Solid sorbents for CO2 capture interface engineering and surface modification
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications in response to growing environmental concerns. The development of solid sorbents represents a critical advancement in this field, offering potentially more energy-efficient and environmentally friendly alternatives to traditional liquid amine scrubbing methods. Early CO2 capture technologies primarily relied on aqueous amine solutions, which despite their effectiveness, presented challenges including high energy requirements for regeneration, corrosion issues, and environmental impacts.
The evolution of solid sorbents began with basic porous materials such as activated carbons and zeolites in the 1990s, progressing to more sophisticated materials including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous polymers in the 2000s and 2010s. This progression has been driven by the need for materials with higher CO2 selectivity, capacity, and stability under various operating conditions.
Interface engineering and surface modification have emerged as pivotal approaches in enhancing the performance of solid sorbents. These techniques focus on tailoring the physical and chemical properties of sorbent surfaces to optimize CO2 adsorption kinetics, selectivity, and capacity. The strategic modification of surface functional groups, pore structures, and interfacial properties has enabled significant improvements in sorbent performance metrics.
The primary objectives of current research in solid sorbents for CO2 capture include developing materials with enhanced CO2 adsorption capacity under relevant industrial conditions, improving selectivity for CO2 over other flue gas components, ensuring mechanical and chemical stability over multiple adsorption-desorption cycles, and reducing the energy penalty associated with sorbent regeneration. Additionally, researchers aim to design sorbents that can function effectively in the presence of contaminants such as SOx, NOx, and water vapor.
Future technological goals include scaling up promising laboratory-scale sorbents for industrial deployment, integrating advanced sorbents into existing and new carbon capture systems, and reducing the overall cost of carbon capture to make it economically viable for widespread implementation. The development of multifunctional sorbents capable of capturing CO2 while simultaneously converting it into value-added products represents an ambitious but potentially transformative objective.
The trajectory of solid sorbent development is increasingly focused on sustainable materials and processes, with growing emphasis on utilizing renewable resources, minimizing environmental footprints, and ensuring the long-term viability of carbon capture technologies as critical components of global climate change mitigation strategies.
The evolution of solid sorbents began with basic porous materials such as activated carbons and zeolites in the 1990s, progressing to more sophisticated materials including metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and functionalized porous polymers in the 2000s and 2010s. This progression has been driven by the need for materials with higher CO2 selectivity, capacity, and stability under various operating conditions.
Interface engineering and surface modification have emerged as pivotal approaches in enhancing the performance of solid sorbents. These techniques focus on tailoring the physical and chemical properties of sorbent surfaces to optimize CO2 adsorption kinetics, selectivity, and capacity. The strategic modification of surface functional groups, pore structures, and interfacial properties has enabled significant improvements in sorbent performance metrics.
The primary objectives of current research in solid sorbents for CO2 capture include developing materials with enhanced CO2 adsorption capacity under relevant industrial conditions, improving selectivity for CO2 over other flue gas components, ensuring mechanical and chemical stability over multiple adsorption-desorption cycles, and reducing the energy penalty associated with sorbent regeneration. Additionally, researchers aim to design sorbents that can function effectively in the presence of contaminants such as SOx, NOx, and water vapor.
Future technological goals include scaling up promising laboratory-scale sorbents for industrial deployment, integrating advanced sorbents into existing and new carbon capture systems, and reducing the overall cost of carbon capture to make it economically viable for widespread implementation. The development of multifunctional sorbents capable of capturing CO2 while simultaneously converting it into value-added products represents an ambitious but potentially transformative objective.
The trajectory of solid sorbent development is increasingly focused on sustainable materials and processes, with growing emphasis on utilizing renewable resources, minimizing environmental footprints, and ensuring the long-term viability of carbon capture technologies as critical components of global climate change mitigation strategies.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.3 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.6 billion by the end of the decade. This growth trajectory is particularly relevant for solid sorbent technologies, which are gaining traction as an alternative to traditional liquid amine-based capture systems.
