Solid sorbents for CO2 capture in industrial and power plant applications
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Capture Technology Background and Objectives
Carbon dioxide (CO2) capture has emerged as a critical technology in the global effort to mitigate climate change. The historical development of CO2 capture technologies dates back to the 1930s when absorption processes using amines were first developed for natural gas sweetening. However, the application of these technologies specifically for climate change mitigation gained momentum only in the late 20th century as scientific consensus on anthropogenic global warming strengthened.
Industrial and power generation sectors collectively account for approximately 45% of global CO2 emissions, making them prime targets for carbon capture implementation. Traditional CO2 capture methods have predominantly relied on liquid solvents, particularly amine-based solutions, which despite their effectiveness, suffer from high energy penalties during regeneration, solvent degradation issues, and equipment corrosion challenges.
Solid sorbents represent a promising alternative pathway that has gained significant research attention over the past two decades. These materials offer several potential advantages including lower regeneration energy requirements, reduced corrosion issues, and greater stability under various operating conditions. The evolution of solid sorbent technology has progressed from basic materials like activated carbon and zeolites to more sophisticated engineered sorbents including metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcite-based materials.
The primary technical objective in solid sorbent development is to create materials with high CO2 selectivity, substantial adsorption capacity, rapid adsorption/desorption kinetics, and excellent cyclic stability under realistic industrial conditions. Additionally, these materials must demonstrate tolerance to common flue gas contaminants such as SOx, NOx, and water vapor, which have historically poisoned many promising sorbent candidates.
Current research aims to bridge the gap between laboratory-scale demonstrations and industrial implementation by addressing challenges related to sorbent manufacturing at scale, mechanical stability during repeated cycling, and integration into existing industrial infrastructure. The ultimate goal is to reduce the energy penalty of carbon capture to below 20% while maintaining capture rates above 90% and keeping costs under $40 per ton of CO2 captured.
The technology roadmap for solid sorbents envisions progressive improvements in material performance, process design, and system integration over the next decade. This includes the development of advanced reactor configurations such as moving bed systems, fluidized beds, and innovative temperature or pressure swing processes that can maximize the inherent advantages of solid sorbents while minimizing operational complexities.
Industrial and power generation sectors collectively account for approximately 45% of global CO2 emissions, making them prime targets for carbon capture implementation. Traditional CO2 capture methods have predominantly relied on liquid solvents, particularly amine-based solutions, which despite their effectiveness, suffer from high energy penalties during regeneration, solvent degradation issues, and equipment corrosion challenges.
Solid sorbents represent a promising alternative pathway that has gained significant research attention over the past two decades. These materials offer several potential advantages including lower regeneration energy requirements, reduced corrosion issues, and greater stability under various operating conditions. The evolution of solid sorbent technology has progressed from basic materials like activated carbon and zeolites to more sophisticated engineered sorbents including metal-organic frameworks (MOFs), amine-functionalized silicas, and hydrotalcite-based materials.
The primary technical objective in solid sorbent development is to create materials with high CO2 selectivity, substantial adsorption capacity, rapid adsorption/desorption kinetics, and excellent cyclic stability under realistic industrial conditions. Additionally, these materials must demonstrate tolerance to common flue gas contaminants such as SOx, NOx, and water vapor, which have historically poisoned many promising sorbent candidates.
Current research aims to bridge the gap between laboratory-scale demonstrations and industrial implementation by addressing challenges related to sorbent manufacturing at scale, mechanical stability during repeated cycling, and integration into existing industrial infrastructure. The ultimate goal is to reduce the energy penalty of carbon capture to below 20% while maintaining capture rates above 90% and keeping costs under $40 per ton of CO2 captured.
The technology roadmap for solid sorbents envisions progressive improvements in material performance, process design, and system integration over the next decade. This includes the development of advanced reactor configurations such as moving bed systems, fluidized beds, and innovative temperature or pressure swing processes that can maximize the inherent advantages of solid sorbents while minimizing operational complexities.
Market Analysis for Industrial Carbon Capture Solutions
The global carbon capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the industrial carbon capture market is valued at approximately $2.1 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $7.0 billion by that time. This growth trajectory is particularly pronounced in regions with stringent carbon pricing mechanisms, such as the European Union, where carbon prices have exceeded €80 per tonne.
