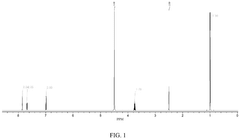
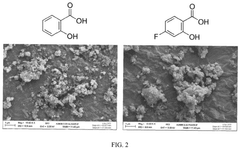
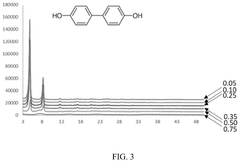
What material parameters are critical for Solid sorbents for CO2 capture performance
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Sorbent Evolution and Objectives
Carbon dioxide capture technology has evolved significantly over the past decades, driven by the urgent need to mitigate climate change impacts. The journey began in the 1970s with basic absorption processes using liquid amines, primarily focused on natural gas purification rather than climate concerns. By the 1990s, as global warming awareness increased, research expanded to include various solid sorbent materials as alternatives to traditional liquid-based systems.
The evolution of solid sorbents for CO2 capture represents a critical technological advancement path. Early materials included basic activated carbons and zeolites, which offered limited capacity and selectivity. The 2000s witnessed the emergence of metal-organic frameworks (MOFs), which revolutionized the field by providing unprecedented surface areas and tunable pore structures. Concurrently, amine-functionalized silica materials gained prominence for their enhanced CO2 affinity.
The 2010s marked a significant shift toward rational material design, with computational screening accelerating discovery processes. This period saw the development of covalent organic frameworks (COFs), porous polymer networks (PPNs), and advanced carbon-based materials with tailored surface chemistry. Each generation of materials addressed specific limitations of its predecessors, gradually improving capacity, selectivity, and regeneration efficiency.
Recent developments have focused on hierarchical porous structures, composite materials combining multiple capture mechanisms, and materials specifically designed for direct air capture applications. The integration of nanotechnology has enabled precise control over material architecture at multiple length scales, optimizing mass transfer and sorption kinetics.
The primary objectives in solid sorbent development center on several key performance parameters. First, achieving high CO2 capacity under relevant operating conditions (temperature, pressure, and gas composition) remains fundamental. Second, enhancing selectivity for CO2 over other flue gas components, particularly nitrogen, water vapor, and sulfur compounds, is crucial for practical applications. Third, materials must demonstrate mechanical and chemical stability over thousands of adsorption-desorption cycles.
Additional objectives include reducing regeneration energy requirements, which directly impacts operational costs, and developing materials compatible with existing industrial infrastructure. For emerging direct air capture applications, sorbents must function effectively at ultra-low CO2 concentrations (approximately 400 ppm) while maintaining reasonable energy efficiency. Finally, environmental sustainability and cost-effectiveness have become increasingly important considerations, driving research toward earth-abundant materials and scalable synthesis methods.
The evolution of solid sorbents for CO2 capture represents a critical technological advancement path. Early materials included basic activated carbons and zeolites, which offered limited capacity and selectivity. The 2000s witnessed the emergence of metal-organic frameworks (MOFs), which revolutionized the field by providing unprecedented surface areas and tunable pore structures. Concurrently, amine-functionalized silica materials gained prominence for their enhanced CO2 affinity.
The 2010s marked a significant shift toward rational material design, with computational screening accelerating discovery processes. This period saw the development of covalent organic frameworks (COFs), porous polymer networks (PPNs), and advanced carbon-based materials with tailored surface chemistry. Each generation of materials addressed specific limitations of its predecessors, gradually improving capacity, selectivity, and regeneration efficiency.
Recent developments have focused on hierarchical porous structures, composite materials combining multiple capture mechanisms, and materials specifically designed for direct air capture applications. The integration of nanotechnology has enabled precise control over material architecture at multiple length scales, optimizing mass transfer and sorption kinetics.
The primary objectives in solid sorbent development center on several key performance parameters. First, achieving high CO2 capacity under relevant operating conditions (temperature, pressure, and gas composition) remains fundamental. Second, enhancing selectivity for CO2 over other flue gas components, particularly nitrogen, water vapor, and sulfur compounds, is crucial for practical applications. Third, materials must demonstrate mechanical and chemical stability over thousands of adsorption-desorption cycles.
