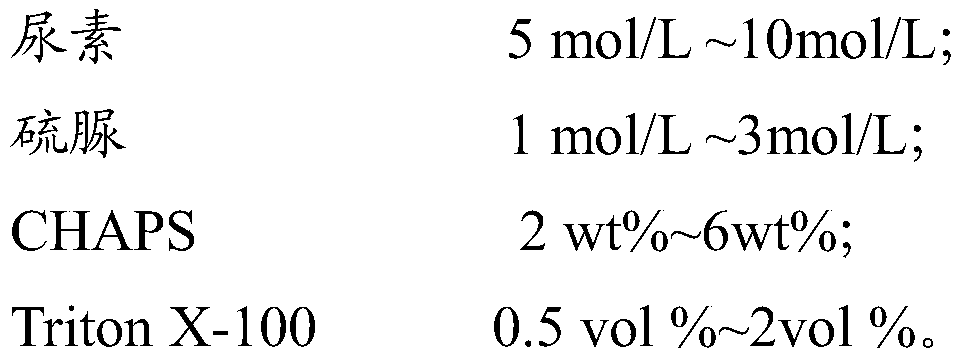Comparative Study of Triton X-100 and DMSO in Protein Solubilization
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Solubilization Background and Objectives
Protein solubilization is a critical process in biochemistry and molecular biology, essential for various applications including protein purification, structural studies, and functional analyses. The ability to effectively solubilize proteins from their native environments while maintaining their structural integrity and biological activity is paramount in advancing our understanding of protein function and developing novel therapeutic approaches.
Historically, protein solubilization techniques have evolved from harsh, denaturing methods to more gentle approaches that aim to preserve protein structure and function. The development of detergents and chaotropic agents has played a crucial role in this evolution, with researchers continually seeking to optimize solubilization protocols for diverse protein types and experimental needs.
In recent years, there has been a growing interest in comparing the efficacy of different solubilizing agents, particularly non-ionic detergents like Triton X-100 and organic solvents such as dimethyl sulfoxide (DMSO). These compounds represent two distinct approaches to protein solubilization, each with its own advantages and limitations.
Triton X-100, a non-ionic surfactant, has long been a staple in protein biochemistry due to its ability to disrupt lipid-lipid and lipid-protein interactions without denaturing protein structure. Its mild nature makes it particularly suitable for solubilizing membrane proteins and other hydrophobic proteins while maintaining their native conformation.
DMSO, on the other hand, is an amphipathic molecule that can penetrate both hydrophilic and hydrophobic environments. Its unique properties allow it to interact with proteins in ways that can enhance solubility without necessarily disrupting secondary and tertiary structures. DMSO has gained attention for its potential to solubilize proteins that are resistant to traditional detergent-based methods.
The comparative study of Triton X-100 and DMSO in protein solubilization aims to address several key objectives. Firstly, it seeks to evaluate the relative effectiveness of these agents in solubilizing a diverse range of proteins, including those that are traditionally difficult to work with, such as membrane proteins and aggregation-prone proteins.
Secondly, the study aims to assess the impact of these solubilizing agents on protein structure and function. This includes investigating their effects on protein stability, enzymatic activity, and the preservation of protein-protein interactions.
Furthermore, the research intends to explore the mechanisms by which Triton X-100 and DMSO facilitate protein solubilization, providing insights into the molecular interactions that govern the process. This understanding could lead to the development of more targeted and efficient solubilization strategies.
Ultimately, this comparative study seeks to provide researchers with a comprehensive understanding of when and how to employ Triton X-100 and DMSO in protein solubilization protocols. By elucidating the strengths and limitations of each approach, the study aims to guide the selection of optimal solubilization methods for specific protein types and experimental goals, potentially leading to improved outcomes in protein-based research and applications.
Historically, protein solubilization techniques have evolved from harsh, denaturing methods to more gentle approaches that aim to preserve protein structure and function. The development of detergents and chaotropic agents has played a crucial role in this evolution, with researchers continually seeking to optimize solubilization protocols for diverse protein types and experimental needs.