Power generation and industrial manufacturing sectors represent the largest market segments for solid sorbent-based carbon capture technologies. Cement production, steel manufacturing, and fossil fuel power plants collectively account for over 70% of the current market demand. The cement industry alone contributes roughly 8% of global CO2 emissions, creating substantial market potential for retrofit carbon capture solutions.
Market penetration of solid sorbent technologies remains relatively low compared to conventional amine-based liquid absorption systems, currently holding approximately 15% market share. However, this segment is expected to grow at a faster rate (23% CAGR) than the overall carbon capture market due to performance advantages in specific applications and lower energy penalties.
Regional analysis reveals that North America currently leads the industrial carbon capture market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). China and India represent the fastest-growing markets, with anticipated growth rates exceeding 25% annually as these nations balance industrial expansion with climate commitments.
Customer segmentation shows three distinct market tiers: large industrial emitters seeking compliance with regulations (55% of market), forward-thinking corporations pursuing carbon neutrality goals (30%), and government-subsidized demonstration projects (15%). The willingness to pay varies significantly across these segments, with price sensitivity decreasing as regulatory pressures intensify.
The economic viability of solid sorbent technologies is approaching an inflection point. Current capture costs range from $40-80 per tonne of CO2, with next-generation sorbents potentially reducing this to $30-50 per tonne. Market adoption accelerates significantly when capture costs fall below regional carbon prices or tax penalties, creating geographically distinct adoption patterns.
Competitive analysis indicates that the market remains fragmented, with over 50 companies offering various carbon capture solutions. However, consolidation is expected as technologies mature, with strategic partnerships between technology providers and industrial end-users becoming increasingly common.
Power generation and industrial manufacturing sectors represent the largest market segments for solid sorbent-based carbon capture technologies. Cement production, steel manufacturing, and fossil fuel power plants collectively account for over 70% of the current market demand. The cement industry alone contributes roughly 8% of global CO2 emissions, creating substantial market potential for retrofit carbon capture solutions.
Market penetration of solid sorbent technologies remains relatively low compared to conventional amine-based liquid absorption systems, currently holding approximately 15% market share. However, this segment is expected to grow at a faster rate (23% CAGR) than the overall carbon capture market due to performance advantages in specific applications and lower energy penalties.
Regional analysis reveals that North America currently leads the industrial carbon capture market with 42% share, followed by Europe (31%) and Asia-Pacific (21%). China and India represent the fastest-growing markets, with anticipated growth rates exceeding 25% annually as these nations balance industrial expansion with climate commitments.
Customer segmentation shows three distinct market tiers: large industrial emitters seeking compliance with regulations (55% of market), forward-thinking corporations pursuing carbon neutrality goals (30%), and government-subsidized demonstration projects (15%). The willingness to pay varies significantly across these segments, with price sensitivity decreasing as regulatory pressures intensify.
The economic viability of solid sorbent technologies is approaching an inflection point. Current capture costs range from $40-80 per tonne of CO2, with next-generation sorbents potentially reducing this to $30-50 per tonne. Market adoption accelerates significantly when capture costs fall below regional carbon prices or tax penalties, creating geographically distinct adoption patterns.
Competitive analysis indicates that the market remains fragmented, with over 50 companies offering various carbon capture solutions. However, consolidation is expected as technologies mature, with strategic partnerships between technology providers and industrial end-users becoming increasingly common.
Current State and Challenges of Solid Sorbent Technologies
Solid sorbent technologies for CO2 capture have advanced significantly in recent years, with various materials being developed and tested at different scales. Currently, the most widely studied solid sorbents include amine-functionalized materials, metal-organic frameworks (MOFs), zeolites, activated carbons, and alkali metal-based sorbents. Each category offers distinct advantages in terms of CO2 selectivity, adsorption capacity, and regeneration energy requirements.
Amine-functionalized materials, particularly those supported on mesoporous silica, have demonstrated high CO2 selectivity and reasonable working capacities under industrial conditions. Commercial implementations include Inventys' VeloxoTherm™ process and Carbon Clean Solutions' technology, which have been tested at pilot scales in power plants and industrial facilities. These technologies have shown promising results with capture costs potentially below $40/ton CO2.
