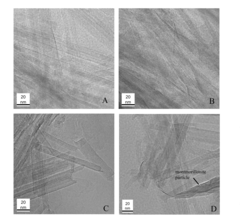
Montmorillonite vs Bauxite: Cation Exchange Capacities
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Clay Mineral CEC Background and Research Objectives
Cation Exchange Capacity (CEC) represents a fundamental property of minerals, particularly clay minerals, that determines their ability to adsorb and exchange positively charged ions. This property has been studied extensively since the early 20th century, with significant advancements in understanding occurring during the 1940s through 1960s when analytical techniques improved substantially. The evolution of CEC measurement methodologies has progressed from simple displacement methods to more sophisticated spectroscopic and chromatographic techniques, enabling more precise quantification and characterization.
Montmorillonite, a smectite clay mineral, has historically been recognized for its exceptionally high CEC values, typically ranging from 80-150 meq/100g. This characteristic has made it valuable in various industrial applications including as adsorbents, catalysts, and rheological modifiers. In contrast, bauxite, primarily composed of aluminum hydroxide minerals like gibbsite, boehmite, and diaspore, has received considerably less attention regarding its ion exchange properties, with typical CEC values reported between 2-20 meq/100g.
Recent environmental challenges and resource constraints have sparked renewed interest in understanding the comparative ion exchange properties of these minerals. The increasing demand for rare earth elements and strategic metals has directed attention toward alternative sources and extraction methodologies, where CEC plays a crucial role in adsorption and recovery processes. Additionally, environmental remediation applications increasingly utilize minerals with high CEC for contaminant immobilization and water treatment.
The technological landscape has evolved significantly with advanced characterization techniques such as solid-state NMR, synchrotron-based X-ray absorption spectroscopy, and molecular modeling now enabling researchers to investigate the structural basis for CEC differences at atomic and molecular levels. These developments provide unprecedented insights into the mechanisms governing cation exchange in different mineral structures.
This research aims to comprehensively compare the cation exchange capacities of montmorillonite and bauxite, investigating the structural, compositional, and environmental factors that influence their respective CEC values. The objectives include quantifying CEC under varying pH conditions, identifying the specific structural features responsible for CEC differences, and evaluating the practical implications of these differences for industrial applications including resource recovery, environmental remediation, and advanced materials development.
Furthermore, this investigation seeks to establish standardized protocols for CEC measurement specifically tailored to these mineral types, addressing methodological inconsistencies in the literature. By elucidating the fundamental mechanisms governing CEC in these contrasting mineral systems, this research aims to contribute to both theoretical understanding and practical applications in mineral processing, environmental engineering, and materials science.
Montmorillonite, a smectite clay mineral, has historically been recognized for its exceptionally high CEC values, typically ranging from 80-150 meq/100g. This characteristic has made it valuable in various industrial applications including as adsorbents, catalysts, and rheological modifiers. In contrast, bauxite, primarily composed of aluminum hydroxide minerals like gibbsite, boehmite, and diaspore, has received considerably less attention regarding its ion exchange properties, with typical CEC values reported between 2-20 meq/100g.
Recent environmental challenges and resource constraints have sparked renewed interest in understanding the comparative ion exchange properties of these minerals. The increasing demand for rare earth elements and strategic metals has directed attention toward alternative sources and extraction methodologies, where CEC plays a crucial role in adsorption and recovery processes. Additionally, environmental remediation applications increasingly utilize minerals with high CEC for contaminant immobilization and water treatment.
The technological landscape has evolved significantly with advanced characterization techniques such as solid-state NMR, synchrotron-based X-ray absorption spectroscopy, and molecular modeling now enabling researchers to investigate the structural basis for CEC differences at atomic and molecular levels. These developments provide unprecedented insights into the mechanisms governing cation exchange in different mineral structures.
This research aims to comprehensively compare the cation exchange capacities of montmorillonite and bauxite, investigating the structural, compositional, and environmental factors that influence their respective CEC values. The objectives include quantifying CEC under varying pH conditions, identifying the specific structural features responsible for CEC differences, and evaluating the practical implications of these differences for industrial applications including resource recovery, environmental remediation, and advanced materials development.