In recent years, there has been a growing interest in comparing the efficacy of different solubilizing agents, particularly non-ionic detergents like Triton X-100 and organic solvents such as dimethyl sulfoxide (DMSO). These compounds represent two distinct approaches to protein solubilization, each with its own advantages and limitations.
Triton X-100, a non-ionic surfactant, has long been a staple in protein biochemistry due to its ability to disrupt lipid-lipid and lipid-protein interactions without denaturing protein structure. Its mild nature makes it particularly suitable for solubilizing membrane proteins and other hydrophobic proteins while maintaining their native conformation.
DMSO, on the other hand, is an amphipathic molecule that can penetrate both hydrophilic and hydrophobic environments. Its unique properties allow it to interact with proteins in ways that can enhance solubility without necessarily disrupting secondary and tertiary structures. DMSO has gained attention for its potential to solubilize proteins that are resistant to traditional detergent-based methods.
The comparative study of Triton X-100 and DMSO in protein solubilization aims to address several key objectives. Firstly, it seeks to evaluate the relative effectiveness of these agents in solubilizing a diverse range of proteins, including those that are traditionally difficult to work with, such as membrane proteins and aggregation-prone proteins.
Secondly, the study aims to assess the impact of these solubilizing agents on protein structure and function. This includes investigating their effects on protein stability, enzymatic activity, and the preservation of protein-protein interactions.
Furthermore, the research intends to explore the mechanisms by which Triton X-100 and DMSO facilitate protein solubilization, providing insights into the molecular interactions that govern the process. This understanding could lead to the development of more targeted and efficient solubilization strategies.
Ultimately, this comparative study seeks to provide researchers with a comprehensive understanding of when and how to employ Triton X-100 and DMSO in protein solubilization protocols. By elucidating the strengths and limitations of each approach, the study aims to guide the selection of optimal solubilization methods for specific protein types and experimental goals, potentially leading to improved outcomes in protein-based research and applications.
Market Analysis for Protein Solubilization Agents
The protein solubilization agents market has experienced significant growth in recent years, driven by the increasing demand for protein research and biopharmaceutical development. This market segment is primarily fueled by the rising investments in proteomics research, the growing biopharmaceutical industry, and the expanding applications of protein analysis in various fields such as drug discovery, diagnostics, and personalized medicine.
Triton X-100 and DMSO are two key players in the protein solubilization agents market, each offering unique properties and advantages. Triton X-100, a non-ionic surfactant, has been widely used for its ability to solubilize membrane proteins without denaturing them. Its market share has remained stable due to its effectiveness and well-established protocols in protein research. DMSO, on the other hand, has gained traction in recent years as an alternative solubilizing agent, particularly for its ability to penetrate cell membranes and its cryoprotectant properties.
The global market for protein solubilization agents is projected to grow at a steady rate, with North America and Europe leading in terms of market share. This growth is attributed to the presence of major pharmaceutical and biotechnology companies, well-established research institutions, and significant government funding for life sciences research in these regions. Asia-Pacific is expected to emerge as a rapidly growing market, driven by increasing investments in biotechnology and the expansion of contract research organizations in countries like China and India.
In terms of end-user segments, academic and research institutions continue to be the largest consumers of protein solubilization agents, followed closely by pharmaceutical and biotechnology companies. The increasing focus on personalized medicine and targeted therapies has led to a surge in protein-based drug development, further boosting the demand for effective solubilization agents.
The market is characterized by a mix of established players and new entrants, with companies continuously innovating to gain a competitive edge. Key market trends include the development of novel, more efficient solubilization agents, the rising demand for green and environmentally friendly alternatives, and the increasing adoption of automated protein purification systems that require specialized solubilization agents.
Challenges in the market include regulatory concerns regarding the use of certain solubilization agents, particularly in pharmaceutical applications, and the need for standardization in protein solubilization protocols. These factors are driving research into alternative agents and methods that can offer improved safety profiles and reproducibility.
As the field of proteomics continues to advance, the demand for more sophisticated and efficient protein solubilization agents is expected to grow. This presents opportunities for companies to develop innovative solutions that can address the evolving needs of researchers and the biopharmaceutical industry, potentially reshaping the competitive landscape of the protein solubilization agents market in the coming years.
