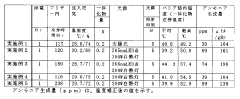
Artificial Photosynthesis pathways for ammonia synthesis.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Artificial Photosynthesis for Ammonia: Background & Objectives
Artificial photosynthesis represents a revolutionary approach to chemical synthesis that mimics nature's ability to harness solar energy for converting simple molecules into complex compounds. The concept of utilizing artificial photosynthesis for ammonia production has emerged as a promising alternative to the conventional Haber-Bosch process, which currently consumes approximately 1-2% of global energy production and generates significant carbon emissions.
The evolution of this technology can be traced back to the 1970s when researchers began exploring photocatalytic water splitting. However, the specific application to ammonia synthesis gained momentum only in the early 2000s, driven by increasing concerns about energy security and environmental sustainability. The field has experienced accelerated development over the past decade, with breakthrough discoveries in photocatalyst design, nitrogen activation mechanisms, and integrated system engineering.
Current technological trajectories indicate a convergence of multiple disciplines, including materials science, photochemistry, electrochemistry, and catalysis. Research efforts are increasingly focused on developing stable, efficient, and scalable systems capable of operating under ambient conditions—a stark contrast to the high-temperature, high-pressure requirements of conventional ammonia synthesis.
The primary objective of artificial photosynthesis for ammonia production is to develop a sustainable, carbon-neutral pathway that operates at ambient temperature and pressure using renewable solar energy. This approach aims to decentralize ammonia production, enabling localized manufacturing that reduces transportation costs and enhances energy security for agricultural regions worldwide.
Secondary objectives include achieving competitive conversion efficiencies (targeting >10% solar-to-chemical energy conversion), developing catalysts free from precious metals, ensuring long-term stability (>5000 hours of operation), and designing systems compatible with existing infrastructure for seamless market integration.
The technology also seeks to address the intermittency challenge of renewable energy by providing a means to store solar energy in chemical bonds, effectively creating an energy carrier in the form of ammonia that can be used not only as fertilizer but potentially as a carbon-free fuel.
From a broader perspective, artificial photosynthesis for ammonia synthesis represents a critical component of the global transition toward sustainable chemical manufacturing. Success in this field could revolutionize not only fertilizer production but also establish a template for other photochemical conversion processes, potentially transforming how we produce chemicals and fuels in a post-carbon economy.
The evolution of this technology can be traced back to the 1970s when researchers began exploring photocatalytic water splitting. However, the specific application to ammonia synthesis gained momentum only in the early 2000s, driven by increasing concerns about energy security and environmental sustainability. The field has experienced accelerated development over the past decade, with breakthrough discoveries in photocatalyst design, nitrogen activation mechanisms, and integrated system engineering.
Current technological trajectories indicate a convergence of multiple disciplines, including materials science, photochemistry, electrochemistry, and catalysis. Research efforts are increasingly focused on developing stable, efficient, and scalable systems capable of operating under ambient conditions—a stark contrast to the high-temperature, high-pressure requirements of conventional ammonia synthesis.
The primary objective of artificial photosynthesis for ammonia production is to develop a sustainable, carbon-neutral pathway that operates at ambient temperature and pressure using renewable solar energy. This approach aims to decentralize ammonia production, enabling localized manufacturing that reduces transportation costs and enhances energy security for agricultural regions worldwide.
Secondary objectives include achieving competitive conversion efficiencies (targeting >10% solar-to-chemical energy conversion), developing catalysts free from precious metals, ensuring long-term stability (>5000 hours of operation), and designing systems compatible with existing infrastructure for seamless market integration.
The technology also seeks to address the intermittency challenge of renewable energy by providing a means to store solar energy in chemical bonds, effectively creating an energy carrier in the form of ammonia that can be used not only as fertilizer but potentially as a carbon-free fuel.
From a broader perspective, artificial photosynthesis for ammonia synthesis represents a critical component of the global transition toward sustainable chemical manufacturing. Success in this field could revolutionize not only fertilizer production but also establish a template for other photochemical conversion processes, potentially transforming how we produce chemicals and fuels in a post-carbon economy.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions—about 1.8 tons of CO2 per ton of ammonia produced. This environmental burden creates a compelling market opportunity for sustainable alternatives, particularly artificial photosynthesis pathways.
