Comparative analysis of Solid sorbents for CO2 capture metal oxides versus amine functionalized
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades in response to growing concerns about climate change and greenhouse gas emissions. The journey began in the 1930s with the first commercial applications of amine scrubbing for natural gas sweetening. By the 1970s, this technology had been adapted for flue gas treatment, marking the first generation of CO2 capture solutions primarily based on liquid amine absorbents.
The early 2000s witnessed a paradigm shift with increased research into solid sorbents, driven by the limitations of liquid amine systems, including high energy requirements for regeneration, equipment corrosion, and solvent degradation. This second generation of capture technologies explored various solid materials including activated carbons, zeolites, and the first iterations of metal oxides and amine-functionalized sorbents.
The technological evolution accelerated after 2010 with the emergence of advanced materials science and nanotechnology. Metal oxides, particularly calcium, magnesium, and sodium-based compounds, gained attention for their high theoretical CO2 uptake capacity and favorable thermodynamics. Concurrently, amine-functionalized materials evolved from simple impregnation methods to sophisticated molecular engineering approaches, creating highly efficient supported amine sorbents.
Current technological development focuses on addressing the fundamental challenges of both material classes. For metal oxides, researchers aim to enhance cycling stability, improve reaction kinetics, and reduce energy penalties during regeneration. For amine-functionalized sorbents, efforts concentrate on increasing CO2 loading capacity, improving moisture tolerance, and extending operational lifetimes under industrial conditions.
The primary objectives of modern CO2 capture technology development include reducing the energy penalty associated with capture processes to below 1.5 GJ/ton CO2, achieving capture costs under $40/ton, and ensuring sorbent stability for thousands of cycles in industrial environments. Additionally, there is growing emphasis on developing materials compatible with direct air capture applications, which require effective operation at ultra-low CO2 concentrations.
Looking forward, the field is moving toward hybrid systems that combine the advantages of both metal oxides and amine-functionalized materials, along with process intensification strategies. The ultimate goal remains developing economically viable carbon capture solutions that can be deployed at scale across various industries, from power generation to cement production, supporting global decarbonization efforts while maintaining energy security and industrial competitiveness.
The early 2000s witnessed a paradigm shift with increased research into solid sorbents, driven by the limitations of liquid amine systems, including high energy requirements for regeneration, equipment corrosion, and solvent degradation. This second generation of capture technologies explored various solid materials including activated carbons, zeolites, and the first iterations of metal oxides and amine-functionalized sorbents.
The technological evolution accelerated after 2010 with the emergence of advanced materials science and nanotechnology. Metal oxides, particularly calcium, magnesium, and sodium-based compounds, gained attention for their high theoretical CO2 uptake capacity and favorable thermodynamics. Concurrently, amine-functionalized materials evolved from simple impregnation methods to sophisticated molecular engineering approaches, creating highly efficient supported amine sorbents.
Current technological development focuses on addressing the fundamental challenges of both material classes. For metal oxides, researchers aim to enhance cycling stability, improve reaction kinetics, and reduce energy penalties during regeneration. For amine-functionalized sorbents, efforts concentrate on increasing CO2 loading capacity, improving moisture tolerance, and extending operational lifetimes under industrial conditions.
The primary objectives of modern CO2 capture technology development include reducing the energy penalty associated with capture processes to below 1.5 GJ/ton CO2, achieving capture costs under $40/ton, and ensuring sorbent stability for thousands of cycles in industrial environments. Additionally, there is growing emphasis on developing materials compatible with direct air capture applications, which require effective operation at ultra-low CO2 concentrations.
Looking forward, the field is moving toward hybrid systems that combine the advantages of both metal oxides and amine-functionalized materials, along with process intensification strategies. The ultimate goal remains developing economically viable carbon capture solutions that can be deployed at scale across various industries, from power generation to cement production, supporting global decarbonization efforts while maintaining energy security and industrial competitiveness.
Market Demand for Carbon Capture Solutions
The global carbon capture market is experiencing unprecedented growth, driven by increasing environmental concerns and stringent regulatory frameworks aimed at reducing greenhouse gas emissions. Current market valuations indicate the carbon capture technology sector reached approximately 7 billion USD in 2022, with projections suggesting a compound annual growth rate of 15-20% through 2030, potentially reaching 30 billion USD by the end of the decade.