Metal-organic frameworks represent another promising category, with materials such as Mg-MOF-74 and HKUST-1 showing exceptional CO2 uptake capacities exceeding 5 mmol/g under certain conditions. However, their implementation at industrial scale remains limited due to stability issues in the presence of moisture and other flue gas contaminants.
Despite these advancements, solid sorbent technologies face several critical challenges. Stability under real operating conditions remains a significant hurdle, as many promising materials degrade when exposed to SOx, NOx, and moisture present in industrial flue gases. For instance, studies have shown that some MOFs can lose up to 50% of their adsorption capacity after just 10-20 cycles in the presence of these contaminants.
Scalability presents another major challenge. While laboratory results are promising, manufacturing high-performance sorbents at the multi-ton scale required for industrial implementation remains difficult and costly. Current production methods for advanced materials like MOFs can cost $100-1000/kg, far above the $5-10/kg threshold considered economically viable for large-scale deployment.
Heat management during adsorption and desorption cycles also poses significant engineering challenges. The exothermic nature of CO2 adsorption can lead to temperature increases that reduce working capacity, while providing sufficient heat for desorption requires careful system design to maintain energy efficiency.
Mechanical stability represents another critical concern, as solid sorbents must withstand thousands of pressure or temperature swing cycles without significant attrition. Studies have shown that some promising materials can lose up to 2-5% of their mass per 100 cycles due to mechanical degradation, making long-term operation problematic.
Addressing these challenges requires interdisciplinary approaches combining materials science, chemical engineering, and process design. Recent research directions include developing composite materials with enhanced stability, exploring novel regeneration methods to reduce energy penalties, and designing innovative reactor configurations to optimize heat and mass transfer.
Amine-functionalized materials, particularly those supported on mesoporous silica, have demonstrated high CO2 selectivity and reasonable working capacities under industrial conditions. Commercial implementations include Inventys' VeloxoTherm™ process and Carbon Clean Solutions' technology, which have been tested at pilot scales in power plants and industrial facilities. These technologies have shown promising results with capture costs potentially below $40/ton CO2.
Metal-organic frameworks represent another promising category, with materials such as Mg-MOF-74 and HKUST-1 showing exceptional CO2 uptake capacities exceeding 5 mmol/g under certain conditions. However, their implementation at industrial scale remains limited due to stability issues in the presence of moisture and other flue gas contaminants.
Despite these advancements, solid sorbent technologies face several critical challenges. Stability under real operating conditions remains a significant hurdle, as many promising materials degrade when exposed to SOx, NOx, and moisture present in industrial flue gases. For instance, studies have shown that some MOFs can lose up to 50% of their adsorption capacity after just 10-20 cycles in the presence of these contaminants.
Scalability presents another major challenge. While laboratory results are promising, manufacturing high-performance sorbents at the multi-ton scale required for industrial implementation remains difficult and costly. Current production methods for advanced materials like MOFs can cost $100-1000/kg, far above the $5-10/kg threshold considered economically viable for large-scale deployment.
Heat management during adsorption and desorption cycles also poses significant engineering challenges. The exothermic nature of CO2 adsorption can lead to temperature increases that reduce working capacity, while providing sufficient heat for desorption requires careful system design to maintain energy efficiency.
Mechanical stability represents another critical concern, as solid sorbents must withstand thousands of pressure or temperature swing cycles without significant attrition. Studies have shown that some promising materials can lose up to 2-5% of their mass per 100 cycles due to mechanical degradation, making long-term operation problematic.
Addressing these challenges requires interdisciplinary approaches combining materials science, chemical engineering, and process design. Recent research directions include developing composite materials with enhanced stability, exploring novel regeneration methods to reduce energy penalties, and designing innovative reactor configurations to optimize heat and mass transfer.
Current Solid Sorbent Solutions for CO2 Capture
01 Metal-organic frameworks (MOFs) for CO2 capture
Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. Their high surface area, tunable pore size, and chemical functionality make them effective for selective CO2 adsorption. MOFs can be designed with specific binding sites for CO2 molecules, enhancing capture capacity and selectivity even at low CO2 concentrations. These materials can be regenerated with minimal energy input, making them promising for industrial carbon capture applications.- Metal-organic frameworks (MOFs) for CO2 capture: Metal-organic frameworks are crystalline porous materials composed of metal ions or clusters coordinated with organic ligands. They have high surface areas, tunable pore sizes, and can be designed with specific functional groups to enhance CO2 adsorption capacity and selectivity. MOFs can be modified to improve stability under various conditions and can be regenerated with lower energy requirements compared to traditional sorbents.