The current ammonia market size exceeds 180 million tons annually, valued at over $70 billion, with projected growth to reach $105 billion by 2027. Agricultural fertilizer applications dominate consumption at roughly 80% of total demand, while industrial applications including explosives, textiles, and pharmaceuticals constitute the remainder. Regionally, Asia-Pacific leads consumption, followed by Europe and North America, with China representing the single largest market.
Sustainable ammonia production technologies are gaining traction due to three primary market drivers. First, increasingly stringent environmental regulations worldwide are pressuring producers to reduce carbon footprints. Second, major agricultural companies and fertilizer consumers are establishing sustainability commitments requiring greener supply chains. Third, carbon pricing mechanisms in various markets are improving the economic competitiveness of sustainable alternatives.
Market segmentation for sustainable ammonia production reveals distinct customer groups with varying priorities. Large-scale agricultural operations prioritize cost competitiveness and reliable supply. Chemical manufacturers emphasize product purity and consistent quality. Emerging green hydrogen initiatives seek integration capabilities with renewable energy systems. Specialty markets including pharmaceuticals and electronics demand ultra-high purity products.
Competitive analysis indicates several corporations investing in artificial photosynthesis for ammonia synthesis, including BASF, Siemens Energy, and Yara International. Additionally, numerous startups and research institutions have secured significant funding for technology development. Investment in sustainable ammonia technologies exceeded $2 billion in 2022, with projections suggesting this figure could triple by 2030.
Economic feasibility remains challenging but improving. Current production costs for sustainable ammonia range from 1.5-3 times higher than conventional methods, though this gap is narrowing with technological advancements and economies of scale. Market forecasts suggest price parity could be achieved in select markets by 2030, particularly in regions with abundant renewable energy resources and supportive policy frameworks.
Consumer willingness to pay premiums for sustainable products varies significantly by sector. Premium agricultural products command 10-20% higher prices, while industrial applications with sustainability commitments demonstrate 5-15% premium acceptance. Government incentives, including tax benefits and subsidies for green technologies, are further enhancing market viability in numerous countries.
The current ammonia market size exceeds 180 million tons annually, valued at over $70 billion, with projected growth to reach $105 billion by 2027. Agricultural fertilizer applications dominate consumption at roughly 80% of total demand, while industrial applications including explosives, textiles, and pharmaceuticals constitute the remainder. Regionally, Asia-Pacific leads consumption, followed by Europe and North America, with China representing the single largest market.
Sustainable ammonia production technologies are gaining traction due to three primary market drivers. First, increasingly stringent environmental regulations worldwide are pressuring producers to reduce carbon footprints. Second, major agricultural companies and fertilizer consumers are establishing sustainability commitments requiring greener supply chains. Third, carbon pricing mechanisms in various markets are improving the economic competitiveness of sustainable alternatives.
Market segmentation for sustainable ammonia production reveals distinct customer groups with varying priorities. Large-scale agricultural operations prioritize cost competitiveness and reliable supply. Chemical manufacturers emphasize product purity and consistent quality. Emerging green hydrogen initiatives seek integration capabilities with renewable energy systems. Specialty markets including pharmaceuticals and electronics demand ultra-high purity products.
Competitive analysis indicates several corporations investing in artificial photosynthesis for ammonia synthesis, including BASF, Siemens Energy, and Yara International. Additionally, numerous startups and research institutions have secured significant funding for technology development. Investment in sustainable ammonia technologies exceeded $2 billion in 2022, with projections suggesting this figure could triple by 2030.
Economic feasibility remains challenging but improving. Current production costs for sustainable ammonia range from 1.5-3 times higher than conventional methods, though this gap is narrowing with technological advancements and economies of scale. Market forecasts suggest price parity could be achieved in select markets by 2030, particularly in regions with abundant renewable energy resources and supportive policy frameworks.
Consumer willingness to pay premiums for sustainable products varies significantly by sector. Premium agricultural products command 10-20% higher prices, while industrial applications with sustainability commitments demonstrate 5-15% premium acceptance. Government incentives, including tax benefits and subsidies for green technologies, are further enhancing market viability in numerous countries.