Industrial sectors, particularly power generation, cement production, and steel manufacturing, represent the primary demand drivers for carbon capture solutions. These industries collectively contribute over 40% of global CO2 emissions, creating an urgent need for effective capture technologies. The cement industry alone accounts for roughly 8% of global CO2 emissions, making it a critical target market for advanced sorbent technologies.
Geographically, North America and Europe currently lead market demand, with the United States, Canada, United Kingdom, and Norway implementing significant carbon capture projects. However, rapid industrialization in Asia-Pacific regions, particularly China and India, is creating emerging markets with substantial growth potential. China's commitment to carbon neutrality by 2060 has accelerated investments in carbon capture technologies across its extensive industrial base.
Policy frameworks are significantly influencing market dynamics. The implementation of carbon pricing mechanisms in over 40 countries has created economic incentives for carbon capture adoption. Tax credits like the 45Q in the United States, offering up to 85 USD per metric ton for captured and sequestered CO2, have substantially improved the financial viability of carbon capture projects.
The demand for solid sorbents specifically has grown due to their operational advantages over traditional liquid amine scrubbing technologies. Market analysis indicates that solid sorbents could capture 25-30% of the overall carbon capture technology market by 2030, representing a significant shift from current deployment levels of approximately 10%.
End-user preferences are increasingly favoring technologies with lower energy penalties and operational costs. This trend benefits advanced solid sorbents, particularly in retrofit applications where space constraints and integration with existing infrastructure are critical considerations. Industries with high-temperature flue gases show particular interest in metal oxide sorbents, while applications requiring lower temperature operation tend toward amine-functionalized materials.
Investment patterns reveal growing venture capital interest in solid sorbent technologies, with funding rounds for startups in this space increasing by approximately 200% between 2018 and 2022. This investment surge reflects market confidence in the commercial potential of next-generation carbon capture materials and their essential role in meeting global climate targets.
Industrial sectors, particularly power generation, cement production, and steel manufacturing, represent the primary demand drivers for carbon capture solutions. These industries collectively contribute over 40% of global CO2 emissions, creating an urgent need for effective capture technologies. The cement industry alone accounts for roughly 8% of global CO2 emissions, making it a critical target market for advanced sorbent technologies.
Geographically, North America and Europe currently lead market demand, with the United States, Canada, United Kingdom, and Norway implementing significant carbon capture projects. However, rapid industrialization in Asia-Pacific regions, particularly China and India, is creating emerging markets with substantial growth potential. China's commitment to carbon neutrality by 2060 has accelerated investments in carbon capture technologies across its extensive industrial base.
Policy frameworks are significantly influencing market dynamics. The implementation of carbon pricing mechanisms in over 40 countries has created economic incentives for carbon capture adoption. Tax credits like the 45Q in the United States, offering up to 85 USD per metric ton for captured and sequestered CO2, have substantially improved the financial viability of carbon capture projects.
The demand for solid sorbents specifically has grown due to their operational advantages over traditional liquid amine scrubbing technologies. Market analysis indicates that solid sorbents could capture 25-30% of the overall carbon capture technology market by 2030, representing a significant shift from current deployment levels of approximately 10%.
End-user preferences are increasingly favoring technologies with lower energy penalties and operational costs. This trend benefits advanced solid sorbents, particularly in retrofit applications where space constraints and integration with existing infrastructure are critical considerations. Industries with high-temperature flue gases show particular interest in metal oxide sorbents, while applications requiring lower temperature operation tend toward amine-functionalized materials.
Investment patterns reveal growing venture capital interest in solid sorbent technologies, with funding rounds for startups in this space increasing by approximately 200% between 2018 and 2022. This investment surge reflects market confidence in the commercial potential of next-generation carbon capture materials and their essential role in meeting global climate targets.