Additional objectives include reducing regeneration energy requirements, which directly impacts operational costs, and developing materials compatible with existing industrial infrastructure. For emerging direct air capture applications, sorbents must function effectively at ultra-low CO2 concentrations (approximately 400 ppm) while maintaining reasonable energy efficiency. Finally, environmental sustainability and cost-effectiveness have become increasingly important considerations, driving research toward earth-abundant materials and scalable synthesis methods.
Market Analysis for Carbon Capture Technologies
The global carbon capture and storage (CCS) market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $7.3 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $35.9 billion by the end of the decade.
Solid sorbent technologies for CO2 capture represent a rapidly expanding segment within this market. Unlike traditional liquid amine scrubbing methods, solid sorbents offer potential advantages in energy efficiency and operational costs, driving their increasing market adoption. Current market penetration remains limited at roughly 8% of total carbon capture implementations, but this share is expected to grow substantially as material science advances continue to improve performance parameters.
Regional analysis reveals that North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 23.5% annually, primarily driven by China's aggressive decarbonization targets and industrial expansion.
Key market segments for solid sorbent applications include power generation (34% of potential applications), cement production (22%), steel manufacturing (18%), and chemical processing (15%). The power generation sector presents the largest immediate opportunity due to concentrated emission sources and established regulatory frameworks.
Customer demand analysis indicates shifting priorities among industrial adopters. While cost efficiency remains paramount (cited by 78% of potential customers), performance metrics specifically related to material parameters are increasingly important. Surveys of industrial end-users reveal that adsorption capacity, selectivity for CO2, and regeneration energy requirements are the three most valued performance characteristics, mentioned by 82%, 76%, and 71% of respondents respectively.
Market barriers include high initial capital costs, with solid sorbent systems currently commanding a 15-30% premium over conventional technologies. However, this gap is narrowing as material science advances improve sorbent durability and performance. Regulatory uncertainty also impacts market growth, with 65% of industry stakeholders citing unclear carbon pricing mechanisms as a significant barrier to technology adoption.
The competitive landscape features both established industrial gas companies expanding into solid sorbents and specialized startups focused exclusively on advanced materials development. Recent market consolidation has seen five major acquisitions of materials technology startups by larger industrial players, indicating growing recognition of the strategic importance of optimized sorbent materials.
Solid sorbent technologies for CO2 capture represent a rapidly expanding segment within this market. Unlike traditional liquid amine scrubbing methods, solid sorbents offer potential advantages in energy efficiency and operational costs, driving their increasing market adoption. Current market penetration remains limited at roughly 8% of total carbon capture implementations, but this share is expected to grow substantially as material science advances continue to improve performance parameters.
Regional analysis reveals that North America currently leads the carbon capture market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 23.5% annually, primarily driven by China's aggressive decarbonization targets and industrial expansion.
Key market segments for solid sorbent applications include power generation (34% of potential applications), cement production (22%), steel manufacturing (18%), and chemical processing (15%). The power generation sector presents the largest immediate opportunity due to concentrated emission sources and established regulatory frameworks.
Customer demand analysis indicates shifting priorities among industrial adopters. While cost efficiency remains paramount (cited by 78% of potential customers), performance metrics specifically related to material parameters are increasingly important. Surveys of industrial end-users reveal that adsorption capacity, selectivity for CO2, and regeneration energy requirements are the three most valued performance characteristics, mentioned by 82%, 76%, and 71% of respondents respectively.
Market barriers include high initial capital costs, with solid sorbent systems currently commanding a 15-30% premium over conventional technologies. However, this gap is narrowing as material science advances improve sorbent durability and performance. Regulatory uncertainty also impacts market growth, with 65% of industry stakeholders citing unclear carbon pricing mechanisms as a significant barrier to technology adoption.
The competitive landscape features both established industrial gas companies expanding into solid sorbents and specialized startups focused exclusively on advanced materials development. Recent market consolidation has seen five major acquisitions of materials technology startups by larger industrial players, indicating growing recognition of the strategic importance of optimized sorbent materials.