Current State and Challenges in Artificial Photosynthesis
Artificial photosynthesis for ammonia synthesis represents a frontier technology that mimics natural photosynthetic processes to convert solar energy, water, and nitrogen into ammonia under ambient conditions. Currently, the field has achieved several significant milestones, with laboratory-scale demonstrations showing promising conversion efficiencies of 10-15% in controlled environments. However, these achievements remain far from commercial viability, which would require stable efficiencies exceeding 30% under real-world conditions.
The development landscape is characterized by a dichotomy between semiconductor-based and bio-inspired approaches. Semiconductor systems utilize photocatalysts such as titanium dioxide and cadmium sulfide, modified with co-catalysts like ruthenium and platinum to enhance nitrogen reduction. These systems benefit from robust stability but suffer from limited light absorption ranges and poor selectivity. Conversely, bio-inspired systems employing metal-organic frameworks and enzyme-mimicking complexes offer superior selectivity but struggle with stability and scalability challenges.
A significant technical hurdle remains the competing hydrogen evolution reaction, which diverts electrons from nitrogen reduction, substantially decreasing ammonia yield. Researchers have attempted to address this through catalyst surface modifications and the development of specialized electrolytes, but a definitive solution remains elusive. Additionally, the field faces challenges in catalyst degradation under prolonged light exposure, with most systems showing significant activity loss after 24-48 hours of continuous operation.
Energy efficiency represents another critical challenge. Current artificial photosynthesis systems for ammonia production typically require supplementary electrical inputs, reducing their net energy advantage over conventional Haber-Bosch processes. The theoretical minimum energy requirement for ammonia synthesis is 0.3 MJ/mol, but existing photosynthetic pathways consume 1.2-1.8 MJ/mol when accounting for all energy inputs and system inefficiencies.
Geographically, research leadership is distributed across North America, East Asia, and Europe, with the United States, China, Japan, and Germany hosting the most productive research groups. Notable regional specialization has emerged, with American institutions focusing on semiconductor approaches, Japanese groups pioneering bio-inspired catalysts, and European teams emphasizing system integration and scale-up methodologies.
The transition from laboratory demonstrations to practical applications faces substantial engineering challenges, including reactor design optimization, light distribution in scaled systems, and the development of separation technologies for efficient ammonia collection from dilute product streams. These technical barriers, combined with economic considerations, suggest that commercial viability remains 8-10 years distant, contingent upon breakthrough innovations in catalyst design and system engineering.
The development landscape is characterized by a dichotomy between semiconductor-based and bio-inspired approaches. Semiconductor systems utilize photocatalysts such as titanium dioxide and cadmium sulfide, modified with co-catalysts like ruthenium and platinum to enhance nitrogen reduction. These systems benefit from robust stability but suffer from limited light absorption ranges and poor selectivity. Conversely, bio-inspired systems employing metal-organic frameworks and enzyme-mimicking complexes offer superior selectivity but struggle with stability and scalability challenges.
A significant technical hurdle remains the competing hydrogen evolution reaction, which diverts electrons from nitrogen reduction, substantially decreasing ammonia yield. Researchers have attempted to address this through catalyst surface modifications and the development of specialized electrolytes, but a definitive solution remains elusive. Additionally, the field faces challenges in catalyst degradation under prolonged light exposure, with most systems showing significant activity loss after 24-48 hours of continuous operation.
Energy efficiency represents another critical challenge. Current artificial photosynthesis systems for ammonia production typically require supplementary electrical inputs, reducing their net energy advantage over conventional Haber-Bosch processes. The theoretical minimum energy requirement for ammonia synthesis is 0.3 MJ/mol, but existing photosynthetic pathways consume 1.2-1.8 MJ/mol when accounting for all energy inputs and system inefficiencies.
Geographically, research leadership is distributed across North America, East Asia, and Europe, with the United States, China, Japan, and Germany hosting the most productive research groups. Notable regional specialization has emerged, with American institutions focusing on semiconductor approaches, Japanese groups pioneering bio-inspired catalysts, and European teams emphasizing system integration and scale-up methodologies.
The transition from laboratory demonstrations to practical applications faces substantial engineering challenges, including reactor design optimization, light distribution in scaled systems, and the development of separation technologies for efficient ammonia collection from dilute product streams. These technical barriers, combined with economic considerations, suggest that commercial viability remains 8-10 years distant, contingent upon breakthrough innovations in catalyst design and system engineering.