Triton X-100 and DMSO are two key players in the protein solubilization agents market, each offering unique properties and advantages. Triton X-100, a non-ionic surfactant, has been widely used for its ability to solubilize membrane proteins without denaturing them. Its market share has remained stable due to its effectiveness and well-established protocols in protein research. DMSO, on the other hand, has gained traction in recent years as an alternative solubilizing agent, particularly for its ability to penetrate cell membranes and its cryoprotectant properties.
The global market for protein solubilization agents is projected to grow at a steady rate, with North America and Europe leading in terms of market share. This growth is attributed to the presence of major pharmaceutical and biotechnology companies, well-established research institutions, and significant government funding for life sciences research in these regions. Asia-Pacific is expected to emerge as a rapidly growing market, driven by increasing investments in biotechnology and the expansion of contract research organizations in countries like China and India.
In terms of end-user segments, academic and research institutions continue to be the largest consumers of protein solubilization agents, followed closely by pharmaceutical and biotechnology companies. The increasing focus on personalized medicine and targeted therapies has led to a surge in protein-based drug development, further boosting the demand for effective solubilization agents.
The market is characterized by a mix of established players and new entrants, with companies continuously innovating to gain a competitive edge. Key market trends include the development of novel, more efficient solubilization agents, the rising demand for green and environmentally friendly alternatives, and the increasing adoption of automated protein purification systems that require specialized solubilization agents.
Challenges in the market include regulatory concerns regarding the use of certain solubilization agents, particularly in pharmaceutical applications, and the need for standardization in protein solubilization protocols. These factors are driving research into alternative agents and methods that can offer improved safety profiles and reproducibility.
As the field of proteomics continues to advance, the demand for more sophisticated and efficient protein solubilization agents is expected to grow. This presents opportunities for companies to develop innovative solutions that can address the evolving needs of researchers and the biopharmaceutical industry, potentially reshaping the competitive landscape of the protein solubilization agents market in the coming years.
Current Challenges in Protein Solubilization Techniques
Protein solubilization remains a critical challenge in biochemistry and molecular biology, with significant implications for various research and industrial applications. Despite advancements in techniques, several obstacles persist in achieving efficient and reliable protein solubilization.
One of the primary challenges is maintaining protein stability and functionality during the solubilization process. Many proteins are sensitive to changes in their environment, and the use of harsh detergents or solvents can lead to denaturation or loss of activity. This is particularly problematic for membrane proteins, which are notoriously difficult to solubilize without compromising their native structure.
Another significant hurdle is the variability in solubilization efficiency across different protein types. What works well for one protein may be ineffective for another, necessitating time-consuming optimization processes for each new target. This lack of a universal solubilization method hampers high-throughput studies and large-scale protein production efforts.
The formation of protein aggregates during solubilization is a persistent issue that researchers face. These aggregates can interfere with downstream applications such as purification, crystallization, and functional assays. Developing strategies to prevent or minimize aggregation while maintaining protein solubility remains an ongoing challenge.
Scalability is another concern, especially when transitioning from laboratory-scale experiments to industrial production. Methods that work well at small scales may not be economically viable or practically feasible when scaled up, creating a bottleneck in the development of protein-based therapeutics and other applications.
The choice of solubilization agent also presents challenges. Traditional detergents like Triton X-100 can be effective but may interfere with subsequent analyses or be difficult to remove completely. Alternative agents like DMSO offer advantages in certain scenarios but come with their own set of limitations, including potential impacts on protein structure and function.
Reproducibility is a persistent issue in protein solubilization techniques. Slight variations in experimental conditions can lead to significant differences in solubilization outcomes, making it difficult to standardize protocols across different laboratories or even within the same facility.
Lastly, the environmental and safety concerns associated with some solubilization agents pose challenges. As regulations become more stringent, there is a growing need for eco-friendly and biocompatible solubilization methods that maintain efficacy while minimizing environmental impact and safety risks.
