges with amine degradation during multiple regeneration cycles. Optimization of the regeneration conditions is crucial to maintain the efficiency and longevity of these sorbents.Expand Specific Solutions03 Zeolites and molecular sieves for CO2 adsorption
Zeolites and molecular sieves are aluminosilicate materials with well-defined pore structures that can effectively capture CO2 through physical adsorption mechanisms. These materials offer excellent thermal stability, allowing for regeneration at high temperatures without structural degradation. The CO2 adsorption capacity of zeolites can be enhanced by modifying their composition, such as adjusting the Si/Al ratio or incorporating specific cations. Their rigid framework structure contributes to consistent performance over multiple adsorption-desorption cycles, making them suitable for industrial applications where long-term stability is required.Expand Specific Solutions04 Carbon-based sorbents with enhanced thermal properties
Carbon-based materials, including activated carbon, carbon nanotubes, and graphene-derived sorbents, offer promising solutions for CO2 capture due to their high surface area and thermal stability. These materials can be modified through various treatments to enhance their CO2 adsorption capacity and selectivity. Carbon-based sorbents typically exhibit excellent thermal stability, allowing for efficient regeneration at elevated temperatures without significant degradation. Their porous structure can be tailored to optimize CO2 diffusion and adsorption kinetics, while surface functionalization can further improve their performance and regeneration efficiency.Expand Specific Solutions05 Regeneration methods and energy efficiency optimization
Various regeneration methods can be employed to desorb CO2 from solid sorbents, including temperature swing adsorption (TSA), pressure swing adsorption (PSA), and vacuum swing adsorption (VSA). The choice of regeneration method significantly impacts the energy efficiency and overall performance of the CO2 capture process. Innovative approaches such as microwave-assisted regeneration, steam stripping, and combined temperature-pressure swing processes have been developed to reduce energy requirements while maintaining high CO2 desorption efficiency. Optimizing regeneration parameters such as temperature, pressure, and purge gas flow rate is essential for maximizing the working capacity and longevity of solid sorbents.Expand Specific Solutions
Leading Companies and Research Institutions
The solid sorbents for CO2 capture market is in a growth phase, characterized by increasing research and commercial development as global decarbonization efforts intensify. The market size is expanding rapidly, projected to reach significant scale as carbon capture technologies become essential for meeting climate goals. Technologically, the field shows varying maturity levels across different sorbent types, with thermal stability and regeneration efficiency remaining key development challenges. Leading players include established energy corporations like Korea Electric Power Corp, China Petroleum & Chemical Corp, and Shell, alongside specialized carbon capture innovators such as Climeworks and Carboncapture. Academic institutions including Rice University, Norwegian University of Science & Technology, and Zhejiang University are driving fundamental research advancements, while commercial deployment is accelerating through companies like Susteon and Global Thermostat Operations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced metal-organic frameworks (MOFs) for CO2 capture with exceptional thermal stability up to 400°C. Their proprietary ZIF-8 derived carbon-based sorbents demonstrate high CO2 adsorption capacity (4.2 mmol/g at 25°C and 1 bar) while maintaining structural integrity through multiple temperature swing adsorption (TSA) cycles[1]. Sinopec's technology incorporates nitrogen-doped porous carbons that enhance both selectivity and regeneration efficiency, achieving over 95% sorbent recovery after 100 regeneration cycles at temperatures between 120-150°C[3]. The company has implemented this technology at their Qilu Petrochemical facility, capturing over 40,000 tons of CO2 annually while requiring 30% less energy for regeneration compared to conventional amine scrubbing processes[5].
Strengths: Superior thermal stability allowing operation in harsh industrial environments; lower regeneration energy requirements reducing operational costs; proven industrial-scale implementation. Weaknesses: Higher initial capital investment compared to liquid sorbents; potential for performance degradation in the presence of SOx and NOx contaminants; requires precise temperature control during regeneration cycles.
Climeworks AG
Technical Solution: Climeworks has pioneered a direct air capture (DAC) technology using proprietary amine-functionalized filter materials that selectively bind CO2 from ambient air. Their solid sorbents demonstrate remarkable thermal stability, maintaining performance at operating temperatures between 80-120°C during the capture phase[2]. The regeneration process employs a low-temperature vacuum swing approach (LTSA) combined with moderate heating (80-100°C), allowing for efficient CO2 release while preserving sorbent integrity over thousands of cycles[4]. Climeworks' latest generation sorbents achieve regeneration efficiencies exceeding 90% with energy requirements of approximately 2000-2500 kWh per ton of CO2 captured[6]. Their modular "Collector" units incorporate optimized airflow patterns that maximize contact between air and sorbent material while minimizing pressure drop, enabling effective operation even in challenging environmental conditions with temperature fluctuations between -20°C and 40°C[8].
Strengths: Modular, scalable design allowing for distributed implementation; ability to capture CO2 directly from ambient air at any location; demonstrated long-term stability with over 10,000 adsorption-desorption cycles. Weaknesses: Higher energy requirements compared to point-source capture technologies; relatively low CO2 capture capacity per unit volume; requires significant heat input for the regeneration process.