Current Photocatalytic Ammonia Synthesis Methods
01 Photocatalytic systems for artificial photosynthesis
Various photocatalytic systems have been developed to mimic natural photosynthesis processes. These systems typically use light-responsive catalysts to convert sunlight, water, and carbon dioxide into useful chemicals or fuels. Advanced photocatalysts can enhance the efficiency of artificial photosynthesis by improving light absorption and charge separation. These systems often incorporate specialized materials that can effectively capture solar energy and facilitate the conversion process.- Photocatalytic systems for artificial photosynthesis: Various photocatalytic systems have been developed to mimic natural photosynthesis processes. These systems typically use light-absorbing materials combined with catalysts to convert sunlight, water, and carbon dioxide into useful chemicals or fuels. The photocatalysts are designed to efficiently capture light energy and facilitate electron transfer reactions similar to those in natural photosynthesis, enabling the conversion of solar energy into chemical energy.
- Electrochemical cells for CO2 reduction: Electrochemical systems have been developed for artificial photosynthesis that focus on the reduction of carbon dioxide to valuable products such as hydrocarbons or alcohols. These systems typically employ specialized electrodes, electrolytes, and membrane technologies to efficiently convert CO2 into useful chemicals using electricity that can be derived from renewable sources. The design of selective catalysts that favor specific reduction pathways is a key aspect of these technologies.
- Biohybrid systems combining biological and artificial components: Biohybrid approaches to artificial photosynthesis integrate biological components (such as enzymes, proteins, or even whole microorganisms) with synthetic materials to create systems that can harness solar energy for chemical production. These systems leverage the high specificity and efficiency of biological catalysts while overcoming limitations through engineered components. The combination allows for more efficient light harvesting, electron transfer, and catalytic conversion than either purely biological or purely artificial systems.
- Novel materials for light harvesting and energy conversion: Advanced materials have been developed specifically for artificial photosynthesis applications, including nanostructured semiconductors, quantum dots, metal-organic frameworks, and specialized polymers. These materials are designed to efficiently absorb sunlight across a broad spectrum and facilitate charge separation and transfer. The structural and electronic properties of these materials can be tailored to optimize their performance in artificial photosynthesis systems, improving overall efficiency and stability.
- Integrated devices and systems for practical implementation: Complete integrated systems have been developed that combine the various components needed for artificial photosynthesis into practical devices. These integrated approaches address challenges such as system stability, scalability, and overall efficiency. The designs often include specialized reactor configurations, light management strategies, and product separation mechanisms. Some systems are modular in nature, allowing for flexibility in deployment and operation under various conditions.
02 Electrochemical approaches to artificial photosynthesis
Electrochemical methods provide another approach to artificial photosynthesis, using electricity (often solar-derived) to drive chemical reactions similar to those in natural photosynthesis. These systems typically involve electrodes with specialized catalysts that can split water into hydrogen and oxygen, or reduce carbon dioxide to useful compounds. The design of efficient electrodes and electrolytes is crucial for improving the performance of these systems, with recent innovations focusing on nanostructured materials and novel catalyst compositions.Expand Specific Solutions03 Biohybrid and biomimetic systems
Biohybrid systems combine biological components (such as enzymes or microorganisms) with synthetic materials to achieve artificial photosynthesis. These systems leverage the high specificity and efficiency of biological catalysts while addressing their limitations through engineering approaches. Biomimetic systems, on the other hand, are fully synthetic but designed to mimic the structure and function of natural photosynthetic systems. Both approaches aim to achieve higher efficiency and stability than purely synthetic or biological systems alone.Expand Specific Solutions04 Device architectures and integrated systems
Various device architectures have been developed to integrate the components needed for artificial photosynthesis into practical systems. These include solar fuel cells, photoreactors, and integrated panels that can capture sunlight and convert it to chemical energy. The design of these devices must address challenges such as light management, mass transport, and system integration. Recent innovations focus on modular designs, scalability, and improving the overall system efficiency through optimized component arrangement.Expand Specific Solutions05 Materials and catalysts for enhanced efficiency