The market for solid sorbents specifically is emerging as a high-potential segment within the broader CCS landscape. Current estimates place this sub-segment at about $1.2 billion, with expectations of accelerated growth as interface engineering and surface modification technologies mature. These advanced materials are attracting substantial investment due to their promise of reduced energy penalties and operational costs compared to conventional capture methods.
Geographically, North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate in the coming years, particularly in China and India where rapid industrialization coincides with increasing environmental concerns.
From an end-user perspective, the power generation sector represents the largest market for carbon capture technologies at 45% of total deployments, followed by industrial applications (30%) and oil and gas operations (15%). The cement and steel industries are emerging as particularly promising growth sectors for solid sorbent applications due to their high emission profiles and technical compatibility with these capture systems.
Investor interest in solid sorbent technologies has seen remarkable growth, with venture capital funding increasing by 215% between 2020 and 2023. Strategic partnerships between technology developers and industrial end-users have become increasingly common, with over 75 major collaboration agreements announced in the past two years focused specifically on piloting advanced sorbent technologies.
Market barriers include high initial capital costs, with current estimates suggesting solid sorbent systems require $60-80 per ton of CO2 captured. However, ongoing research in interface engineering and surface modification is expected to reduce these costs to $40-50 per ton by 2028, potentially disrupting the competitive landscape and accelerating market adoption across multiple industrial sectors.
The market for solid sorbents specifically is emerging as a high-potential segment within the broader CCS landscape. Current estimates place this sub-segment at about $1.2 billion, with expectations of accelerated growth as interface engineering and surface modification technologies mature. These advanced materials are attracting substantial investment due to their promise of reduced energy penalties and operational costs compared to conventional capture methods.
Geographically, North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate in the coming years, particularly in China and India where rapid industrialization coincides with increasing environmental concerns.
From an end-user perspective, the power generation sector represents the largest market for carbon capture technologies at 45% of total deployments, followed by industrial applications (30%) and oil and gas operations (15%). The cement and steel industries are emerging as particularly promising growth sectors for solid sorbent applications due to their high emission profiles and technical compatibility with these capture systems.
Investor interest in solid sorbent technologies has seen remarkable growth, with venture capital funding increasing by 215% between 2020 and 2023. Strategic partnerships between technology developers and industrial end-users have become increasingly common, with over 75 major collaboration agreements announced in the past two years focused specifically on piloting advanced sorbent technologies.
Market barriers include high initial capital costs, with current estimates suggesting solid sorbent systems require $60-80 per ton of CO2 captured. However, ongoing research in interface engineering and surface modification is expected to reduce these costs to $40-50 per ton by 2028, potentially disrupting the competitive landscape and accelerating market adoption across multiple industrial sectors.
Current Solid Sorbents Landscape and Barriers
The current landscape of solid sorbents for CO2 capture is characterized by a diverse array of materials with varying properties and performance metrics. Amine-functionalized materials, particularly those based on silica supports, dominate commercial applications due to their high selectivity for CO2 and relatively mature manufacturing processes. Metal-organic frameworks (MOFs) represent another significant category, with materials like Mg-MOF-74 and HKUST-1 demonstrating exceptional CO2 uptake capacities under specific conditions. Zeolites, activated carbons, and hydrotalcites also maintain substantial market presence, each offering unique advantages in different capture scenarios.
Despite these advancements, several critical barriers impede widespread implementation of solid sorbent technologies. Thermal stability remains a primary concern, with many high-performance materials experiencing significant degradation after multiple adsorption-desorption cycles at elevated temperatures. This degradation manifests as reduced CO2 uptake capacity and structural collapse, particularly in amine-functionalized materials where amine leaching occurs during regeneration cycles.
Moisture sensitivity presents another substantial challenge, as water vapor can competitively adsorb on active sites, dramatically reducing CO2 capture efficiency in real-world flue gas conditions. This is especially problematic for zeolites and certain MOFs, where water can permanently alter the material structure or block pore networks.
Manufacturing scalability constitutes a significant barrier, particularly for advanced materials like MOFs and custom-designed porous polymers. Current synthesis methods often involve expensive precursors, environmentally harmful solvents, or energy-intensive processes that limit industrial viability. The gap between laboratory performance and industrial implementation remains substantial, with few materials successfully transitioning to commercial-scale production.