- Amine-functionalized solid sorbents: Solid sorbents functionalized with amine groups demonstrate high affinity for CO2 through chemical adsorption mechanisms. These materials can be prepared by impregnating porous supports with amine compounds or by grafting amine groups onto the surface of various substrates. The amine functionality creates strong binding sites for CO2, enabling efficient capture even at low CO2 concentrations. These sorbents can be designed with different amine types to optimize capture performance under specific conditions.
- Zeolite-based CO2 capture materials: Zeolites are aluminosilicate minerals with highly ordered microporous structures that can effectively adsorb CO2. Their crystalline framework contains cavities and channels that can selectively capture CO2 molecules based on size and polarity. Zeolites can be modified through ion exchange, dealumination, or incorporation of functional groups to enhance their CO2 capture performance. These materials offer advantages including thermal stability, mechanical strength, and resistance to degradation in various industrial environments.
- Carbon-based sorbents for CO2 capture: Carbon-based materials including activated carbon, carbon nanotubes, graphene, and carbon molecular sieves can be used as effective CO2 sorbents. These materials can be produced from various precursors including biomass, coal, or synthetic sources. Their high surface area, tunable pore structure, and surface chemistry can be optimized for CO2 adsorption. Carbon-based sorbents can be functionalized with nitrogen, oxygen, or metal species to enhance their CO2 capture capacity and selectivity.
- Regeneration methods for solid CO2 sorbents: Various regeneration techniques can be employed to release captured CO2 from solid sorbents and prepare them for reuse. These methods include temperature swing adsorption (TSA), pressure swing adsorption (PSA), vacuum swing adsorption (VSA), and combinations thereof. Novel approaches such as microwave-assisted regeneration, electrical swing adsorption, and steam stripping can reduce energy requirements. The regeneration process must be optimized to maintain sorbent integrity over multiple capture-release cycles while minimizing energy consumption.
02 Amine-functionalized solid sorbents
Amine-functionalized materials represent a significant class of solid sorbents for CO2 capture. These materials combine the high surface area of porous supports with amine groups that chemically bind to CO2 molecules. Common supports include silica, activated carbon, and polymeric materials. The amine functionalization can be achieved through impregnation, grafting, or polymerization methods. These materials offer advantages such as high CO2 selectivity, good working capacity, and relatively low regeneration energy requirements compared to traditional liquid amine scrubbing.Expand Specific Solutions03 Zeolite-based CO2 capture systems
Zeolites are crystalline aluminosilicate materials with well-defined pore structures that can effectively separate CO2 from gas mixtures. Their molecular sieving properties, thermal stability, and resistance to harsh conditions make them suitable for industrial carbon capture applications. Zeolites can be modified with various cations to enhance CO2 adsorption capacity and selectivity. These materials work through physical adsorption mechanisms and can be regenerated through pressure or temperature swing processes, allowing for continuous operation in carbon capture systems.Expand Specific Solutions04 Carbon-based sorbents for CO2 capture
Carbon-based materials, including activated carbon, carbon nanotubes, and graphene derivatives, offer promising solutions for CO2 capture. These materials feature high surface area, tunable pore structure, and surface chemistry that can be optimized for CO2 adsorption. Carbon-based sorbents can be functionalized with nitrogen-containing groups or metal particles to enhance their CO2 capture performance. Their advantages include low cost, good stability, and relatively simple regeneration processes, making them attractive for large-scale carbon capture applications.Expand Specific Solutions05 Hybrid and composite sorbent materials
Hybrid and composite materials combine different types of sorbents to leverage their complementary properties for enhanced CO2 capture performance. These materials may integrate organic and inorganic components, such as polymer-inorganic composites, mixed matrix materials, or layered structures. By combining multiple capture mechanisms, these hybrid sorbents can achieve higher capacity, selectivity, and stability than single-component materials. They can be designed to operate effectively under specific conditions, such as high temperature, high pressure, or in the presence of contaminants, making them versatile for various carbon capture applications.Expand Specific Solutions
Key Industry Players in Solid Sorbent Development