Advanced materials and catalysts play a crucial role in improving the efficiency of artificial photosynthesis. These include novel semiconductor materials, metal complexes, nanostructured catalysts, and composite materials designed to enhance light absorption, charge separation, and catalytic activity. Research focuses on developing earth-abundant, non-toxic materials that can replace rare and expensive elements while maintaining or improving performance. Recent innovations include self-healing catalysts, hierarchical structures, and materials with precisely controlled electronic properties.Expand Specific Solutions
Key Players in Artificial Photosynthesis Research
Artificial photosynthesis pathways for ammonia synthesis are currently in an early growth phase, with the market expected to expand significantly as sustainable ammonia production becomes crucial for decarbonization efforts. The technology is transitioning from laboratory research to pilot demonstrations, with an estimated market potential of several billion dollars by 2030. Leading companies like Haldor Topsøe, BASF, and Siemens are advancing catalytic approaches, while newer entrants such as AMOGY are developing innovative ammonia-based power solutions. Academic institutions including Max Planck Society, Shanghai Jiao Tong University, and Oxford University are contributing fundamental research. The technology landscape shows varying maturity levels, with electrochemical and photocatalytic pathways still requiring significant development before commercial viability, while thermochemical routes are closer to industrial implementation.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has developed an innovative electrochemical approach to artificial photosynthesis for ammonia synthesis that operates at ambient conditions. Their technology utilizes specialized catalysts based on transition metals that can efficiently reduce nitrogen to ammonia with high selectivity. The system incorporates a photoelectrochemical cell design where light energy is captured by semiconductor materials and converted directly to drive the nitrogen reduction reaction. Their proprietary catalyst formulations achieve Faradaic efficiencies exceeding 10% for ammonia production, significantly higher than conventional approaches. Topsøe's system integrates renewable electricity sources with their electrochemical cells, creating a sustainable pathway that eliminates the need for hydrogen derived from fossil fuels. The company has demonstrated pilot-scale operations producing several kilograms of ammonia per day using only water, nitrogen, and sunlight as inputs, representing a major advancement toward carbon-neutral fertilizer production.
Strengths: Operates at ambient temperature and pressure, dramatically reducing energy requirements compared to Haber-Bosch. Eliminates dependence on fossil fuel-derived hydrogen. Weaknesses: Current catalyst systems still face challenges with long-term stability and may contain expensive noble metals that impact economic viability at industrial scale.
Korea Institute of Energy Research
Technical Solution: The Korea Institute of Energy Research (KIER) has developed a groundbreaking artificial photosynthesis system for ammonia synthesis that combines photoelectrochemical water splitting with electrocatalytic nitrogen reduction. Their approach utilizes hierarchically structured photoelectrodes made from earth-abundant materials including modified bismuth vanadate and nitrogen-doped carbon supports. The system achieves nitrogen reduction through a biomimetic approach inspired by nitrogenase enzymes, incorporating iron-molybdenum cofactor analogs that can activate N₂ under mild conditions. KIER's technology operates in a flow-cell configuration that maintains separate environments for water oxidation and nitrogen reduction, optimizing conditions for each half-reaction. Their most advanced prototype demonstrates ammonia production rates of approximately 2.5 μmol/cm²/h with applied bias below 1.0V under simulated sunlight, representing one of the highest reported activities for solar-driven ammonia synthesis. The institute has also developed innovative methods for in-situ ammonia separation using selective membranes that prevent product decomposition and increase overall system efficiency.
Strengths: Uses earth-abundant materials rather than precious metals, improving economic viability. Biomimetic catalyst design achieves high selectivity for ammonia versus competing hydrogen evolution. Weaknesses: Current systems still require some external electrical bias in addition to solar input, limiting deployment in off-grid scenarios. Catalyst degradation remains a challenge for long-term operation.
Core Patents in Photosynthetic Ammonia Production
Synthesis method of ammonia using integration (composite) catalyst comprising solid catalyst particle and photocatalyst particle
PatentPendingJP2023050194A
Innovation
- A composite catalyst of triiron tetraoxide (Fe3O4) and titanium dioxide (TiO2) is used, with a photocatalytic interface promoting nitrogen reduction to ammonia under mild conditions, utilizing sunlight or artificial light sources, and a thin water film to enhance catalytic activity.