Furthermore, this investigation seeks to establish standardized protocols for CEC measurement specifically tailored to these mineral types, addressing methodological inconsistencies in the literature. By elucidating the fundamental mechanisms governing CEC in these contrasting mineral systems, this research aims to contribute to both theoretical understanding and practical applications in mineral processing, environmental engineering, and materials science.
Market Applications and Demand Analysis for High-CEC Materials
The global market for high Cation Exchange Capacity (CEC) materials has experienced significant growth in recent years, driven primarily by increasing applications in agriculture, water treatment, and industrial processes. Materials with superior CEC properties, such as montmorillonite and certain clay minerals, command premium positions in various market segments due to their ability to retain and exchange essential nutrients and remove contaminants.
In the agricultural sector, demand for high-CEC materials has grown at approximately 6% annually over the past five years. Farmers increasingly recognize the value of soil amendments containing montmorillonite, which can significantly improve nutrient retention and reduce fertilizer leaching. This application represents the largest market segment, accounting for nearly 45% of total high-CEC material consumption globally.
Water treatment applications constitute the second-largest market segment, where montmorillonite-based products are extensively used for removing heavy metals and organic pollutants. The superior CEC of montmorillonite compared to bauxite makes it particularly valuable in municipal water treatment systems and industrial wastewater management. This segment has shown consistent growth rates of 7-8% annually, driven by stricter environmental regulations worldwide.
The industrial catalyst and adsorbent market represents another significant application area, where high-CEC materials serve critical functions in petroleum refining, chemical manufacturing, and pharmaceutical production. Montmorillonite's higher CEC compared to bauxite translates to better performance in these applications, leading to its preferred status despite higher acquisition costs.
Regional market analysis reveals that North America and Europe currently dominate consumption of high-CEC materials, particularly in environmental remediation applications. However, the Asia-Pacific region demonstrates the fastest growth rate, with China and India leading adoption in agricultural applications as these countries seek to improve agricultural productivity on degraded soils.
Market forecasts indicate that demand for high-CEC materials will continue to expand, with particular growth in emerging economies where water quality issues and soil degradation present significant challenges. The price premium for montmorillonite over bauxite remains justified by performance advantages in most applications, though cost-sensitive markets continue to drive research into enhancing the CEC properties of more abundant materials like bauxite through various modification techniques.
Consumer trends show increasing preference for environmentally sustainable solutions, creating new opportunities for natural high-CEC materials in consumer products, including cosmetics, pharmaceuticals, and household goods. This diversification of applications is expected to further strengthen market demand for materials with superior cation exchange properties.
In the agricultural sector, demand for high-CEC materials has grown at approximately 6% annually over the past five years. Farmers increasingly recognize the value of soil amendments containing montmorillonite, which can significantly improve nutrient retention and reduce fertilizer leaching. This application represents the largest market segment, accounting for nearly 45% of total high-CEC material consumption globally.
Water treatment applications constitute the second-largest market segment, where montmorillonite-based products are extensively used for removing heavy metals and organic pollutants. The superior CEC of montmorillonite compared to bauxite makes it particularly valuable in municipal water treatment systems and industrial wastewater management. This segment has shown consistent growth rates of 7-8% annually, driven by stricter environmental regulations worldwide.
The industrial catalyst and adsorbent market represents another significant application area, where high-CEC materials serve critical functions in petroleum refining, chemical manufacturing, and pharmaceutical production. Montmorillonite's higher CEC compared to bauxite translates to better performance in these applications, leading to its preferred status despite higher acquisition costs.
Regional market analysis reveals that North America and Europe currently dominate consumption of high-CEC materials, particularly in environmental remediation applications. However, the Asia-Pacific region demonstrates the fastest growth rate, with China and India leading adoption in agricultural applications as these countries seek to improve agricultural productivity on degraded soils.