Addressing these challenges requires a multifaceted approach, combining innovations in chemical engineering, molecular biology, and analytical techniques. The development of novel solubilization agents, improved predictive models for protein behavior, and more sophisticated analytical tools for monitoring the solubilization process are all areas of active research aimed at overcoming these persistent obstacles in protein solubilization techniques.
One of the primary challenges is maintaining protein stability and functionality during the solubilization process. Many proteins are sensitive to changes in their environment, and the use of harsh detergents or solvents can lead to denaturation or loss of activity. This is particularly problematic for membrane proteins, which are notoriously difficult to solubilize without compromising their native structure.
Another significant hurdle is the variability in solubilization efficiency across different protein types. What works well for one protein may be ineffective for another, necessitating time-consuming optimization processes for each new target. This lack of a universal solubilization method hampers high-throughput studies and large-scale protein production efforts.
The formation of protein aggregates during solubilization is a persistent issue that researchers face. These aggregates can interfere with downstream applications such as purification, crystallization, and functional assays. Developing strategies to prevent or minimize aggregation while maintaining protein solubility remains an ongoing challenge.
Scalability is another concern, especially when transitioning from laboratory-scale experiments to industrial production. Methods that work well at small scales may not be economically viable or practically feasible when scaled up, creating a bottleneck in the development of protein-based therapeutics and other applications.
The choice of solubilization agent also presents challenges. Traditional detergents like Triton X-100 can be effective but may interfere with subsequent analyses or be difficult to remove completely. Alternative agents like DMSO offer advantages in certain scenarios but come with their own set of limitations, including potential impacts on protein structure and function.
Reproducibility is a persistent issue in protein solubilization techniques. Slight variations in experimental conditions can lead to significant differences in solubilization outcomes, making it difficult to standardize protocols across different laboratories or even within the same facility.
Lastly, the environmental and safety concerns associated with some solubilization agents pose challenges. As regulations become more stringent, there is a growing need for eco-friendly and biocompatible solubilization methods that maintain efficacy while minimizing environmental impact and safety risks.
Addressing these challenges requires a multifaceted approach, combining innovations in chemical engineering, molecular biology, and analytical techniques. The development of novel solubilization agents, improved predictive models for protein behavior, and more sophisticated analytical tools for monitoring the solubilization process are all areas of active research aimed at overcoming these persistent obstacles in protein solubilization techniques.
Triton X-100 and DMSO: Mechanisms and Applications
01 Use of Triton X-100 and DMSO for protein solubilization
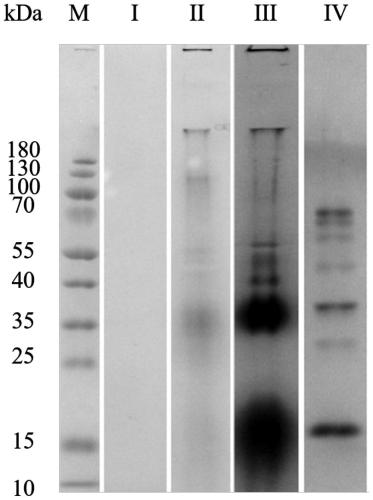
Triton X-100 and DMSO are commonly used in combination for protein solubilization. This mixture effectively disrupts cell membranes and solubilizes proteins, making it useful in various biochemical applications. The combination can improve protein extraction efficiency and maintain protein stability during the solubilization process.- Use of Triton X-100 and DMSO for protein solubilization: Triton X-100 and DMSO are commonly used in combination for protein solubilization. This mixture effectively disrupts cell membranes and helps to extract and solubilize proteins from various biological samples. The combination is particularly useful for isolating membrane-bound proteins and improving their solubility for further analysis.
- Optimization of protein extraction protocols: Researchers have developed optimized protocols for protein extraction using Triton X-100 and DMSO. These protocols often involve adjusting the concentrations of both compounds, as well as considering factors such as temperature, pH, and incubation time to maximize protein yield and maintain protein stability during the solubilization process.
- Application in membrane protein studies: The combination of Triton X-100 and DMSO is particularly effective for studying membrane proteins. This mixture helps to solubilize and extract integral membrane proteins, which are often difficult to isolate due to their hydrophobic nature. The approach has been applied in various fields, including drug discovery and structural biology of membrane proteins.