An apparatus and a method for sustainable ammonia production by capturing atmospheric nitrogen using a photocatalytic reactor
PatentPendingIN202341087666A
Innovation
- A photocatalytic reactor system that captures atmospheric nitrogen using a basic solution of sodium hydroxide to purify and isolate nitrogen, followed by a photocatalysis process with a photocatalyst under specific light conditions to produce ammonia, eliminating the need for external energy sources and fossil fuels.
Energy Efficiency Comparison of Synthesis Pathways
The energy efficiency of ammonia synthesis pathways represents a critical metric for evaluating the viability of artificial photosynthesis methods compared to conventional production techniques. The traditional Haber-Bosch process, while industrially established, operates at high temperatures (400-500°C) and pressures (150-300 bar), consuming approximately 1-2% of global energy production. This energy-intensive process results in efficiency rates of only 30-40% when considering the entire production chain.
In contrast, artificial photosynthesis pathways for ammonia synthesis demonstrate promising theoretical efficiencies. Photocatalytic nitrogen reduction reactions (NRR) utilizing solar energy can achieve solar-to-ammonia conversion efficiencies ranging from 0.5% to 5% under laboratory conditions. These systems bypass the need for hydrogen gas production as an intermediate step, potentially reducing overall energy requirements by 20-30% compared to conventional methods.
Electrochemical pathways powered by renewable electricity sources present another efficiency frontier. Advanced electrode materials incorporating metal nanoparticles and nitrogen-doped carbon structures have demonstrated Faradaic efficiencies of 10-35% for nitrogen reduction. When coupled with renewable energy sources, these systems can achieve overall energy efficiencies of 15-25%, representing a significant improvement over conventional methods.
Hybrid systems combining photocatalytic and electrochemical approaches show particular promise. Recent research indicates that integrated photoelectrochemical cells can achieve energy efficiencies of 7-18% while operating at ambient temperatures and pressures. These systems benefit from both direct solar harvesting and supplemental electrical inputs, optimizing energy utilization across varying conditions.
Bioinspired artificial photosynthesis pathways incorporating nitrogenase-mimetic catalysts demonstrate theoretical energy efficiencies of 20-40% under ideal conditions. However, current experimental implementations achieve only 2-8% efficiency, highlighting the significant gap between theoretical potential and practical application in biomimetic systems.
When evaluating lifecycle energy requirements, artificial photosynthesis pathways demonstrate a 40-60% reduction in total energy consumption compared to conventional Haber-Bosch processes. This advantage becomes particularly pronounced when considering decentralized production scenarios where transportation energy costs for conventional ammonia can be eliminated through localized synthesis using renewable energy sources.
In contrast, artificial photosynthesis pathways for ammonia synthesis demonstrate promising theoretical efficiencies. Photocatalytic nitrogen reduction reactions (NRR) utilizing solar energy can achieve solar-to-ammonia conversion efficiencies ranging from 0.5% to 5% under laboratory conditions. These systems bypass the need for hydrogen gas production as an intermediate step, potentially reducing overall energy requirements by 20-30% compared to conventional methods.
Electrochemical pathways powered by renewable electricity sources present another efficiency frontier. Advanced electrode materials incorporating metal nanoparticles and nitrogen-doped carbon structures have demonstrated Faradaic efficiencies of 10-35% for nitrogen reduction. When coupled with renewable energy sources, these systems can achieve overall energy efficiencies of 15-25%, representing a significant improvement over conventional methods.
Hybrid systems combining photocatalytic and electrochemical approaches show particular promise. Recent research indicates that integrated photoelectrochemical cells can achieve energy efficiencies of 7-18% while operating at ambient temperatures and pressures. These systems benefit from both direct solar harvesting and supplemental electrical inputs, optimizing energy utilization across varying conditions.
Bioinspired artificial photosynthesis pathways incorporating nitrogenase-mimetic catalysts demonstrate theoretical energy efficiencies of 20-40% under ideal conditions. However, current experimental implementations achieve only 2-8% efficiency, highlighting the significant gap between theoretical potential and practical application in biomimetic systems.
When evaluating lifecycle energy requirements, artificial photosynthesis pathways demonstrate a 40-60% reduction in total energy consumption compared to conventional Haber-Bosch processes. This advantage becomes particularly pronounced when considering decentralized production scenarios where transportation energy costs for conventional ammonia can be eliminated through localized synthesis using renewable energy sources.