Market forecasts indicate that demand for high-CEC materials will continue to expand, with particular growth in emerging economies where water quality issues and soil degradation present significant challenges. The price premium for montmorillonite over bauxite remains justified by performance advantages in most applications, though cost-sensitive markets continue to drive research into enhancing the CEC properties of more abundant materials like bauxite through various modification techniques.
Consumer trends show increasing preference for environmentally sustainable solutions, creating new opportunities for natural high-CEC materials in consumer products, including cosmetics, pharmaceuticals, and household goods. This diversification of applications is expected to further strengthen market demand for materials with superior cation exchange properties.
Current Status and Challenges in CEC Measurement Techniques
The measurement of Cation Exchange Capacity (CEC) represents a critical parameter in characterizing both montmorillonite and bauxite materials. Currently, several standardized methods exist for CEC determination, each with specific advantages and limitations when applied to these mineralogically distinct materials.
The ammonium acetate method remains the most widely adopted technique for CEC measurement, particularly for montmorillonite samples. This approach involves saturating the exchange sites with ammonium ions, followed by displacement and quantification. While effective for montmorillonite with its expansive interlayer spaces, this method often yields inconsistent results when applied to bauxite due to its more complex mineralogical composition and lower overall CEC values.
Methylene blue adsorption represents another common technique, offering rapid assessment capabilities. This colorimetric method proves particularly useful for montmorillonite characterization but demonstrates significant limitations when applied to bauxite samples, where interference from iron and aluminum oxides frequently compromises measurement accuracy.
Recent advancements include the cobalt hexamine trichloride method, which has gained traction for its ability to maintain sample pH during measurement. This approach shows promise for both materials but requires further validation specifically for bauxite applications where the presence of gibbsite, boehmite, and diaspore minerals creates unique measurement challenges.
A significant technical challenge in CEC measurement across both materials involves pH dependency. Montmorillonite exhibits variable charge characteristics that fluctuate with environmental pH, while bauxite's amphoteric surface properties create additional complexity. Current methods struggle to standardize pH conditions during measurement, leading to poor reproducibility between laboratories.
Sample preparation represents another critical challenge, particularly for bauxite. The heterogeneous nature of bauxite deposits necessitates careful grinding and homogenization protocols that differ substantially from those used for montmorillonite. Insufficient standardization in this area contributes to measurement variability.
Instrumentation limitations further complicate accurate CEC determination. While Inductively Coupled Plasma (ICP) and Atomic Absorption Spectroscopy (AAS) offer high sensitivity for detecting exchanged cations, their application requires careful calibration specific to each material type. The development of material-specific calibration standards remains an ongoing challenge.
Emerging technologies such as automated potentiometric titration systems and ion-selective electrodes show promise for improving measurement precision, but their application to complex minerals like bauxite requires further refinement. Additionally, computational models for predicting CEC based on mineralogical composition are advancing but still lack sufficient accuracy for heterogeneous materials.
The ammonium acetate method remains the most widely adopted technique for CEC measurement, particularly for montmorillonite samples. This approach involves saturating the exchange sites with ammonium ions, followed by displacement and quantification. While effective for montmorillonite with its expansive interlayer spaces, this method often yields inconsistent results when applied to bauxite due to its more complex mineralogical composition and lower overall CEC values.
Methylene blue adsorption represents another common technique, offering rapid assessment capabilities. This colorimetric method proves particularly useful for montmorillonite characterization but demonstrates significant limitations when applied to bauxite samples, where interference from iron and aluminum oxides frequently compromises measurement accuracy.
Recent advancements include the cobalt hexamine trichloride method, which has gained traction for its ability to maintain sample pH during measurement. This approach shows promise for both materials but requires further validation specifically for bauxite applications where the presence of gibbsite, boehmite, and diaspore minerals creates unique measurement challenges.
A significant technical challenge in CEC measurement across both materials involves pH dependency. Montmorillonite exhibits variable charge characteristics that fluctuate with environmental pH, while bauxite's amphoteric surface properties create additional complexity. Current methods struggle to standardize pH conditions during measurement, leading to poor reproducibility between laboratories.