Current Solid Sorbents and Technical Barriers
Currently, several types of solid sorbents are being investigated for CO2 capture, each with distinct advantages and limitations. Amine-functionalized materials, including supported amine sorbents and amine-grafted porous materials, demonstrate high CO2 selectivity and capacity at low partial pressures, making them suitable for post-combustion capture. However, they face challenges including thermal degradation during regeneration cycles, amine leaching, and oxidative degradation in the presence of oxygen and moisture.
Metal-Organic Frameworks (MOFs) represent another promising category with exceptional surface areas and tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which exhibit high CO2 uptake capacities. Despite these advantages, MOFs often suffer from hydrolytic instability in humid conditions and relatively high production costs, limiting their industrial-scale implementation.
Zeolites, particularly those with high aluminum content like 13X and 5A, show good CO2 adsorption performance at moderate temperatures. Their main drawbacks include significant capacity reduction in humid conditions and relatively high regeneration energy requirements due to strong CO2 binding.
Activated carbons offer benefits including low cost, hydrophobicity, and good stability, but generally display lower CO2 selectivity compared to other sorbents, particularly at low CO2 partial pressures relevant to flue gas conditions.
Hydrotalcites and layered double hydroxides perform well at elevated temperatures (200-400°C), making them suitable for pre-combustion capture, but exhibit limited capacity at ambient conditions and can require complex synthesis procedures.
Several technical barriers currently impede the widespread deployment of solid sorbents. Stability issues during multiple adsorption-desorption cycles remain a significant challenge, with many materials showing performance degradation after extended operation. The energy penalty associated with sorbent regeneration represents another major barrier, as high regeneration temperatures increase operational costs and reduce overall process efficiency.
Scalability and manufacturing challenges also persist, with many high-performing materials currently limited to laboratory-scale synthesis. The translation of these materials to industrial production volumes while maintaining performance characteristics presents significant engineering challenges.
Additionally, the mechanical properties of many sorbents are suboptimal for practical applications. Issues such as attrition, crushing, and dusting during handling and cycling can lead to material losses and reduced performance in fixed or fluidized bed configurations.
Water co-adsorption remains problematic for many materials, with moisture in flue gas streams often competing with CO2 for adsorption sites, significantly reducing effective capacity in real-world conditions.
Metal-Organic Frameworks (MOFs) represent another promising category with exceptional surface areas and tunable pore structures. Notable examples include Mg-MOF-74 and HKUST-1, which exhibit high CO2 uptake capacities. Despite these advantages, MOFs often suffer from hydrolytic instability in humid conditions and relatively high production costs, limiting their industrial-scale implementation.
Zeolites, particularly those with high aluminum content like 13X and 5A, show good CO2 adsorption performance at moderate temperatures. Their main drawbacks include significant capacity reduction in humid conditions and relatively high regeneration energy requirements due to strong CO2 binding.
Activated carbons offer benefits including low cost, hydrophobicity, and good stability, but generally display lower CO2 selectivity compared to other sorbents, particularly at low CO2 partial pressures relevant to flue gas conditions.
Hydrotalcites and layered double hydroxides perform well at elevated temperatures (200-400°C), making them suitable for pre-combustion capture, but exhibit limited capacity at ambient conditions and can require complex synthesis procedures.
Several technical barriers currently impede the widespread deployment of solid sorbents. Stability issues during multiple adsorption-desorption cycles remain a significant challenge, with many materials showing performance degradation after extended operation. The energy penalty associated with sorbent regeneration represents another major barrier, as high regeneration temperatures increase operational costs and reduce overall process efficiency.
Scalability and manufacturing challenges also persist, with many high-performing materials currently limited to laboratory-scale synthesis. The translation of these materials to industrial production volumes while maintaining performance characteristics presents significant engineering challenges.
Additionally, the mechanical properties of many sorbents are suboptimal for practical applications. Issues such as attrition, crushing, and dusting during handling and cycling can lead to material losses and reduced performance in fixed or fluidized bed configurations.
Water co-adsorption remains problematic for many materials, with moisture in flue gas streams often competing with CO2 for adsorption sites, significantly reducing effective capacity in real-world conditions.