The solid sorbents for CO2 capture market is in a growth phase, with increasing adoption across industrial and power plant applications. The market size is expanding rapidly due to stringent carbon emission regulations and growing corporate sustainability commitments. Technologically, the field shows varying maturity levels, with companies like Sinopec, CHN Energy, and Korea Electric Power Corp leading commercial deployment in Asia. Western players including Climeworks, Carboncapture, and Cabot Corp are advancing innovative sorbent technologies. Research institutions such as Zhejiang University, Rice University, and University of Wyoming collaborate with industry partners to develop next-generation materials. The competitive landscape features both established energy conglomerates investing in carbon capture infrastructure and specialized startups focusing on novel sorbent formulations and direct air capture applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced solid sorbent technologies for CO2 capture in industrial applications, focusing on metal-organic frameworks (MOFs) and amine-functionalized mesoporous silica materials. Their proprietary MOF-based sorbents demonstrate exceptional CO2 selectivity and capacity under flue gas conditions, with adsorption capacities reaching 4-5 mmol/g at typical power plant operating conditions. Sinopec has implemented pilot-scale testing at several coal-fired power plants, demonstrating regeneration energy requirements approximately 30% lower than conventional amine scrubbing technologies. Their solid sorbent system employs a temperature-vacuum swing adsorption (TVSA) process that allows for efficient sorbent regeneration at temperatures below 120°C, significantly reducing the energy penalty associated with CO2 capture. The company has also developed specialized fixed-bed reactor configurations that minimize pressure drop while maximizing gas-solid contact efficiency, addressing one of the key engineering challenges in solid sorbent deployment.
Strengths: Sinopec's solid sorbents demonstrate excellent stability over multiple adsorption-desorption cycles (>1000 cycles with minimal degradation), lower regeneration energy requirements compared to liquid amines, and reduced equipment corrosion issues. Weaknesses: The technology faces challenges in scaling up production of specialized sorbent materials at competitive costs and requires complex process engineering to handle large gas volumes efficiently in industrial settings.
Carboncapture, Inc.
Technical Solution: Carboncapture, Inc. has pioneered a modular direct air capture (DAC) system utilizing zeolite-based solid sorbents for CO2 capture from ambient air and industrial sources. Their proprietary CarbonCapture™ technology employs specially modified zeolites that can selectively adsorb CO2 even at the low concentrations found in ambient air (approximately 415 ppm). The company's approach features a temperature-vacuum swing adsorption process where the sorbent is heated to relatively low temperatures (80-120°C) for regeneration, significantly reducing energy requirements compared to high-temperature calcination processes used in other DAC technologies. Their modular design allows for scalable deployment, with each module capable of capturing 100-1000 tons of CO2 annually depending on configuration. The system incorporates advanced process control algorithms that optimize the adsorption-desorption cycle based on ambient conditions, maximizing capture efficiency while minimizing energy consumption. Carboncapture has demonstrated the ability to produce high-purity CO2 (>99%) suitable for utilization or sequestration, and their zeolite sorbents maintain performance over thousands of cycles with minimal degradation.
Strengths: The modular design enables flexible deployment and scaling, while the zeolite-based sorbents offer excellent durability and lower regeneration energy compared to liquid amine systems. The technology works effectively across a wide range of environmental conditions. Weaknesses: Current costs remain relatively high at $250-600 per ton of CO2 captured, and the technology requires significant land area for large-scale deployment. Energy requirements, while lower than some alternatives, still present challenges for net-negative emissions.
Critical Patents and Research in Advanced Sorbent Materials
Solid sorbents for capturing co 2
PatentWO2023232666A1
Innovation
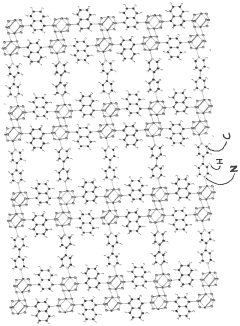
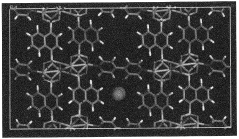
- Development of phosphonate and organoarsonate MOFs with specific molecular formulas, such as [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediphosphonate)] and [{M2(4,4’-bipyridine)0.5}(l,4-naphthalenediarsonate)], which maintain selectivity and stability under harsh conditions, including high humidity and temperatures up to 360°C, by creating a hydrophobic environment that favors CO2 physisorption over H2O.