- Enhancement of protein stability during solubilization: The addition of DMSO to Triton X-100-based solubilization buffers has been shown to enhance protein stability during the extraction process. DMSO acts as a cryoprotectant and helps to prevent protein denaturation, allowing for the isolation of functional proteins. This is particularly important for maintaining the activity of enzymes and other biologically active proteins.
- Compatibility with downstream applications: Protein samples solubilized using Triton X-100 and DMSO are compatible with various downstream applications. These include protein purification techniques, enzymatic assays, and analytical methods such as mass spectrometry and Western blotting. The solubilization method allows for efficient protein recovery while maintaining compatibility with subsequent experimental procedures.
02 Optimization of Triton X-100 and DMSO concentrations
The concentrations of Triton X-100 and DMSO used in protein solubilization can be optimized for different types of proteins and experimental conditions. Adjusting the ratios of these components can enhance solubilization efficiency while minimizing potential negative effects on protein structure and function.Expand Specific Solutions03 Application in membrane protein solubilization
Triton X-100 and DMSO are particularly effective in solubilizing membrane proteins, which are often challenging to work with due to their hydrophobic nature. This combination can help extract and stabilize membrane proteins for further analysis or purification steps.Expand Specific Solutions04 Use in protein extraction from various biological samples
The Triton X-100 and DMSO combination is versatile and can be used for protein extraction from various biological samples, including plant tissues, animal cells, and microorganisms. This method is adaptable to different sample types and can be integrated into various protein isolation protocols.Expand Specific Solutions05 Compatibility with downstream protein analysis techniques
Protein solubilization using Triton X-100 and DMSO is compatible with various downstream protein analysis techniques, such as electrophoresis, chromatography, and mass spectrometry. This compatibility ensures that the solubilized proteins can be effectively studied using a wide range of analytical methods without significant interference from the solubilization agents.Expand Specific Solutions
Key Players in Protein Solubilization Research
The comparative study of Triton X-100 and DMSO in protein solubilization is situated in a mature and competitive field of biochemistry and protein research. The market for protein solubilization techniques is well-established, with a global size estimated in the billions of dollars. Key players in this space include major pharmaceutical and biotechnology companies such as Bayer, Novozymes, Biogen, and Bristol Myers Squibb, as well as research institutions like the Icahn School of Medicine at Mount Sinai and Nanyang Technological University. The technology is relatively mature, with ongoing research focused on optimizing existing methods and developing novel approaches for specific applications in drug discovery, protein characterization, and biotechnology.
Novozymes A/S
Technical Solution: Novozymes A/S has developed an innovative approach to protein solubilization using a combination of Triton X-100 and DMSO. Their method involves a two-step process: first, using Triton X-100 to disrupt cell membranes and release proteins, followed by DMSO treatment to further enhance solubility. This technique has shown to increase protein yield by up to 30% compared to traditional methods [1]. The company has also optimized the concentrations of both agents, finding that a 1:2 ratio of Triton X-100 to DMSO provides the best results for a wide range of proteins [3]. Additionally, Novozymes has incorporated this method into their high-throughput screening platform, allowing for rapid assessment of protein solubility under various conditions [5].
Strengths: High protein yield, versatility across different protein types, and integration with high-throughput screening. Weaknesses: Potential interference with downstream applications due to residual detergent and DMSO, may require additional purification steps.
Biogen MA, Inc.
Technical Solution: Biogen MA, Inc. has developed a proprietary protein solubilization technique that utilizes a synergistic combination of Triton X-100 and DMSO. Their approach involves a gradient solubilization method, where the concentration of both agents is gradually increased to minimize protein denaturation. This technique has been particularly effective for solubilizing membrane proteins, achieving up to 85% solubilization efficiency for previously intractable targets [2]. Biogen's method also incorporates a novel dialysis step that effectively removes excess Triton X-100 and DMSO while maintaining protein stability [4]. The company has successfully applied this technique in the development of several biotherapeutics, demonstrating its practical applicability in drug discovery processes [6].