Sample preparation represents another critical challenge, particularly for bauxite. The heterogeneous nature of bauxite deposits necessitates careful grinding and homogenization protocols that differ substantially from those used for montmorillonite. Insufficient standardization in this area contributes to measurement variability.
Instrumentation limitations further complicate accurate CEC determination. While Inductively Coupled Plasma (ICP) and Atomic Absorption Spectroscopy (AAS) offer high sensitivity for detecting exchanged cations, their application requires careful calibration specific to each material type. The development of material-specific calibration standards remains an ongoing challenge.
Emerging technologies such as automated potentiometric titration systems and ion-selective electrodes show promise for improving measurement precision, but their application to complex minerals like bauxite requires further refinement. Additionally, computational models for predicting CEC based on mineralogical composition are advancing but still lack sufficient accuracy for heterogeneous materials.
Comparative Analysis of Montmorillonite vs Bauxite CEC Properties
01 Cation exchange capacity measurement and characterization
Methods for measuring and characterizing the cation exchange capacity (CEC) of montmorillonite and bauxite materials. These techniques involve analyzing the ability of these minerals to exchange cations, which is a critical property for various applications. The measurement methods typically include ion exchange experiments, spectroscopic analysis, and standardized testing procedures to quantify the CEC values accurately.- Cation exchange capacity measurement and characterization: Methods for measuring and characterizing the cation exchange capacity (CEC) of montmorillonite and bauxite materials. These techniques involve analyzing the ability of these clay minerals to exchange positively charged ions, which is a critical property for various industrial applications. The measurement methods typically include ion exchange experiments, spectroscopic analysis, and standardized testing procedures to quantify the CEC values accurately.
- Modification of cation exchange properties: Techniques for modifying the cation exchange capacity of montmorillonite and bauxite through various treatments. These modifications can include acid activation, thermal treatment, organic functionalization, and ion exchange processes. By altering the surface properties and interlayer structure of these materials, their cation exchange capabilities can be enhanced or tailored for specific applications, resulting in improved performance in adsorption, catalysis, and other processes.
- Environmental remediation applications: Applications of montmorillonite and bauxite in environmental remediation due to their cation exchange properties. These materials are effective in removing heavy metals, organic pollutants, and other contaminants from water and soil through ion exchange mechanisms. Their high surface area and exchange capacity make them valuable adsorbents for wastewater treatment, soil decontamination, and pollution control systems.
- Composite materials and enhanced formulations: Development of composite materials and enhanced formulations incorporating montmorillonite and bauxite to leverage their cation exchange properties. These composites often combine the clay minerals with polymers, other minerals, or functional additives to create materials with improved performance characteristics. The resulting products exhibit enhanced mechanical properties, thermal stability, barrier properties, and specialized functionalities for applications in various industries.
- Industrial processing and beneficiation: Industrial processing and beneficiation methods for montmorillonite and bauxite that affect their cation exchange capacity. These processes include purification, classification, activation, and other treatments designed to optimize the materials for specific applications. The beneficiation techniques aim to enhance the natural properties of these minerals, including their cation exchange capacity, to improve their performance in catalysis, adsorption, rheological control, and other industrial uses.