State-of-the-Art Solid Sorbent Solutions
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks (MOFs) are crystalline porous materials that have shown exceptional performance as solid sorbents for CO2 capture. Their high surface area, tunable pore size, and chemical functionality allow for selective CO2 adsorption. These materials can be designed with specific metal centers and organic linkers to enhance CO2 binding affinity and selectivity. MOFs can operate under various conditions and can be regenerated with minimal energy input, making them promising candidates for industrial-scale carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks represent a promising class of solid sorbents for CO2 capture due to their high surface area, tunable pore size, and customizable chemical functionality. These crystalline porous materials consist of metal ions or clusters coordinated to organic ligands, creating structures with exceptional CO2 adsorption capacity. MOFs can be designed with specific binding sites for CO2 molecules, enhancing selectivity and capture efficiency even at low CO2 concentrations. Their regeneration typically requires less energy compared to traditional liquid amine systems.
- Amine-functionalized solid sorbents: Amine-functionalized solid sorbents combine the advantages of traditional liquid amine scrubbing with the benefits of solid supports. These materials feature amine groups chemically attached to various substrates such as silica, polymers, or carbon-based materials. The amine groups selectively bind CO2 through carbamate formation mechanisms similar to those in liquid amine systems. These sorbents demonstrate high CO2 selectivity even in the presence of moisture and can be regenerated at lower temperatures than conventional systems, reducing energy requirements for the capture process.
- Carbon-based adsorbents for CO2 capture: Carbon-based materials including activated carbons, carbon nanotubes, and graphene derivatives serve as effective CO2 adsorbents. These materials offer advantages such as high surface area, hydrophobicity, and thermal stability. Their porous structure can be optimized through activation processes to enhance CO2 adsorption capacity. Carbon-based sorbents can be further modified with nitrogen-containing functional groups or metal particles to improve CO2 selectivity. Their relatively low cost of production and high stability under various operating conditions make them attractive for large-scale carbon capture applications.
- Zeolites and molecular sieves for selective CO2 adsorption: Zeolites and molecular sieves are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving of CO2 from gas mixtures. These materials can selectively adsorb CO2 based on differences in molecular size, shape, and polarity. The cation composition of zeolites can be modified to enhance CO2 adsorption properties. Their high thermal stability allows for multiple adsorption-desorption cycles without significant degradation in performance. Zeolites demonstrate particularly good performance in pressure swing adsorption systems for CO2 capture from various gas streams.
- Composite and hybrid sorbent materials: Composite and hybrid sorbent materials combine different types of adsorbents to achieve enhanced CO2 capture performance. These materials integrate the advantageous properties of multiple components, such as the high surface area of one material with the selective binding sites of another. Examples include MOF-polymer composites, amine-grafted mesoporous silica, and zeolite-carbon hybrids. The synergistic effects between components often result in improved adsorption capacity, selectivity, and stability compared to single-component sorbents. These materials can be tailored for specific operating conditions and can address limitations of individual sorbent types.