Layered solid sorbents for carbon dioxide capture
PatentActiveUS8889589B2
Innovation
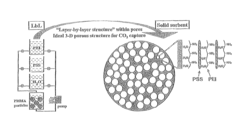
- Development of nano-layered solid sorbents using electrostatic layer-by-layer nanoassembly, where positively charged polyethylenimine and negatively charged polystyrene sulfonate layers are alternately deposited on a porous substrate, enhancing CO2 capture and transport kinetics.
Environmental Impact and Sustainability Assessment
The environmental impact of solid sorbents for CO2 capture extends beyond their primary function of reducing greenhouse gas emissions. Life cycle assessment (LCA) studies reveal that while these technologies significantly decrease direct CO2 emissions, their production, operation, and disposal generate environmental footprints that must be carefully evaluated. The energy requirements for sorbent regeneration represent a substantial environmental concern, as this process typically demands high temperatures or pressure swings that consume considerable energy, potentially offsetting some carbon reduction benefits.
Material sourcing for solid sorbents presents another critical environmental consideration. Amine-functionalized materials, metal-organic frameworks (MOFs), and zeolites each require specific raw materials that may involve energy-intensive mining operations or chemical synthesis processes. The environmental burden of these upstream activities varies significantly depending on the sorbent type, with some advanced materials requiring rare elements or complex manufacturing procedures that generate additional environmental impacts.
Water consumption represents a frequently overlooked environmental factor in solid sorbent technologies. Many sorbents exhibit high water affinity, potentially competing with CO2 for adsorption sites and necessitating additional energy for water removal. Furthermore, water usage in cooling systems and process operations contributes to the overall water footprint of these capture technologies, raising sustainability concerns in water-stressed regions where power plants and industrial facilities often operate.
The sustainability of solid sorbents must be evaluated through their complete lifecycle, including end-of-life management. Current research indicates that most commercial sorbents have finite lifespans, typically degrading after multiple adsorption-desorption cycles. The disposal or regeneration of spent sorbents presents both challenges and opportunities for sustainable materials management. Some materials can be recycled or repurposed, while others may require specialized disposal procedures to prevent secondary environmental contamination.
Land use impacts associated with solid sorbent technologies are generally favorable compared to alternative carbon capture approaches. The compact nature of solid sorbent systems typically requires less physical space than liquid absorption technologies or direct air capture installations. This spatial efficiency becomes particularly advantageous in retrofitting existing industrial facilities where available land may be limited, allowing for carbon capture implementation with minimal additional land disturbance.
The sustainability assessment of solid sorbents must ultimately balance their environmental costs against their carbon mitigation benefits. Recent advancements in bio-based sorbents and materials derived from industrial waste streams show promise for reducing the environmental footprint of these technologies. These innovations, coupled with improvements in energy efficiency and sorbent durability, are gradually enhancing the overall sustainability profile of solid sorbent systems for industrial and power plant applications.
Material sourcing for solid sorbents presents another critical environmental consideration. Amine-functionalized materials, metal-organic frameworks (MOFs), and zeolites each require specific raw materials that may involve energy-intensive mining operations or chemical synthesis processes. The environmental burden of these upstream activities varies significantly depending on the sorbent type, with some advanced materials requiring rare elements or complex manufacturing procedures that generate additional environmental impacts.
Water consumption represents a frequently overlooked environmental factor in solid sorbent technologies. Many sorbents exhibit high water affinity, potentially competing with CO2 for adsorption sites and necessitating additional energy for water removal. Furthermore, water usage in cooling systems and process operations contributes to the overall water footprint of these capture technologies, raising sustainability concerns in water-stressed regions where power plants and industrial facilities often operate.
The sustainability of solid sorbents must be evaluated through their complete lifecycle, including end-of-life management. Current research indicates that most commercial sorbents have finite lifespans, typically degrading after multiple adsorption-desorption cycles. The disposal or regeneration of spent sorbents presents both challenges and opportunities for sustainable materials management. Some materials can be recycled or repurposed, while others may require specialized disposal procedures to prevent secondary environmental contamination.