Current State and Challenges in Solid Sorbent Technology
The field of solid sorbents for CO2 capture has witnessed significant advancements globally, with two primary categories emerging as frontrunners: metal oxides and amine-functionalized materials. Currently, metal oxide sorbents such as calcium oxide (CaO), magnesium oxide (MgO), and lithium zirconate (Li2ZrO3) demonstrate promising CO2 capture capacities ranging from 0.5 to 0.8 g CO2/g sorbent under optimal conditions. These materials operate effectively at elevated temperatures (400-700°C), making them suitable for integration with high-temperature industrial processes.
In contrast, amine-functionalized sorbents, including polyethyleneimine (PEI) supported on silica or metal-organic frameworks (MOFs) with grafted amines, exhibit CO2 capture capacities of 0.1-0.3 g CO2/g sorbent at lower temperatures (40-120°C). This temperature range aligns well with post-combustion capture scenarios, offering energy efficiency advantages in certain applications.
Despite these advancements, both sorbent categories face substantial technical challenges. Metal oxides suffer from rapid performance degradation, with capacity losses of 30-50% observed after just 10-20 cycles due to sintering and structural collapse. Additionally, their high regeneration temperatures (>800°C for CaO) impose significant energy penalties, reducing overall process efficiency by 8-12 percentage points in power generation applications.
Amine-functionalized materials, while operating at lower temperatures, struggle with oxidative degradation in the presence of oxygen and moisture, losing up to 15-25% capacity after extended cycling. Their production also involves complex synthesis procedures, limiting large-scale manufacturing capabilities and increasing costs to $50-100/kg compared to $5-15/kg for metal oxides.
Geographically, research leadership in metal oxide sorbents is concentrated in Europe (particularly Spain and Germany) and China, while amine-functionalized materials development is predominantly led by North American and Japanese research institutions. This distribution reflects regional industrial priorities and existing research infrastructure.
Water stability represents another critical challenge, with humidity causing structural degradation in many metal oxides and competitive adsorption in amine-functionalized materials, reducing CO2 selectivity by 20-40% in humid conditions. Additionally, both sorbent types face challenges with flue gas contaminants such as SOx and NOx, which can irreversibly poison active sites and accelerate performance decline.
The technical landscape is further complicated by scale-up challenges, as most promising materials have only been demonstrated at laboratory or small pilot scales (1-10 kg sorbent), with limited data available on long-term stability under industrial conditions involving thousands of cycles and diverse operating environments.
In contrast, amine-functionalized sorbents, including polyethyleneimine (PEI) supported on silica or metal-organic frameworks (MOFs) with grafted amines, exhibit CO2 capture capacities of 0.1-0.3 g CO2/g sorbent at lower temperatures (40-120°C). This temperature range aligns well with post-combustion capture scenarios, offering energy efficiency advantages in certain applications.
Despite these advancements, both sorbent categories face substantial technical challenges. Metal oxides suffer from rapid performance degradation, with capacity losses of 30-50% observed after just 10-20 cycles due to sintering and structural collapse. Additionally, their high regeneration temperatures (>800°C for CaO) impose significant energy penalties, reducing overall process efficiency by 8-12 percentage points in power generation applications.
Amine-functionalized materials, while operating at lower temperatures, struggle with oxidative degradation in the presence of oxygen and moisture, losing up to 15-25% capacity after extended cycling. Their production also involves complex synthesis procedures, limiting large-scale manufacturing capabilities and increasing costs to $50-100/kg compared to $5-15/kg for metal oxides.
Geographically, research leadership in metal oxide sorbents is concentrated in Europe (particularly Spain and Germany) and China, while amine-functionalized materials development is predominantly led by North American and Japanese research institutions. This distribution reflects regional industrial priorities and existing research infrastructure.
Water stability represents another critical challenge, with humidity causing structural degradation in many metal oxides and competitive adsorption in amine-functionalized materials, reducing CO2 selectivity by 20-40% in humid conditions. Additionally, both sorbent types face challenges with flue gas contaminants such as SOx and NOx, which can irreversibly poison active sites and accelerate performance decline.
The technical landscape is further complicated by scale-up challenges, as most promising materials have only been demonstrated at laboratory or small pilot scales (1-10 kg sorbent), with limited data available on long-term stability under industrial conditions involving thousands of cycles and diverse operating environments.