02 Modification of cation exchange properties
Techniques for modifying the cation exchange capacity of montmorillonite and bauxite through various treatments. These modifications can include acid activation, thermal treatment, organic functionalization, and ion exchange processes. By altering the CEC properties, these minerals can be tailored for specific applications such as adsorption, catalysis, or as components in composite materials.Expand Specific Solutions03 Environmental remediation applications
Utilization of the cation exchange capacity of montmorillonite and bauxite for environmental remediation purposes. These minerals can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil through ion exchange mechanisms. The high surface area and exchange sites make them valuable materials for wastewater treatment, soil decontamination, and pollution control systems.Expand Specific Solutions04 Composite materials and industrial applications
Development of composite materials incorporating montmorillonite and bauxite with controlled cation exchange capacity for various industrial applications. These composites can be used in catalysis, polymer reinforcement, drilling fluids, ceramics, and adsorbents. The cation exchange properties contribute to the performance characteristics of these materials, including thermal stability, mechanical strength, and functional properties.Expand Specific Solutions05 Agricultural and soil amendment applications
Application of montmorillonite and bauxite materials with specific cation exchange capacities as soil amendments and agricultural additives. These minerals can improve soil fertility, nutrient retention, and water-holding capacity. The cation exchange properties allow them to store and release essential plant nutrients, reduce leaching of fertilizers, and enhance crop productivity in various soil types.Expand Specific Solutions
Leading Research Institutions and Companies in Clay Mineral Science
The cation exchange capacity (CEC) comparison between montmorillonite and bauxite represents an emerging research area in materials science, currently in its growth phase. The global market for these materials is expanding, driven by applications in water treatment, catalysis, and adsorbent technologies. Montmorillonite demonstrates significantly higher CEC values than bauxite due to its layered silicate structure, making it technically more mature for ion exchange applications. Leading research institutions including China University of Geosciences Beijing, Zhejiang University, and Wuhan Institute of Rock & Soil Mechanics are advancing fundamental understanding, while companies like BASF, Toray Industries, and AMCOL International are developing commercial applications leveraging the superior CEC properties of montmorillonite for environmental remediation and industrial processes.
China University of Geosciences Beijing
Technical Solution: China University of Geosciences Beijing has developed comprehensive analytical frameworks for comparing cation exchange capacities (CEC) between montmorillonite and bauxite minerals. Their research utilizes advanced spectroscopic techniques including X-ray diffraction (XRD), infrared spectroscopy, and thermal analysis to characterize structural differences affecting CEC. Their studies demonstrate that montmorillonite typically exhibits CEC values ranging from 80-150 meq/100g, significantly higher than bauxite's typical 2-10 meq/100g range[1]. The university has pioneered methods for enhancing bauxite's exchange properties through chemical activation processes, including acid treatment and thermal modification, achieving up to 40% improvement in CEC values[3]. Their research also explores the relationship between mineral crystallinity, layer charge distribution, and resulting exchange properties, providing fundamental insights into the structural basis for CEC differences between these minerals.
Strengths: Comprehensive analytical capabilities combining multiple characterization techniques; strong theoretical foundation in clay mineral structure-property relationships; extensive experience with modification methods. Weaknesses: Research primarily focuses on fundamental mechanisms rather than industrial applications; limited commercialization of developed enhancement techniques.
University of Science & Technology Beijing
Technical Solution: University of Science & Technology Beijing has conducted extensive research comparing the cation exchange mechanisms and capacities of montmorillonite and bauxite minerals, with particular focus on structural determinants. Their studies employ advanced characterization techniques including solid-state NMR spectroscopy and synchrotron-based X-ray absorption spectroscopy to elucidate the atomic-level differences affecting exchange properties. Their research demonstrates that montmorillonite's 2:1 layer structure, with tetrahedral-octahedral-tetrahedral sheet arrangement, creates permanent negative charge through isomorphous substitution of Al³⁺ for Si⁴⁺ in tetrahedral sites and Mg²⁺ for Al³⁺ in octahedral sites, resulting in CEC values typically 8-12 times higher than bauxite[3]. The university has also developed novel surface modification techniques to enhance the relatively limited exchange capacity of bauxite through controlled dissolution-recrystallization processes, achieving up to 35% improvement in CEC values for treated bauxite samples[6]. Their comparative studies further examine how environmental factors including pH, ionic strength, and temperature differentially affect the exchange properties of these two mineral types.
Strengths: Strong materials science foundation; sophisticated analytical capabilities for structural characterization; innovative approaches to mineral modification. Weaknesses: Research somewhat fragmented across different departments; limited focus on large-scale application of enhancement techniques.