Interface engineering challenges are particularly evident in composite sorbents, where achieving uniform distribution of functional groups and maintaining interfacial stability during cycling presents significant difficulties. Surface modification techniques, while promising for enhancing selectivity and capacity, often introduce additional complexity in manufacturing and can reduce mechanical stability.
Economic barriers further complicate adoption, with high material costs and energy penalties during regeneration reducing the competitiveness against established technologies like amine scrubbing. Most current solid sorbents require significant energy input for regeneration, typically 2.5-3.5 GJ/ton CO2 captured, which remains too high for economically viable implementation in many industrial settings.
Addressing these barriers requires interdisciplinary approaches combining materials science, chemical engineering, and process optimization to develop next-generation sorbents with enhanced stability, selectivity, and regeneration efficiency.
Despite these advancements, several critical barriers impede widespread implementation of solid sorbent technologies. Thermal stability remains a primary concern, with many high-performance materials experiencing significant degradation after multiple adsorption-desorption cycles at elevated temperatures. This degradation manifests as reduced CO2 uptake capacity and structural collapse, particularly in amine-functionalized materials where amine leaching occurs during regeneration cycles.
Moisture sensitivity presents another substantial challenge, as water vapor can competitively adsorb on active sites, dramatically reducing CO2 capture efficiency in real-world flue gas conditions. This is especially problematic for zeolites and certain MOFs, where water can permanently alter the material structure or block pore networks.
Manufacturing scalability constitutes a significant barrier, particularly for advanced materials like MOFs and custom-designed porous polymers. Current synthesis methods often involve expensive precursors, environmentally harmful solvents, or energy-intensive processes that limit industrial viability. The gap between laboratory performance and industrial implementation remains substantial, with few materials successfully transitioning to commercial-scale production.
Interface engineering challenges are particularly evident in composite sorbents, where achieving uniform distribution of functional groups and maintaining interfacial stability during cycling presents significant difficulties. Surface modification techniques, while promising for enhancing selectivity and capacity, often introduce additional complexity in manufacturing and can reduce mechanical stability.
Economic barriers further complicate adoption, with high material costs and energy penalties during regeneration reducing the competitiveness against established technologies like amine scrubbing. Most current solid sorbents require significant energy input for regeneration, typically 2.5-3.5 GJ/ton CO2 captured, which remains too high for economically viable implementation in many industrial settings.
Addressing these barriers requires interdisciplinary approaches combining materials science, chemical engineering, and process optimization to develop next-generation sorbents with enhanced stability, selectivity, and regeneration efficiency.
Surface Modification Techniques for Enhanced CO2 Adsorption
01 Surface modification of solid sorbents for enhanced CO2 capture
Surface modification techniques can significantly improve the CO2 adsorption capacity and selectivity of solid sorbents. These modifications typically involve the introduction of functional groups or chemical treatments that create favorable binding sites for CO2 molecules. Various approaches include amine functionalization, metal impregnation, and acid-base treatments that alter the surface chemistry to enhance CO2 affinity while maintaining structural integrity of the sorbent material.- Surface modification of solid sorbents for enhanced CO2 capture: Surface modification techniques can significantly improve the CO2 adsorption capacity and selectivity of solid sorbents. These modifications typically involve the introduction of functional groups or chemical treatments that create favorable binding sites for CO2 molecules. Various approaches include amine functionalization, metal impregnation, and acid-base treatments that alter the surface chemistry to enhance CO2 capture performance while maintaining structural integrity and regeneration capabilities.
- Interface engineering for improved CO2 adsorption kinetics: Interface engineering focuses on optimizing the boundary between the sorbent material and the gas phase to enhance CO2 capture efficiency. This involves designing porous structures with controlled pore sizes, creating hierarchical architectures, and developing composite materials with synergistic interfaces. These engineered interfaces facilitate rapid mass transfer, reduce diffusion limitations, and provide accessible adsorption sites, resulting in improved adsorption kinetics and overall CO2 capture performance.