Metal Oxides vs Amine-Functionalized Sorbents: Technical Comparison
01 Metal oxide-based sorbents for CO2 capture
Metal oxide-based materials serve as effective solid sorbents for CO2 capture. These materials, including calcium oxide, magnesium oxide, and transition metal oxides, can capture CO2 through carbonation reactions. The capture efficiency depends on factors such as surface area, porosity, and thermal stability. These sorbents often undergo multiple capture-regeneration cycles, with their performance enhanced through various modification strategies like doping with promoters or controlling particle size distribution.- Metal oxide-based sorbents for CO2 capture: Metal oxide-based materials serve as effective solid sorbents for CO2 capture. These materials, including calcium oxide, magnesium oxide, and transition metal oxides, can capture CO2 through carbonation reactions. The efficiency of these sorbents depends on factors such as surface area, porosity, and thermal stability. Various modifications and composite formations can enhance their CO2 capture capacity and regeneration properties, making them suitable for industrial-scale carbon capture applications.
- Amine-functionalized materials for CO2 adsorption: Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine the high CO2 affinity of amines with the structural advantages of solid supports such as silica, polymers, or MOFs. The amine groups form carbamates or carbonates with CO2, enabling selective capture even at low CO2 concentrations. The efficiency of these materials depends on amine loading, accessibility of functional groups, and stability during multiple adsorption-desorption cycles.
- Novel composite and hybrid sorbent materials: Hybrid and composite materials combine the advantages of different types of sorbents to enhance CO2 capture efficiency. These include metal oxide/amine hybrids, polymer-inorganic composites, and multi-component systems that leverage synergistic effects. The composite approach allows for tailoring properties such as selectivity, capacity, and regeneration energy requirements. These materials often demonstrate improved stability and performance under various operating conditions compared to single-component sorbents.
- Structured and engineered sorbents for improved mass transfer: Structured and engineered sorbents are designed to overcome mass transfer limitations in CO2 capture processes. These include monolithic structures, hierarchical porous materials, and 3D-printed sorbents with optimized geometries. By controlling pore architecture and creating dedicated transport channels, these materials facilitate faster CO2 diffusion and adsorption kinetics. The improved mass transfer properties lead to higher CO2 capture rates and efficiencies, particularly in rapid-cycle operations.
- Regeneration methods and cyclic stability enhancement: Enhancing the regeneration efficiency and cyclic stability of solid sorbents is crucial for practical CO2 capture applications. Various approaches include thermal, pressure, and concentration swing processes, as well as novel methods like microwave and electrical swing adsorption. Material modifications such as dopants, stabilizers, and core-shell structures can prevent degradation mechanisms like sintering and amine leaching. These improvements extend sorbent lifetime and maintain high CO2 capture efficiency over multiple cycles, reducing operational costs.
02 Amine-functionalized materials for CO2 adsorption
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine support structures (such as silica, polymers, or MOFs) with various amine groups that chemically bind CO2. The capture efficiency is influenced by the type of amine, loading density, and accessibility of binding sites. These sorbents typically operate at lower temperatures compared to metal oxides and show high selectivity for CO2 even in humid conditions, making them suitable for post-combustion capture applications.Expand Specific Solutions03 Hybrid and composite sorbent systems
Hybrid and composite sorbent systems combine different materials to enhance CO2 capture efficiency. These systems often integrate metal oxides with amine-functionalized components or incorporate multiple functional materials into structured supports. The synergistic effects between components can improve adsorption capacity, selectivity, and regeneration properties. These hybrid systems frequently demonstrate better stability during cycling and can be tailored for specific operating conditions, such as temperature ranges or gas compositions.Expand Specific Solutions04 Structural and morphological optimization
The structural and morphological characteristics of solid sorbents significantly impact CO2 capture efficiency. Optimization strategies include developing hierarchical pore structures, controlling particle size and shape, and creating high-surface-area materials. Techniques such as templating, hydrothermal synthesis, and sol-gel processing are employed to engineer sorbents with enhanced gas diffusion properties and accessible binding sites. These structural modifications can substantially improve adsorption kinetics and overall capture capacity.Expand Specific Solutions05 Regeneration and cycling performance enhancement