02 Amine-functionalized sorbents for enhanced CO2 adsorption
Amine-functionalized solid sorbents leverage the chemical affinity between amine groups and CO2 molecules to achieve high capture efficiency. These materials typically consist of a porous support structure (such as silica, alumina, or polymers) impregnated or grafted with various amine compounds. The amine groups form carbamates or carbonates upon reaction with CO2, enabling selective capture even at low CO2 concentrations. These sorbents demonstrate good stability over multiple adsorption-desorption cycles and can operate effectively under humid conditions, which is advantageous for real-world applications.Expand Specific Solutions03 Zeolite-based materials for selective CO2 adsorption
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that enable molecular sieving for selective CO2 capture. Their performance can be enhanced through ion exchange, framework modification, and surface functionalization to increase CO2 adsorption capacity and selectivity. Zeolite-based sorbents typically exhibit good thermal stability, allowing for efficient regeneration at elevated temperatures. These materials can be tailored to operate effectively in the presence of moisture and other gas components, making them suitable for various carbon capture applications including post-combustion flue gas treatment.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbons, carbon nanotubes, and graphene derivatives, serve as effective solid sorbents for CO2 capture due to their high surface area and porous structure. These materials can be modified through chemical activation, nitrogen doping, or incorporation of metal particles to enhance their CO2 adsorption capacity and selectivity. Carbon-based sorbents typically demonstrate good mechanical stability, low cost, and ease of regeneration. Their hydrophobic nature can be advantageous in humid conditions, and they can be produced from sustainable precursors including biomass and waste materials.Expand Specific Solutions05 Composite and hybrid sorbents for improved CO2 capture performance
Composite and hybrid sorbents combine different materials to leverage their complementary properties for enhanced CO2 capture performance. These may include polymer-inorganic composites, MOF-polymer hybrids, or multi-component systems that integrate various functional materials. Such combinations can address limitations of individual materials, resulting in improved adsorption capacity, selectivity, stability, and regeneration efficiency. These advanced sorbents often feature hierarchical pore structures, multiple adsorption mechanisms, and synergistic effects that optimize CO2 capture under various operating conditions, including temperature swings, pressure variations, and presence of contaminants.Expand Specific Solutions
Leading Organizations in CO2 Capture Materials
The solid sorbent CO2 capture market is in a growth phase, with increasing demand driven by global decarbonization efforts. Key material parameters critical for performance include high CO2 adsorption capacity, selectivity, stability through multiple cycles, fast kinetics, and low regeneration energy requirements. Leading research institutions (Shanghai Advanced Research Institute, Dalian Institute, Norwegian University of Science & Technology) and commercial players (Climeworks, ExxonMobil, Susteon) are advancing the technology across different maturity levels. While established companies like Shell and ExxonMobil focus on industrial-scale applications, specialized firms like Climeworks have deployed commercial direct air capture plants. The technology is progressing from lab-scale to demonstration projects, with significant R&D investment improving cost-effectiveness and efficiency.
Climeworks AG
Technical Solution: Climeworks has developed proprietary Direct Air Capture (DAC) technology utilizing specialized solid sorbents with optimized porosity and surface chemistry. Their approach focuses on amine-functionalized filter materials that selectively bind CO2 from ambient air. The technology employs a modular collector design where air is drawn through the sorbent by fans, with captured CO2 released through temperature-swing adsorption (TSA) at approximately 100°C. Their materials are engineered for high CO2 selectivity, rapid adsorption-desorption cycling, and long-term stability under repeated thermal cycling conditions. Climeworks' sorbents demonstrate particular attention to water tolerance, as their systems operate in ambient conditions where moisture competition effects must be managed effectively. The company has identified surface area, pore structure, and functional group density as critical parameters for optimizing capture efficiency while minimizing energy requirements.
Strengths: Highly selective CO2 capture from ultra-dilute atmospheric concentrations; sorbents designed for thousands of adsorption-desorption cycles; low-temperature regeneration requirements. Weaknesses: Higher energy consumption per ton of CO2 compared to point-source capture; material degradation from contaminants in ambient air; relatively high capital costs for sorbent production and replacement.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed advanced Metal-Organic Framework (MOF) based solid sorbents for CO2 capture with precisely engineered pore structures and functionalized binding sites. Their proprietary materials focus on optimizing the trade-off between CO2 selectivity and working capacity through careful tuning of metal centers and organic linkers. ExxonMobil's approach emphasizes materials with high CO2 adsorption enthalpy (30-40 kJ/mol) to achieve strong but reversible binding, enabling efficient capture from flue gas streams with CO2 concentrations of 5-15%. Their research has identified framework stability under moisture and contaminant exposure as critical parameters, developing hydrophobic MOFs that maintain performance in real-world industrial conditions. The company has also pioneered composite sorbent structures that integrate high-capacity MOFs with thermally conductive supports to enhance heat transfer during temperature swing regeneration, significantly reducing energy penalties. Their materials are designed for pressure-temperature swing adsorption processes that can be integrated with existing industrial infrastructure.
Strengths: Exceptional CO2/N2 selectivity ratios exceeding 100:1; materials engineered for rapid adsorption kinetics; sorbents designed for integration with existing industrial processes. Weaknesses: Some materials require complex synthesis procedures increasing production costs; potential for performance degradation in the presence of SOx and NOx contaminants; higher regeneration temperatures compared to some competing technologies.