- Novel materials and composites for CO2 capture: Advanced materials and composites are being developed specifically for CO2 capture applications. These include metal-organic frameworks (MOFs), zeolites, activated carbons, and polymer-based sorbents with tailored properties. By combining different materials into composites, synergistic effects can be achieved that enhance adsorption capacity, selectivity, and stability. These novel materials often feature high surface areas, tunable pore structures, and specific binding sites for CO2 molecules.
- Stability and regeneration enhancement of CO2 sorbents: Improving the stability and regeneration capabilities of solid sorbents is crucial for practical CO2 capture applications. Various approaches include thermal stabilization treatments, incorporation of support materials, and development of core-shell structures that protect active sites. These modifications aim to prevent degradation during multiple adsorption-desorption cycles, maintain structural integrity under harsh conditions, and reduce energy requirements for regeneration while preserving CO2 capture performance.
- Integration of CO2 capture sorbents in industrial systems: Effective integration of solid sorbents into industrial CO2 capture systems requires specialized engineering approaches. This includes designing appropriate contacting methods between flue gas and sorbents, developing efficient heat management strategies, and creating scalable reactor configurations. Surface modifications and interface engineering must be optimized not only for laboratory performance but also for real-world conditions, considering factors such as presence of contaminants, pressure fluctuations, and temperature variations in industrial settings.
02 Interface engineering of porous materials for CO2 capture
Interface engineering focuses on optimizing the boundary regions between different phases or components in solid sorbents to maximize CO2 capture efficiency. This approach involves controlling pore structure, size distribution, and connectivity to enhance gas diffusion and adsorption kinetics. By engineering interfaces at the nanoscale, researchers can create materials with improved mass transfer properties, reduced diffusion limitations, and optimized contact between the CO2 molecules and active adsorption sites.Expand Specific Solutions03 Composite and hybrid sorbent materials for CO2 capture
Composite and hybrid materials combine different components to create synergistic effects for enhanced CO2 capture. These materials often integrate organic and inorganic components, such as metal-organic frameworks (MOFs), polymer-inorganic hybrids, or supported amine materials. The interface between these components is carefully engineered to maximize adsorption capacity, selectivity, and stability under various operating conditions, while minimizing energy requirements for regeneration.Expand Specific Solutions04 Thermal and chemical stability enhancement of CO2 sorbents
Improving the thermal and chemical stability of solid sorbents is crucial for their practical application in CO2 capture systems. Surface modification techniques, such as hydrophobic treatments, cross-linking, and incorporation of stabilizing agents, can protect the sorbent from degradation during multiple adsorption-desorption cycles. These modifications help maintain the structural integrity and adsorption performance of the sorbents under harsh conditions, including high temperatures, presence of moisture, and exposure to contaminants.Expand Specific Solutions05 Nano-engineered sorbents with controlled morphology
Nano-engineering approaches focus on controlling the morphology, particle size, and surface properties of solid sorbents at the nanoscale to optimize CO2 capture performance. These techniques include template-assisted synthesis, self-assembly processes, and precise control of nucleation and growth conditions. By tailoring the nanoscale architecture, researchers can create materials with high surface area, optimized pore structure, and enhanced accessibility to active sites, resulting in improved adsorption capacity and faster kinetics.Expand Specific Solutions
Leading Organizations in CO2 Capture Sorbent Research
The solid sorbents for CO2 capture market is currently in a growth phase, with increasing global focus on carbon reduction technologies. The market size is expanding rapidly, projected to reach several billion dollars by 2030 as carbon capture becomes essential for climate goals. Technologically, the field shows varying maturity levels across different approaches. Leading companies like Climeworks AG and Global Thermostat have deployed commercial direct air capture facilities, while Carboncapture Inc. is advancing scalable modular systems. Research institutions including Georgia Tech Research Corp. and Arizona State University are developing next-generation sorbent materials. Meanwhile, industrial players such as Schlumberger, China Petroleum & Chemical Corp., and Wacker Chemie AG are integrating surface modification techniques to enhance capture efficiency and selectivity, indicating a competitive landscape with both specialized startups and established corporations.