Enhancing regeneration capabilities and cycling stability is crucial for practical CO2 capture applications. This involves developing sorbents that maintain their capture efficiency over multiple adsorption-desorption cycles. Strategies include incorporating stabilizing agents, optimizing regeneration conditions, and designing materials resistant to thermal and chemical degradation. Advanced regeneration methods, such as pressure-swing, temperature-swing, or combined approaches, are employed to minimize energy requirements while maximizing CO2 recovery and sorbent longevity.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Capture
The CO2 capture technology market using solid sorbents is currently in a growth phase, with metal oxides and amine-functionalized materials representing two competing technological approaches. The global carbon capture market is expanding rapidly, projected to reach $7-10 billion by 2030, driven by increasing climate change mitigation efforts. Metal oxide sorbents, championed by companies like Climeworks AG and Global Thermostat, offer high temperature stability and regeneration efficiency, while amine-functionalized materials, advanced by ExxonMobil and Schlumberger, provide superior selectivity at lower temperatures. Academic institutions including Cornell University and King Abdullah University collaborate with industry leaders like China Petroleum & Chemical Corp. to bridge fundamental research with commercial applications. The technology remains in early commercial deployment, with cost reduction and scalability representing key challenges for widespread adoption.
Climeworks AG
Technical Solution: Climeworks has developed a Direct Air Capture (DAC) technology using solid sorbents for CO2 capture. Their approach employs amine-functionalized filter materials that selectively capture CO2 from ambient air. The process involves air flowing through collectors where the proprietary sorbent material binds with CO2 molecules. Once saturated, the collectors are heated to approximately 100°C, releasing concentrated CO2 that can be permanently stored underground or utilized in various applications. Climeworks' technology operates in a modular fashion with stackable "CO2 collectors" that can be scaled according to capture requirements. Their latest plants, including the Orca facility in Iceland, demonstrate commercial-scale implementation of this technology, capturing thousands of tons of CO2 annually with the captured carbon being mineralized and stored permanently underground through partnership with Carbfix.
Strengths: Modular and scalable design allows for flexible deployment; direct integration with renewable energy sources; proven commercial implementation with operational plants. Weaknesses: Relatively high energy requirements for the thermal regeneration process; higher cost per ton of CO2 captured compared to point-source capture technologies; limited by geographical constraints for optimal renewable energy access.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced metal oxide-based sorbents for CO2 capture, particularly focusing on carbonate looping technology. Their approach utilizes calcium oxide (CaO) and magnesium oxide (MgO) based sorbents that undergo carbonation-calcination cycles. The company has engineered these metal oxide sorbents to maintain structural integrity over multiple capture-regeneration cycles, addressing the common degradation issues associated with traditional metal oxide sorbents. ExxonMobil's research has focused on enhancing the cyclic stability through novel material compositions and structural modifications, including the incorporation of support materials and promoters to prevent sintering. Their technology is particularly targeted at high-temperature industrial applications, such as power plants and refineries, where the heat from industrial processes can be integrated with the sorbent regeneration requirements, improving overall energy efficiency. The company has also explored hybrid systems combining metal oxides with amine functionalization to leverage the advantages of both approaches.
Strengths: High temperature operation capability makes integration with industrial processes more efficient; potentially lower regeneration energy requirements compared to pure amine systems; robust performance in the presence of contaminants found in industrial flue gases. Weaknesses: Metal oxide sorbents typically suffer from capacity degradation over multiple cycles; higher operating temperatures require specialized materials and equipment; slower kinetics compared to some amine-functionalized sorbents.
Key Patents and Breakthroughs in Solid Sorbent Development
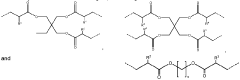
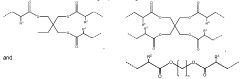
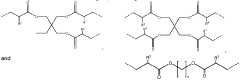
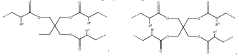
Metal oxide foam, amine functional solid sorbent, methods and applications
PatentActiveUS20130338001A1
Innovation
- Amine functional solid sorbents supported by metal oxide foam structures, specifically silica foam, with ultra-large mesopores, are used for CO2 capture, where the amine material is either physically or chemically incorporated or covalently bonded to the sorbent support, optimizing the structure and morphology to enhance capture efficiency.