Critical Material Parameters Analysis
Solid sorbent materials functionalized with polyamines having oxygen-containing units selected from carbonyl units, hydroxyl units, and combinations thereof
PatentPendingEP4616940A1
Innovation
- Development of sorbent materials functionalized with polyamines containing oxygen-containing units such as carbonyl and hydroxyl groups, which exhibit high CO2 adsorption capacity and low water adsorption, allowing for efficient CO2 capture with minimal desorption residuals under mild conditions.
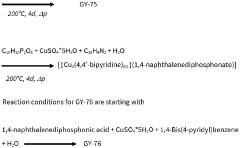
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Environmental Impact Assessment
The environmental implications of solid sorbent materials for CO2 capture extend far beyond their primary function. When evaluating these materials, lifecycle assessment reveals significant environmental considerations across production, operation, and disposal phases. The manufacturing of advanced sorbents often requires energy-intensive processes and specialized chemicals, potentially generating substantial carbon footprints that must be accounted for in overall capture efficiency calculations.
Material toxicity represents another critical environmental parameter. Many promising sorbents contain heavy metals, amines, or other compounds that could pose ecological risks if released. For instance, amine-functionalized materials may degrade over time, potentially leaching harmful compounds into surrounding ecosystems. Comprehensive toxicological assessments are therefore essential when developing new sorbent technologies.
Water consumption and contamination risks must also be evaluated, particularly for hydrophilic materials that may compete with other essential water uses in water-stressed regions. Some sorbents require significant water for regeneration cycles or can alter water chemistry through ion exchange or leaching processes, potentially affecting aquatic ecosystems if wastewater is improperly managed.
The regeneration energy requirements of sorbents directly impact their environmental viability. Materials requiring high-temperature regeneration contribute to indirect emissions unless powered by renewable energy sources. This energy penalty must be minimized to ensure the net environmental benefit of the capture process exceeds its environmental costs.
Durability and degradation characteristics significantly influence environmental impact through replacement frequency and waste generation. Sorbents with longer operational lifespans reduce material consumption and waste disposal challenges. The development of recyclable or biodegradable sorbent materials represents an important frontier in minimizing end-of-life environmental impacts.
Land use considerations become increasingly relevant as carbon capture technologies scale up. Large-scale deployment of solid sorbent systems requires substantial space, potentially competing with other land uses. The physical footprint of these systems must be optimized, particularly in densely populated or ecologically sensitive areas.
Finally, the potential for secondary environmental benefits should be explored. Some sorbent materials can simultaneously capture multiple pollutants or be designed for beneficial reuse applications, creating cascading environmental advantages beyond CO2 reduction. These co-benefits may significantly enhance the overall environmental value proposition of specific sorbent technologies.
Material toxicity represents another critical environmental parameter. Many promising sorbents contain heavy metals, amines, or other compounds that could pose ecological risks if released. For instance, amine-functionalized materials may degrade over time, potentially leaching harmful compounds into surrounding ecosystems. Comprehensive toxicological assessments are therefore essential when developing new sorbent technologies.
Water consumption and contamination risks must also be evaluated, particularly for hydrophilic materials that may compete with other essential water uses in water-stressed regions. Some sorbents require significant water for regeneration cycles or can alter water chemistry through ion exchange or leaching processes, potentially affecting aquatic ecosystems if wastewater is improperly managed.
The regeneration energy requirements of sorbents directly impact their environmental viability. Materials requiring high-temperature regeneration contribute to indirect emissions unless powered by renewable energy sources. This energy penalty must be minimized to ensure the net environmental benefit of the capture process exceeds its environmental costs.
Durability and degradation characteristics significantly influence environmental impact through replacement frequency and waste generation. Sorbents with longer operational lifespans reduce material consumption and waste disposal challenges. The development of recyclable or biodegradable sorbent materials represents an important frontier in minimizing end-of-life environmental impacts.
Land use considerations become increasingly relevant as carbon capture technologies scale up. Large-scale deployment of solid sorbent systems requires substantial space, potentially competing with other land uses. The physical footprint of these systems must be optimized, particularly in densely populated or ecologically sensitive areas.