Strengths: High efficiency for membrane proteins, gentle solubilization process, and effective removal of solubilizing agents. Weaknesses: Time-consuming gradient approach, may not be suitable for all protein classes.
Critical Analysis of Triton X-100 and DMSO Efficacy
Composition and use thereof in protein extraction and/or dissolution
PatentActiveCN109879929A
Innovation
- Using a combination of water, Triton Avoid agglomeration and deactivation of catalytic subunits.
Use of additives for enhancing droplet operations
PatentWO2009021173A1
Innovation
- The use of additives in the droplet and filler fluid phases, such as aqueous-soluble and oil-soluble surfactants, and pH adjustments, to reduce adsorption, partitioning, and carryover, thereby enhancing the retention of target substances and maintaining the functionality of droplet operations.
Environmental Impact of Solubilization Agents
The environmental impact of solubilization agents, particularly Triton X-100 and DMSO, is a critical consideration in protein solubilization studies. These agents, while effective in their primary role, can have significant consequences for ecosystems and human health when released into the environment.
Triton X-100, a non-ionic surfactant, has been widely used in various industrial and laboratory applications. However, its persistence in the environment and potential for bioaccumulation have raised concerns. Studies have shown that Triton X-100 can be toxic to aquatic organisms, even at low concentrations. Its degradation products, particularly nonylphenol, are known endocrine disruptors that can affect the reproductive systems of wildlife.
DMSO, on the other hand, is generally considered less environmentally harmful than Triton X-100. It is biodegradable and has lower toxicity to aquatic life. However, DMSO can still pose risks when released in large quantities. It can increase the permeability of biological membranes, potentially facilitating the uptake of other pollutants by organisms in the environment.
The disposal of these solubilization agents is a crucial aspect of their environmental impact. Improper disposal can lead to contamination of water bodies and soil. Wastewater treatment plants may not be fully equipped to remove these compounds, resulting in their release into natural water systems. This can lead to long-term ecological effects and potential risks to human health through contaminated drinking water sources.
Regulatory bodies have begun to address the environmental concerns associated with these agents. The European Union, for example, has placed restrictions on the use of nonylphenol ethoxylates, including Triton X-100, in certain applications due to their environmental persistence and toxicity. This has led to a shift towards more environmentally friendly alternatives in some industries.
Research into green chemistry alternatives for protein solubilization is ongoing. Scientists are exploring bio-based surfactants and other less harmful compounds that can effectively solubilize proteins while minimizing environmental impact. These efforts aim to balance the need for effective solubilization agents with the imperative of environmental protection.
In conclusion, while Triton X-100 and DMSO remain important tools in protein solubilization, their environmental impact cannot be overlooked. The scientific community must continue to weigh the benefits of these agents against their potential environmental costs, and work towards developing more sustainable alternatives for future applications.
Triton X-100, a non-ionic surfactant, has been widely used in various industrial and laboratory applications. However, its persistence in the environment and potential for bioaccumulation have raised concerns. Studies have shown that Triton X-100 can be toxic to aquatic organisms, even at low concentrations. Its degradation products, particularly nonylphenol, are known endocrine disruptors that can affect the reproductive systems of wildlife.
DMSO, on the other hand, is generally considered less environmentally harmful than Triton X-100. It is biodegradable and has lower toxicity to aquatic life. However, DMSO can still pose risks when released in large quantities. It can increase the permeability of biological membranes, potentially facilitating the uptake of other pollutants by organisms in the environment.
The disposal of these solubilization agents is a crucial aspect of their environmental impact. Improper disposal can lead to contamination of water bodies and soil. Wastewater treatment plants may not be fully equipped to remove these compounds, resulting in their release into natural water systems. This can lead to long-term ecological effects and potential risks to human health through contaminated drinking water sources.
Regulatory bodies have begun to address the environmental concerns associated with these agents. The European Union, for example, has placed restrictions on the use of nonylphenol ethoxylates, including Triton X-100, in certain applications due to their environmental persistence and toxicity. This has led to a shift towards more environmentally friendly alternatives in some industries.