Sorbent materials for carbon dioxide separation
PatentWO2023237579A1
Innovation
- A sorbent material comprising cross-linked and substituted branched polyethylenimine with specific molecular weight and substitution degrees, allowing for isothermal CO2 separation at temperatures between ambient and 70 °C, reducing energy consumption and costs.
Environmental Impact and Sustainability Assessment
The environmental impact assessment of solid sorbents for CO2 capture reveals significant differences between metal oxides and amine-functionalized materials. Metal oxide sorbents generally require high regeneration temperatures (600-900°C), resulting in substantial energy consumption and associated greenhouse gas emissions. This energy penalty can partially offset the environmental benefits of carbon capture, particularly when power sources are fossil fuel-based. However, metal oxides typically demonstrate longer operational lifespans and better thermal stability, reducing the frequency of replacement and associated material waste.
In contrast, amine-functionalized sorbents operate at lower temperatures (60-120°C), offering potential energy savings during regeneration. This translates to reduced indirect CO2 emissions from the capture process itself. However, these materials face challenges including amine leaching and degradation over multiple cycles, potentially releasing harmful compounds into the environment. The production of amine-functionalized materials also involves complex chemical processes that may generate hazardous waste streams requiring careful management.
Life cycle assessment (LCA) studies indicate that the environmental footprint of both sorbent types extends beyond operational considerations. The extraction of raw materials, particularly rare earth elements sometimes used in metal oxide formulations, can involve intensive mining operations with significant land disturbance and water pollution risks. Similarly, the chemical precursors for amine functionalization may derive from petroleum sources, linking these materials to fossil fuel supply chains.
Water usage patterns differ markedly between the two sorbent categories. Metal oxides typically require less water during operation but may generate more particulate matter. Amine-functionalized materials often involve aqueous processing steps and may contribute to water contamination if degradation products are not properly contained. Both sorbent types face end-of-life management challenges, though metal oxides generally offer better recyclability potential.
From a sustainability perspective, recent innovations are addressing these environmental concerns. Advanced metal oxide formulations are being developed with lower regeneration temperatures, while more stable amine structures are reducing degradation issues. Biobased precursors for amine functionalization represent a promising direction for reducing the carbon footprint of these materials. Additionally, hybrid systems combining the advantages of both sorbent types are emerging as potentially more sustainable solutions.
The environmental trade-offs between these sorbent classes ultimately depend on specific deployment contexts, including available energy sources, water constraints, and waste management infrastructure. A holistic sustainability assessment must consider these factors alongside technical performance metrics to identify optimal carbon capture solutions for different applications and regions.
In contrast, amine-functionalized sorbents operate at lower temperatures (60-120°C), offering potential energy savings during regeneration. This translates to reduced indirect CO2 emissions from the capture process itself. However, these materials face challenges including amine leaching and degradation over multiple cycles, potentially releasing harmful compounds into the environment. The production of amine-functionalized materials also involves complex chemical processes that may generate hazardous waste streams requiring careful management.
Life cycle assessment (LCA) studies indicate that the environmental footprint of both sorbent types extends beyond operational considerations. The extraction of raw materials, particularly rare earth elements sometimes used in metal oxide formulations, can involve intensive mining operations with significant land disturbance and water pollution risks. Similarly, the chemical precursors for amine functionalization may derive from petroleum sources, linking these materials to fossil fuel supply chains.
Water usage patterns differ markedly between the two sorbent categories. Metal oxides typically require less water during operation but may generate more particulate matter. Amine-functionalized materials often involve aqueous processing steps and may contribute to water contamination if degradation products are not properly contained. Both sorbent types face end-of-life management challenges, though metal oxides generally offer better recyclability potential.
From a sustainability perspective, recent innovations are addressing these environmental concerns. Advanced metal oxide formulations are being developed with lower regeneration temperatures, while more stable amine structures are reducing degradation issues. Biobased precursors for amine functionalization represent a promising direction for reducing the carbon footprint of these materials. Additionally, hybrid systems combining the advantages of both sorbent types are emerging as potentially more sustainable solutions.