Land use impacts associated with solid sorbent technologies are generally favorable compared to alternative carbon capture approaches. The compact nature of solid sorbent systems typically requires less physical space than liquid absorption technologies or direct air capture installations. This spatial efficiency becomes particularly advantageous in retrofitting existing industrial facilities where available land may be limited, allowing for carbon capture implementation with minimal additional land disturbance.
The sustainability assessment of solid sorbents must ultimately balance their environmental costs against their carbon mitigation benefits. Recent advancements in bio-based sorbents and materials derived from industrial waste streams show promise for reducing the environmental footprint of these technologies. These innovations, coupled with improvements in energy efficiency and sorbent durability, are gradually enhancing the overall sustainability profile of solid sorbent systems for industrial and power plant applications.
Economic Viability and Scalability Analysis
The economic viability of solid sorbents for CO2 capture depends significantly on their cost-effectiveness compared to traditional carbon capture technologies. Current analysis indicates that while amine-based liquid solvents remain the industry standard, solid sorbents offer potential cost advantages through reduced energy requirements for regeneration, which typically accounts for 70-80% of operational expenses in carbon capture systems. Economic modeling suggests that advanced metal-organic frameworks (MOFs) and functionalized porous materials could reduce capture costs from the current $40-60 per ton of CO2 to approximately $30-40 per ton when implemented at scale.
Capital expenditure considerations reveal that solid sorbent systems may require higher initial investment due to specialized equipment needs, but this is potentially offset by lower operational costs over the system lifetime. Sensitivity analyses demonstrate that sorbent durability significantly impacts economic viability, with materials maintaining performance over 1,000+ cycles showing substantially improved return on investment profiles compared to those requiring frequent replacement.
Scalability remains a critical challenge for widespread industrial adoption. Laboratory-scale successes with novel sorbents often face significant hurdles during scale-up, including decreased performance metrics, manufacturing complexities, and increased costs. Current production capacities for advanced sorbents like covalent organic frameworks (COFs) and certain zeolites remain limited to kilogram quantities, whereas industrial applications require metric tons.
Process integration analysis indicates that retrofitting existing power plants with solid sorbent systems presents fewer technical challenges than complete system redesigns, potentially accelerating market penetration. However, the economic case strengthens significantly when designing new facilities with integrated carbon capture systems from inception.
Market projections suggest that with continued research and development investment, production scale economies could reduce sorbent manufacturing costs by 40-60% over the next decade. This cost trajectory, combined with increasingly stringent carbon regulations and potential carbon pricing mechanisms, creates a favorable economic outlook for solid sorbent technologies in the medium to long term.
Risk assessment models highlight that economic viability remains sensitive to policy frameworks, with carbon pricing thresholds of approximately $35-50 per ton needed to make most current solid sorbent technologies economically competitive without additional subsidies or incentives in power generation applications.
Capital expenditure considerations reveal that solid sorbent systems may require higher initial investment due to specialized equipment needs, but this is potentially offset by lower operational costs over the system lifetime. Sensitivity analyses demonstrate that sorbent durability significantly impacts economic viability, with materials maintaining performance over 1,000+ cycles showing substantially improved return on investment profiles compared to those requiring frequent replacement.
Scalability remains a critical challenge for widespread industrial adoption. Laboratory-scale successes with novel sorbents often face significant hurdles during scale-up, including decreased performance metrics, manufacturing complexities, and increased costs. Current production capacities for advanced sorbents like covalent organic frameworks (COFs) and certain zeolites remain limited to kilogram quantities, whereas industrial applications require metric tons.
Process integration analysis indicates that retrofitting existing power plants with solid sorbent systems presents fewer technical challenges than complete system redesigns, potentially accelerating market penetration. However, the economic case strengthens significantly when designing new facilities with integrated carbon capture systems from inception.
Market projections suggest that with continued research and development investment, production scale economies could reduce sorbent manufacturing costs by 40-60% over the next decade. This cost trajectory, combined with increasingly stringent carbon regulations and potential carbon pricing mechanisms, creates a favorable economic outlook for solid sorbent technologies in the medium to long term.
Risk assessment models highlight that economic viability remains sensitive to policy frameworks, with carbon pricing thresholds of approximately $35-50 per ton needed to make most current solid sorbent technologies economically competitive without additional subsidies or incentives in power generation applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!