Finally, the potential for secondary environmental benefits should be explored. Some sorbent materials can simultaneously capture multiple pollutants or be designed for beneficial reuse applications, creating cascading environmental advantages beyond CO2 reduction. These co-benefits may significantly enhance the overall environmental value proposition of specific sorbent technologies.
Scalability and Economic Feasibility
The scalability and economic feasibility of solid sorbents for CO2 capture represent critical considerations for their industrial implementation. When evaluating material parameters, their impact on large-scale deployment costs must be thoroughly assessed. Materials with high CO2 adsorption capacity significantly reduce the required equipment size and capital expenditure, creating a direct correlation between capacity and economic viability.
Production costs of solid sorbents vary dramatically based on precursor materials and synthesis complexity. Commercially available materials like activated carbons and zeolites offer cost advantages ($1-5/kg) compared to specialized materials such as metal-organic frameworks (MOFs) or amine-functionalized silicas ($20-100/kg). The economic equation must balance higher-performing but expensive materials against more affordable options with moderate performance.
Mechanical stability emerges as a crucial parameter affecting long-term economics. Materials prone to attrition or degradation require frequent replacement, substantially increasing operational costs. Sorbents maintaining structural integrity through thousands of adsorption-desorption cycles demonstrate superior lifetime value despite potentially higher initial costs.
Energy requirements for regeneration directly impact operational expenses, with lower regeneration temperatures (below 100°C) enabling the use of waste heat streams and significantly reducing energy costs. Materials requiring temperatures above 200°C necessitate dedicated heating systems, dramatically increasing both capital and operational expenditures.
Manufacturing scalability presents another critical dimension, as laboratory-synthesized materials often face challenges in industrial-scale production. Parameters such as synthesis time, solvent requirements, and process complexity can create bottlenecks when scaling from grams to tons. Materials amenable to continuous manufacturing processes demonstrate superior economic feasibility compared to those requiring batch processing.
Environmental considerations increasingly influence economic assessments through regulatory compliance costs and carbon pricing mechanisms. Sorbents with lower environmental footprints in production and disposal phases gain competitive advantages in regions with stringent environmental regulations.
The integration potential with existing infrastructure represents a final critical parameter, as materials compatible with conventional equipment reduce retrofit costs. Sorbents requiring specialized handling equipment or extreme operating conditions face significant implementation barriers regardless of their theoretical performance metrics.
Production costs of solid sorbents vary dramatically based on precursor materials and synthesis complexity. Commercially available materials like activated carbons and zeolites offer cost advantages ($1-5/kg) compared to specialized materials such as metal-organic frameworks (MOFs) or amine-functionalized silicas ($20-100/kg). The economic equation must balance higher-performing but expensive materials against more affordable options with moderate performance.
Mechanical stability emerges as a crucial parameter affecting long-term economics. Materials prone to attrition or degradation require frequent replacement, substantially increasing operational costs. Sorbents maintaining structural integrity through thousands of adsorption-desorption cycles demonstrate superior lifetime value despite potentially higher initial costs.
Energy requirements for regeneration directly impact operational expenses, with lower regeneration temperatures (below 100°C) enabling the use of waste heat streams and significantly reducing energy costs. Materials requiring temperatures above 200°C necessitate dedicated heating systems, dramatically increasing both capital and operational expenditures.
Manufacturing scalability presents another critical dimension, as laboratory-synthesized materials often face challenges in industrial-scale production. Parameters such as synthesis time, solvent requirements, and process complexity can create bottlenecks when scaling from grams to tons. Materials amenable to continuous manufacturing processes demonstrate superior economic feasibility compared to those requiring batch processing.
Environmental considerations increasingly influence economic assessments through regulatory compliance costs and carbon pricing mechanisms. Sorbents with lower environmental footprints in production and disposal phases gain competitive advantages in regions with stringent environmental regulations.
The integration potential with existing infrastructure represents a final critical parameter, as materials compatible with conventional equipment reduce retrofit costs. Sorbents requiring specialized handling equipment or extreme operating conditions face significant implementation barriers regardless of their theoretical performance metrics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!