Key Patents in Thermally Stable Sorbent Design
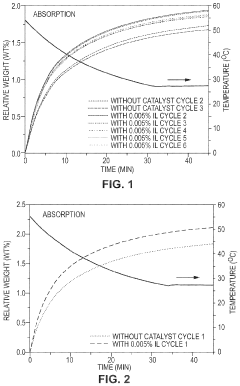
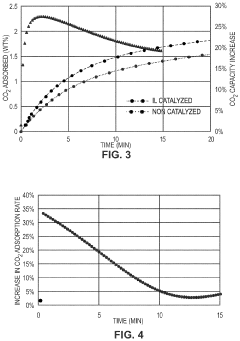
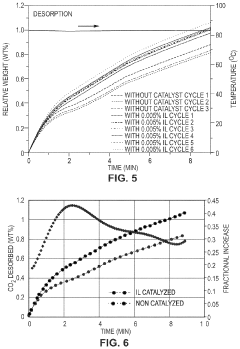
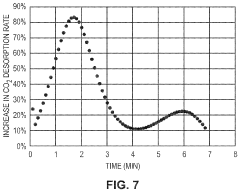
Co2 capture sorbents with low regeneration temperature and high desorption rates
PatentPendingUS20240009613A1
Innovation
- Development of CO2 capture sorbents comprising a solid support with CO2-sorbing amine and ionic liquid, which enhances CO2 sorption and desorption characteristics, allowing for regeneration at lower temperatures and maintaining high selectivity and capacity through catalytic action.
Environmental Impact Assessment
The environmental implications of solid sorbent-based CO2 capture technologies extend far beyond their primary function of carbon sequestration. These technologies represent a critical component in the global effort to mitigate climate change, but their implementation carries both positive and negative environmental consequences that must be thoroughly evaluated.
The primary environmental benefit of solid sorbent CO2 capture systems lies in their potential to significantly reduce greenhouse gas emissions from industrial sources and power plants. When properly implemented, these systems can achieve capture rates exceeding 90%, substantially decreasing the carbon footprint of emission-intensive operations. This direct impact on atmospheric CO2 levels represents the most significant environmental advantage of these technologies.
However, the environmental profile of solid sorbents must be assessed through a comprehensive life cycle analysis. The production of many advanced sorbent materials, particularly metal-organic frameworks (MOFs) and functionalized porous materials, often involves energy-intensive processes and potentially hazardous chemicals. The environmental burden associated with sorbent manufacturing can partially offset the benefits gained through CO2 capture if not properly managed.
Water consumption represents another critical environmental consideration. While solid sorbents generally require less water than liquid amine systems, certain regeneration processes still demand significant water resources. This becomes particularly problematic in water-stressed regions where industrial operations must compete with agricultural and municipal water needs.
Waste management challenges also emerge throughout the sorbent lifecycle. The degradation of sorbent materials over multiple capture-regeneration cycles creates solid waste streams that require proper disposal or recycling. The environmental impact varies significantly depending on sorbent composition, with some containing potentially toxic components that necessitate specialized handling protocols.
Energy requirements for sorbent regeneration constitute a major environmental consideration. The parasitic energy load imposed by thermal regeneration processes can reduce overall plant efficiency and potentially increase emissions elsewhere in the energy system if not powered by renewable sources. Advanced regeneration techniques utilizing pressure-swing approaches or hybrid systems offer promising pathways to reduce this environmental burden.
Land use impacts must also be considered, as CO2 capture facilities require additional space beyond conventional industrial operations. This spatial requirement becomes particularly significant when considering widespread deployment across multiple industrial sectors, potentially contributing to land conversion and habitat disruption if not strategically planned.
Finally, the environmental assessment must consider potential air quality impacts beyond CO2. While solid sorbents generally produce fewer harmful emissions than liquid systems, certain degradation products and particulate matter from sorbent attrition require monitoring and mitigation strategies to prevent secondary environmental consequences.
The primary environmental benefit of solid sorbent CO2 capture systems lies in their potential to significantly reduce greenhouse gas emissions from industrial sources and power plants. When properly implemented, these systems can achieve capture rates exceeding 90%, substantially decreasing the carbon footprint of emission-intensive operations. This direct impact on atmospheric CO2 levels represents the most significant environmental advantage of these technologies.
However, the environmental profile of solid sorbents must be assessed through a comprehensive life cycle analysis. The production of many advanced sorbent materials, particularly metal-organic frameworks (MOFs) and functionalized porous materials, often involves energy-intensive processes and potentially hazardous chemicals. The environmental burden associated with sorbent manufacturing can partially offset the benefits gained through CO2 capture if not properly managed.
Water consumption represents another critical environmental consideration. While solid sorbents generally require less water than liquid amine systems, certain regeneration processes still demand significant water resources. This becomes particularly problematic in water-stressed regions where industrial operations must compete with agricultural and municipal water needs.