Global Thermostat Operations LLC
Technical Solution: Global Thermostat has pioneered a unique solid sorbent CO2 capture technology using amine-functionalized monolithic structures. Their approach features a proprietary ceramic honeycomb substrate with high surface area that is coated with specially formulated amine compounds. This interface engineering creates a three-dimensional capture matrix that maximizes contact between air and active sorbent sites while minimizing pressure drop. The company's surface modification techniques include precise control of amine loading density and distribution to optimize both capture capacity and kinetics. Global Thermostat's process operates at near-ambient temperatures for adsorption and uses low-temperature steam (85-100°C) for regeneration, allowing integration with existing industrial processes and waste heat sources. Their sorbent design incorporates stability enhancers that prevent amine leaching and degradation during repeated thermal cycling, addressing a common challenge with amine-based capture systems. The company has demonstrated their technology at pilot scale facilities and is working toward commercial deployment with partners including ExxonMobil, who invested in scaling the technology for industrial applications.
Strengths: Monolithic structure provides excellent mechanical stability and low pressure drop; can utilize low-grade waste heat for regeneration; modular design allows for flexible scaling. Weaknesses: Manufacturing complex monolithic structures with consistent amine coating presents production challenges; potential for amine degradation over extended operation; higher capital costs compared to some competing technologies.
Carboncapture, Inc.
Technical Solution: CarbonCapture Inc. has developed an advanced modular direct air capture (DAC) system utilizing zeolites as solid sorbents with proprietary surface modifications. Their technology employs specially engineered molecular sieves with tailored pore structures and surface chemistry to optimize CO2 adsorption kinetics and capacity. The company's innovation lies in their temperature-vacuum swing adsorption process that allows for efficient sorbent regeneration using significantly less energy than conventional thermal approaches. CarbonCapture's surface modification techniques include ion exchange processes and post-synthetic treatments that enhance the zeolites' CO2 selectivity and stability under repeated cycling. Their modular design incorporates optimized air-sorbent contact interfaces that maximize mass transfer while minimizing pressure drop. The company has demonstrated that their modified zeolite sorbents maintain performance over thousands of adsorption-desorption cycles, addressing a key challenge in solid sorbent durability. CarbonCapture is deploying their technology in Wyoming as part of Project Bison, which aims to remove 5 million tons of CO2 annually by 2030.
Strengths: Zeolite-based sorbents offer excellent thermal and chemical stability; modular design enables rapid scaling; system can operate effectively in various environmental conditions. Weaknesses: Zeolites generally have lower CO2 capacity than amine-functionalized materials; energy requirements for temperature-vacuum swing process may limit deployment locations; technology is still scaling to prove commercial viability at large scales.
Key Patents in Solid Sorbent Interface Engineering
Layered Solid Sorbents For Carbon Dioxide Capture
PatentActiveUS20140127104A1
Innovation
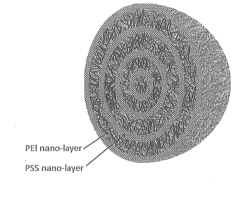
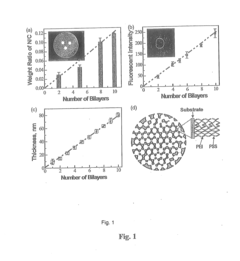
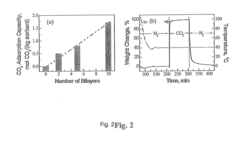
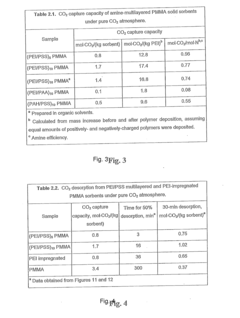
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where CO2-adsorbing polymers like polyethylenimine and oppositely charged polyelectrolytes are alternately deposited on porous substrates, creating bilayers that enhance CO2 capture and transport efficiency.