The environmental trade-offs between these sorbent classes ultimately depend on specific deployment contexts, including available energy sources, water constraints, and waste management infrastructure. A holistic sustainability assessment must consider these factors alongside technical performance metrics to identify optimal carbon capture solutions for different applications and regions.
Techno-Economic Analysis of Sorbent Implementation
The implementation of solid sorbents for CO2 capture requires thorough techno-economic analysis to determine commercial viability. Current cost estimates for metal oxide-based systems range from $40-70 per ton of CO2 captured, while amine-functionalized sorbents typically cost between $45-85 per ton, depending on regeneration energy requirements and sorbent lifetime.
Capital expenditure for metal oxide systems is generally lower due to simpler reactor designs and less expensive raw materials. CaO-based systems, for instance, benefit from abundant limestone resources priced at $10-30 per ton. However, these systems often require higher operating temperatures (600-900°C), resulting in increased energy consumption during regeneration cycles.
Amine-functionalized sorbents, while having higher initial material costs ($500-2,000 per ton), demonstrate superior performance at lower temperatures (40-120°C), reducing overall energy penalties. Their selective CO2 capture capabilities in dilute gas streams provide advantages in various industrial applications, potentially offsetting the higher initial investment through operational savings.
Sorbent durability significantly impacts economic feasibility. Metal oxides typically experience 10-40% capacity degradation over 100 cycles, necessitating more frequent replacement. In contrast, advanced amine-functionalized materials maintain 80-90% capacity after similar cycling, extending operational lifespans and improving long-term economics despite higher upfront costs.
Scale-up considerations reveal different challenges for each sorbent class. Metal oxides benefit from established fluidized bed technologies adapted from other industries, reducing technology risk. Amine-functionalized materials often require more specialized handling and regeneration processes, increasing complexity but potentially offering better integration with existing low-temperature waste heat sources.
Energy integration analysis indicates that metal oxide systems can achieve thermal efficiencies of 25-35% when integrated with power generation, while amine-functionalized sorbents typically reach 30-40% efficiency due to lower regeneration temperatures and better heat recovery potential.
Market sensitivity analysis suggests metal oxides may be more economically viable in regions with low energy costs and high availability of raw materials, while amine-functionalized sorbents demonstrate better economics in applications where selective capture from dilute streams is required or where waste heat at lower temperatures is readily available.
Capital expenditure for metal oxide systems is generally lower due to simpler reactor designs and less expensive raw materials. CaO-based systems, for instance, benefit from abundant limestone resources priced at $10-30 per ton. However, these systems often require higher operating temperatures (600-900°C), resulting in increased energy consumption during regeneration cycles.
Amine-functionalized sorbents, while having higher initial material costs ($500-2,000 per ton), demonstrate superior performance at lower temperatures (40-120°C), reducing overall energy penalties. Their selective CO2 capture capabilities in dilute gas streams provide advantages in various industrial applications, potentially offsetting the higher initial investment through operational savings.
Sorbent durability significantly impacts economic feasibility. Metal oxides typically experience 10-40% capacity degradation over 100 cycles, necessitating more frequent replacement. In contrast, advanced amine-functionalized materials maintain 80-90% capacity after similar cycling, extending operational lifespans and improving long-term economics despite higher upfront costs.
Scale-up considerations reveal different challenges for each sorbent class. Metal oxides benefit from established fluidized bed technologies adapted from other industries, reducing technology risk. Amine-functionalized materials often require more specialized handling and regeneration processes, increasing complexity but potentially offering better integration with existing low-temperature waste heat sources.
Energy integration analysis indicates that metal oxide systems can achieve thermal efficiencies of 25-35% when integrated with power generation, while amine-functionalized sorbents typically reach 30-40% efficiency due to lower regeneration temperatures and better heat recovery potential.
Market sensitivity analysis suggests metal oxides may be more economically viable in regions with low energy costs and high availability of raw materials, while amine-functionalized sorbents demonstrate better economics in applications where selective capture from dilute streams is required or where waste heat at lower temperatures is readily available.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!