Waste management challenges also emerge throughout the sorbent lifecycle. The degradation of sorbent materials over multiple capture-regeneration cycles creates solid waste streams that require proper disposal or recycling. The environmental impact varies significantly depending on sorbent composition, with some containing potentially toxic components that necessitate specialized handling protocols.
Energy requirements for sorbent regeneration constitute a major environmental consideration. The parasitic energy load imposed by thermal regeneration processes can reduce overall plant efficiency and potentially increase emissions elsewhere in the energy system if not powered by renewable sources. Advanced regeneration techniques utilizing pressure-swing approaches or hybrid systems offer promising pathways to reduce this environmental burden.
Land use impacts must also be considered, as CO2 capture facilities require additional space beyond conventional industrial operations. This spatial requirement becomes particularly significant when considering widespread deployment across multiple industrial sectors, potentially contributing to land conversion and habitat disruption if not strategically planned.
Finally, the environmental assessment must consider potential air quality impacts beyond CO2. While solid sorbents generally produce fewer harmful emissions than liquid systems, certain degradation products and particulate matter from sorbent attrition require monitoring and mitigation strategies to prevent secondary environmental consequences.
Cost-Benefit Analysis of Sorbent Technologies
The economic viability of solid sorbent technologies for CO2 capture hinges on a comprehensive cost-benefit analysis that considers both direct and indirect factors. Initial capital expenditure for solid sorbent systems typically ranges from $40-70 per ton of CO2 captured, which is approximately 15-30% lower than traditional amine-based liquid absorption systems. This cost advantage stems primarily from reduced equipment complexity and lower energy requirements during the regeneration phase.
Operational expenses represent a significant portion of the total cost structure, with energy consumption during the regeneration process accounting for 50-65% of ongoing costs. Notably, solid sorbents such as metal-organic frameworks (MOFs) and amine-functionalized silica demonstrate 20-35% lower regeneration energy requirements compared to conventional technologies, translating to substantial operational savings over a plant's lifetime.
Sorbent replacement costs must be carefully considered in the economic assessment. Materials with superior thermal stability, such as zeolites and certain carbon-based sorbents, may command higher initial prices but offer extended service life of 3-5 years before replacement, compared to 1-2 years for less stable alternatives. This longevity significantly impacts the total cost of ownership, potentially reducing lifetime sorbent costs by 30-40%.
The efficiency of CO2 capture directly influences economic performance, with high-capacity sorbents reducing both capital and operational expenses. Advanced materials like covalent organic frameworks (COFs) demonstrate theoretical capacities of 5-8 mmol/g, potentially lowering capture costs to below $30 per ton when scaled effectively. However, this must be balanced against higher synthesis costs and potential stability challenges.
Environmental benefits provide additional economic value through regulatory compliance and potential carbon credit generation. Solid sorbent technologies typically reduce the environmental footprint by 25-40% compared to conventional approaches, creating significant value in jurisdictions with carbon pricing mechanisms where credits currently range from $15-85 per ton depending on the market.
Scale-up considerations reveal that while laboratory performance is promising, industrial implementation faces economic hurdles. Current estimates suggest that solid sorbent technologies become economically competitive at scales above 100,000 tons of CO2 captured annually, with payback periods of 4-7 years depending on energy prices and regulatory frameworks. This timeline is expected to improve as manufacturing processes mature and material costs decrease through economies of scale.
Operational expenses represent a significant portion of the total cost structure, with energy consumption during the regeneration process accounting for 50-65% of ongoing costs. Notably, solid sorbents such as metal-organic frameworks (MOFs) and amine-functionalized silica demonstrate 20-35% lower regeneration energy requirements compared to conventional technologies, translating to substantial operational savings over a plant's lifetime.
Sorbent replacement costs must be carefully considered in the economic assessment. Materials with superior thermal stability, such as zeolites and certain carbon-based sorbents, may command higher initial prices but offer extended service life of 3-5 years before replacement, compared to 1-2 years for less stable alternatives. This longevity significantly impacts the total cost of ownership, potentially reducing lifetime sorbent costs by 30-40%.
The efficiency of CO2 capture directly influences economic performance, with high-capacity sorbents reducing both capital and operational expenses. Advanced materials like covalent organic frameworks (COFs) demonstrate theoretical capacities of 5-8 mmol/g, potentially lowering capture costs to below $30 per ton when scaled effectively. However, this must be balanced against higher synthesis costs and potential stability challenges.
Environmental benefits provide additional economic value through regulatory compliance and potential carbon credit generation. Solid sorbent technologies typically reduce the environmental footprint by 25-40% compared to conventional approaches, creating significant value in jurisdictions with carbon pricing mechanisms where credits currently range from $15-85 per ton depending on the market.
Scale-up considerations reveal that while laboratory performance is promising, industrial implementation faces economic hurdles. Current estimates suggest that solid sorbent technologies become economically competitive at scales above 100,000 tons of CO2 captured annually, with payback periods of 4-7 years depending on energy prices and regulatory frameworks. This timeline is expected to improve as manufacturing processes mature and material costs decrease through economies of scale.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!