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
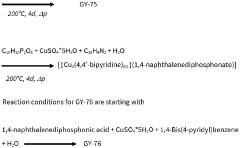
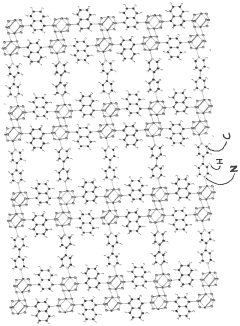
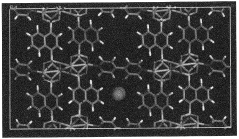
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Environmental Impact Assessment of Sorbent Technologies
The environmental impact assessment of solid sorbents for CO2 capture reveals a complex interplay between potential benefits and drawbacks. When evaluating these technologies from a lifecycle perspective, solid sorbents generally demonstrate lower energy penalties compared to conventional amine-based liquid absorption systems, potentially reducing the overall carbon footprint of capture operations by 15-30% depending on the specific sorbent material and process configuration.
Surface-modified sorbents, particularly those utilizing metal-organic frameworks (MOFs) and functionalized porous carbons, show promising environmental profiles due to their regeneration capabilities and durability. Studies indicate that advanced interface engineering techniques can extend the operational lifespan of these materials by up to 300%, significantly reducing waste generation and resource consumption associated with sorbent replacement cycles.
Water consumption represents a critical environmental consideration, with solid sorbent technologies typically requiring 40-60% less cooling water than liquid absorption systems. This advantage becomes particularly significant in water-stressed regions where traditional carbon capture methods might exacerbate existing resource scarcity issues. However, the production of specialized sorbents with complex surface modifications often involves energy-intensive processes and potentially hazardous chemicals.
The environmental footprint of nanomaterial-based sorbents raises particular concerns regarding potential ecotoxicity. Recent research indicates that nano-scale particles may be released during handling, regeneration, or disposal phases, with uncertain long-term environmental consequences. This highlights the need for comprehensive risk assessment frameworks specifically designed for advanced sorbent materials with engineered interfaces.
Land use impacts vary significantly between sorbent types. Biomass-derived sorbents offer potential carbon sequestration benefits but may compete with food production or natural habitats if scaled extensively. Conversely, synthetic zeolites and metal-organic frameworks require minimal land resources but depend on extractive industries for raw materials.
Waste management challenges persist across the sorbent lifecycle, particularly regarding end-of-life disposal or recycling. Surface-modified materials with complex chemical functionalization may present unique challenges for safe disposal or material recovery. Emerging research suggests that circular economy approaches, including sorbent regeneration and material reclamation, could reduce lifecycle environmental impacts by up to 45% compared to single-use scenarios.
Surface-modified sorbents, particularly those utilizing metal-organic frameworks (MOFs) and functionalized porous carbons, show promising environmental profiles due to their regeneration capabilities and durability. Studies indicate that advanced interface engineering techniques can extend the operational lifespan of these materials by up to 300%, significantly reducing waste generation and resource consumption associated with sorbent replacement cycles.
Water consumption represents a critical environmental consideration, with solid sorbent technologies typically requiring 40-60% less cooling water than liquid absorption systems. This advantage becomes particularly significant in water-stressed regions where traditional carbon capture methods might exacerbate existing resource scarcity issues. However, the production of specialized sorbents with complex surface modifications often involves energy-intensive processes and potentially hazardous chemicals.
The environmental footprint of nanomaterial-based sorbents raises particular concerns regarding potential ecotoxicity. Recent research indicates that nano-scale particles may be released during handling, regeneration, or disposal phases, with uncertain long-term environmental consequences. This highlights the need for comprehensive risk assessment frameworks specifically designed for advanced sorbent materials with engineered interfaces.
Land use impacts vary significantly between sorbent types. Biomass-derived sorbents offer potential carbon sequestration benefits but may compete with food production or natural habitats if scaled extensively. Conversely, synthetic zeolites and metal-organic frameworks require minimal land resources but depend on extractive industries for raw materials.
Waste management challenges persist across the sorbent lifecycle, particularly regarding end-of-life disposal or recycling. Surface-modified materials with complex chemical functionalization may present unique challenges for safe disposal or material recovery. Emerging research suggests that circular economy approaches, including sorbent regeneration and material reclamation, could reduce lifecycle environmental impacts by up to 45% compared to single-use scenarios.