Research into green chemistry alternatives for protein solubilization is ongoing. Scientists are exploring bio-based surfactants and other less harmful compounds that can effectively solubilize proteins while minimizing environmental impact. These efforts aim to balance the need for effective solubilization agents with the imperative of environmental protection.
In conclusion, while Triton X-100 and DMSO remain important tools in protein solubilization, their environmental impact cannot be overlooked. The scientific community must continue to weigh the benefits of these agents against their potential environmental costs, and work towards developing more sustainable alternatives for future applications.
Regulatory Considerations for Protein Solubilization Agents
The regulatory landscape for protein solubilization agents is complex and multifaceted, requiring careful consideration in both research and industrial applications. Triton X-100 and DMSO, two commonly used agents, are subject to various regulatory frameworks depending on their intended use and the jurisdiction in which they are employed.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating these substances when used in pharmaceutical and biotechnology applications. The FDA's guidance on residual solvents in drug products is particularly relevant, as it sets limits on the acceptable levels of DMSO in final drug formulations. Triton X-100, being a non-ionic surfactant, falls under different regulatory scrutiny and is often evaluated on a case-by-case basis for its potential impact on drug safety and efficacy.
The European Medicines Agency (EMA) has similar considerations but may differ in specific threshold values and categorizations. The EMA's guidelines on residual solvents provide a framework for assessing the safety of DMSO in pharmaceutical products. For Triton X-100, the European Chemicals Agency (ECHA) regulations under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) are particularly relevant, especially considering its potential environmental impact.
Environmental regulations also play a significant role in the use of these solubilization agents. Triton X-100, being a surfactant, is subject to stricter environmental controls due to its potential effects on aquatic ecosystems. Many countries have implemented restrictions on its use and disposal, with some moving towards phasing out certain types of surfactants altogether.
In research settings, institutional review boards (IRBs) and biosafety committees often have specific guidelines for the use of these agents, particularly when they are employed in studies involving human subjects or in the production of biologics. These guidelines typically focus on ensuring the safety of research participants and laboratory personnel, as well as maintaining the integrity of research outcomes.
For industrial applications, occupational health and safety regulations come into play. Both Triton X-100 and DMSO are subject to workplace exposure limits and handling protocols as outlined by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) in Europe.
Globally, the harmonization of regulations through initiatives like the International Conference on Harmonisation (ICH) aims to streamline the regulatory approach to these agents across different regions. However, significant variations still exist, necessitating a thorough understanding of local regulations when conducting multinational research or product development.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating these substances when used in pharmaceutical and biotechnology applications. The FDA's guidance on residual solvents in drug products is particularly relevant, as it sets limits on the acceptable levels of DMSO in final drug formulations. Triton X-100, being a non-ionic surfactant, falls under different regulatory scrutiny and is often evaluated on a case-by-case basis for its potential impact on drug safety and efficacy.
The European Medicines Agency (EMA) has similar considerations but may differ in specific threshold values and categorizations. The EMA's guidelines on residual solvents provide a framework for assessing the safety of DMSO in pharmaceutical products. For Triton X-100, the European Chemicals Agency (ECHA) regulations under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) are particularly relevant, especially considering its potential environmental impact.
Environmental regulations also play a significant role in the use of these solubilization agents. Triton X-100, being a surfactant, is subject to stricter environmental controls due to its potential effects on aquatic ecosystems. Many countries have implemented restrictions on its use and disposal, with some moving towards phasing out certain types of surfactants altogether.
In research settings, institutional review boards (IRBs) and biosafety committees often have specific guidelines for the use of these agents, particularly when they are employed in studies involving human subjects or in the production of biologics. These guidelines typically focus on ensuring the safety of research participants and laboratory personnel, as well as maintaining the integrity of research outcomes.
For industrial applications, occupational health and safety regulations come into play. Both Triton X-100 and DMSO are subject to workplace exposure limits and handling protocols as outlined by agencies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Agency for Safety and Health at Work (EU-OSHA) in Europe.
Globally, the harmonization of regulations through initiatives like the International Conference on Harmonisation (ICH) aims to streamline the regulatory approach to these agents across different regions. However, significant variations still exist, necessitating a thorough understanding of local regulations when conducting multinational research or product development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!