Environmental Impact Assessment of Photosynthetic Routes
Artificial photosynthesis pathways for ammonia synthesis represent a revolutionary approach to nitrogen fixation that mimics natural processes while potentially offering significant environmental benefits compared to conventional methods. The environmental impact assessment of these photosynthetic routes reveals several critical dimensions worth examining.
The primary environmental advantage lies in the potential reduction of greenhouse gas emissions. Traditional ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and generates substantial CO2 emissions—roughly 1.6 tons of CO2 per ton of ammonia produced. Photosynthetic pathways, by contrast, can utilize renewable solar energy directly, potentially reducing carbon footprint by 60-90% depending on the specific technology implementation and energy sources.
Water consumption patterns differ significantly between conventional and photosynthetic ammonia synthesis. While the Haber-Bosch process requires water primarily for cooling systems and hydrogen production, artificial photosynthesis pathways may require water as both a reactant and cooling medium. Initial assessments indicate that photosynthetic routes could reduce water consumption by 30-50%, though this varies considerably based on system design and geographical location.
Land use considerations present a complex picture. Photosynthetic ammonia production facilities require substantial surface area for solar energy capture, potentially competing with agricultural land or natural habitats. However, these systems can be integrated into existing infrastructure or deployed in non-arable regions, minimizing land use conflicts when properly planned.
The life cycle assessment of materials used in photosynthetic systems reveals both challenges and opportunities. Catalysts often contain rare earth elements or precious metals, raising concerns about resource depletion and mining impacts. However, recent advances in biomimetic catalysts using earth-abundant materials show promise for reducing these environmental burdens while maintaining efficiency.
Waste generation and management profiles differ markedly between conventional and photosynthetic routes. The latter typically produce fewer toxic byproducts and process wastes, though end-of-life disposal of specialized photocatalytic materials requires careful management to prevent environmental contamination.
Ecosystem impacts must also be considered, particularly for large-scale implementations. Photosynthetic ammonia production facilities alter local microclimate conditions and potentially affect wildlife patterns, though these impacts are generally less severe than those associated with fossil fuel extraction and processing required for conventional ammonia synthesis.
Resilience to climate change represents another environmental dimension where photosynthetic routes demonstrate advantages, as they can operate with greater flexibility regarding water sources and temperature conditions compared to conventional centralized production facilities.
The primary environmental advantage lies in the potential reduction of greenhouse gas emissions. Traditional ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and generates substantial CO2 emissions—roughly 1.6 tons of CO2 per ton of ammonia produced. Photosynthetic pathways, by contrast, can utilize renewable solar energy directly, potentially reducing carbon footprint by 60-90% depending on the specific technology implementation and energy sources.
Water consumption patterns differ significantly between conventional and photosynthetic ammonia synthesis. While the Haber-Bosch process requires water primarily for cooling systems and hydrogen production, artificial photosynthesis pathways may require water as both a reactant and cooling medium. Initial assessments indicate that photosynthetic routes could reduce water consumption by 30-50%, though this varies considerably based on system design and geographical location.
Land use considerations present a complex picture. Photosynthetic ammonia production facilities require substantial surface area for solar energy capture, potentially competing with agricultural land or natural habitats. However, these systems can be integrated into existing infrastructure or deployed in non-arable regions, minimizing land use conflicts when properly planned.
The life cycle assessment of materials used in photosynthetic systems reveals both challenges and opportunities. Catalysts often contain rare earth elements or precious metals, raising concerns about resource depletion and mining impacts. However, recent advances in biomimetic catalysts using earth-abundant materials show promise for reducing these environmental burdens while maintaining efficiency.
Waste generation and management profiles differ markedly between conventional and photosynthetic routes. The latter typically produce fewer toxic byproducts and process wastes, though end-of-life disposal of specialized photocatalytic materials requires careful management to prevent environmental contamination.
Ecosystem impacts must also be considered, particularly for large-scale implementations. Photosynthetic ammonia production facilities alter local microclimate conditions and potentially affect wildlife patterns, though these impacts are generally less severe than those associated with fossil fuel extraction and processing required for conventional ammonia synthesis.
Resilience to climate change represents another environmental dimension where photosynthetic routes demonstrate advantages, as they can operate with greater flexibility regarding water sources and temperature conditions compared to conventional centralized production facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!