Scalability and Industrial Implementation Challenges
The scaling of solid sorbent technologies for CO2 capture from laboratory to industrial scale presents significant challenges that must be addressed for successful implementation. Current pilot-scale demonstrations have revealed that maintaining consistent interface engineering and surface modification properties during scale-up often results in performance degradation. The transition from gram-scale synthesis to ton-scale production introduces variables in reaction conditions, mixing efficiency, and quality control that can alter the critical surface properties essential for optimal CO2 adsorption.
Material production represents a primary bottleneck, as many advanced sorbents with promising laboratory performance utilize complex synthesis procedures or expensive precursors that become economically prohibitive at industrial scale. For instance, amine-functionalized mesoporous silica materials that show excellent CO2 selectivity often require multi-step synthesis protocols that are difficult to replicate in large-scale production environments without compromising surface modification uniformity.
Engineering challenges extend to reactor design and process integration, where maintaining optimal gas-solid contact efficiency becomes increasingly difficult in large-scale operations. The pressure drop across fixed-bed configurations with fine sorbent particles necessitates careful consideration of particle size distribution and bed geometry. Fluidized bed alternatives offer improved heat and mass transfer but introduce attrition concerns that can degrade carefully engineered surface modifications over multiple adsorption-desorption cycles.
Durability under industrial conditions represents another critical challenge. Laboratory testing rarely accounts for the presence of contaminants like SOx, NOx, and moisture that can permanently deactivate active sites on modified surfaces. Thermal cycling and mechanical stress in industrial settings accelerate degradation of surface modifications, potentially reducing the effective lifetime of sorbents from years to months, significantly impacting economic viability.
Economic considerations further complicate implementation, as the cost-benefit analysis must account for not only the initial capital investment but also operational expenses related to sorbent replacement, regeneration energy requirements, and potential revenue from captured CO2. Current estimates suggest that solid sorbent technologies need to achieve regeneration energy requirements below 2.0 GJ/ton CO2 while maintaining sorbent replacement costs under $10/ton CO2 captured to be economically competitive with conventional amine scrubbing.
Regulatory frameworks and standardization efforts are still evolving for solid sorbent technologies, creating uncertainty for industrial adopters. The lack of established performance metrics specifically designed for surface-modified sorbents makes it difficult to compare technologies and establish industry-wide best practices for implementation and operation.
Material production represents a primary bottleneck, as many advanced sorbents with promising laboratory performance utilize complex synthesis procedures or expensive precursors that become economically prohibitive at industrial scale. For instance, amine-functionalized mesoporous silica materials that show excellent CO2 selectivity often require multi-step synthesis protocols that are difficult to replicate in large-scale production environments without compromising surface modification uniformity.
Engineering challenges extend to reactor design and process integration, where maintaining optimal gas-solid contact efficiency becomes increasingly difficult in large-scale operations. The pressure drop across fixed-bed configurations with fine sorbent particles necessitates careful consideration of particle size distribution and bed geometry. Fluidized bed alternatives offer improved heat and mass transfer but introduce attrition concerns that can degrade carefully engineered surface modifications over multiple adsorption-desorption cycles.
Durability under industrial conditions represents another critical challenge. Laboratory testing rarely accounts for the presence of contaminants like SOx, NOx, and moisture that can permanently deactivate active sites on modified surfaces. Thermal cycling and mechanical stress in industrial settings accelerate degradation of surface modifications, potentially reducing the effective lifetime of sorbents from years to months, significantly impacting economic viability.
Economic considerations further complicate implementation, as the cost-benefit analysis must account for not only the initial capital investment but also operational expenses related to sorbent replacement, regeneration energy requirements, and potential revenue from captured CO2. Current estimates suggest that solid sorbent technologies need to achieve regeneration energy requirements below 2.0 GJ/ton CO2 while maintaining sorbent replacement costs under $10/ton CO2 captured to be economically competitive with conventional amine scrubbing.
Regulatory frameworks and standardization efforts are still evolving for solid sorbent technologies, creating uncertainty for industrial adopters. The lack of established performance metrics specifically designed for surface-modified sorbents makes it difficult to compare technologies and establish industry-wide best practices for implementation and operation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!