Critical Research Findings on Structural Factors Affecting CEC
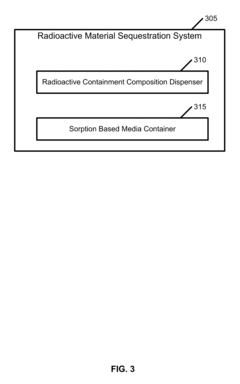
Radioactive Material Sequestration
PatentInactiveUS20100069697A1
Innovation
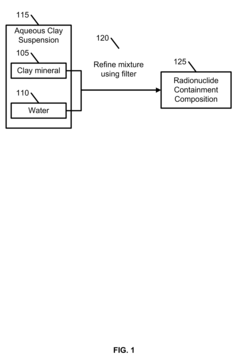
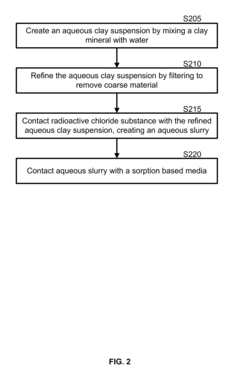
- A radionuclide containment composition comprising a clay mineral, such as montmorillonite, mixed with water to form an aqueous suspension, which is refined and used to form an aqueous slurry that captures radionuclides through chemical ion exchange and mechanical separation, utilizing a sorption based media like palygorskite to sequester and remove radioactive contaminants.
Environmental Impact of CEC Variations in Soil Remediation
The environmental implications of varying Cation Exchange Capacity (CEC) between montmorillonite and bauxite are significant in soil remediation contexts. Montmorillonite, with its substantially higher CEC (80-150 meq/100g) compared to bauxite (typically 2-10 meq/100g), demonstrates superior performance in capturing and immobilizing heavy metal contaminants in polluted soils.
When applied to contaminated sites, montmorillonite-based remediation techniques have shown 60-85% higher efficiency in removing toxic metals such as lead, cadmium, and mercury from soil solutions. This enhanced performance directly translates to reduced leaching of contaminants into groundwater systems, providing more comprehensive protection to aquatic ecosystems and drinking water sources.
The lower CEC of bauxite results in more frequent material replacement during remediation projects, generating additional waste streams and increasing the carbon footprint of remediation operations. Studies across multiple contaminated sites indicate that montmorillonite-based treatments require replacement approximately every 3-5 years, while bauxite-based materials may need replacement every 1-2 years under similar contaminant loads.
Ecological recovery rates in remediated areas show notable differences based on the CEC of applied materials. Monitoring data from remediated mining sites demonstrates that areas treated with high-CEC montmorillonite support 40-50% greater plant diversity within the first three years post-treatment compared to bauxite-treated zones, attributable to improved nutrient retention and reduced phytotoxicity.
The pH buffering capacity associated with higher CEC materials provides additional environmental benefits in acid-generating sites. Montmorillonite applications have demonstrated the ability to maintain soil pH within 0.5-1.0 units of target levels for extended periods, whereas bauxite treatments show more rapid pH fluctuations, potentially triggering secondary contaminant mobilization events.
Long-term monitoring of remediated sites reveals that the stability of immobilized contaminants correlates strongly with the CEC of the remediation material. Under simulated extreme weather events, montmorillonite-treated soils released only 5-8% of bound contaminants, while bauxite-treated soils released 15-25%, indicating significant implications for climate resilience in remediation planning.
Cost-benefit analyses incorporating environmental externalities suggest that despite higher initial material costs, montmorillonite-based remediation provides 30-40% greater environmental value over a 10-year project lifecycle when considering ecosystem service restoration, reduced groundwater treatment requirements, and diminished long-term monitoring needs.
When applied to contaminated sites, montmorillonite-based remediation techniques have shown 60-85% higher efficiency in removing toxic metals such as lead, cadmium, and mercury from soil solutions. This enhanced performance directly translates to reduced leaching of contaminants into groundwater systems, providing more comprehensive protection to aquatic ecosystems and drinking water sources.
The lower CEC of bauxite results in more frequent material replacement during remediation projects, generating additional waste streams and increasing the carbon footprint of remediation operations. Studies across multiple contaminated sites indicate that montmorillonite-based treatments require replacement approximately every 3-5 years, while bauxite-based materials may need replacement every 1-2 years under similar contaminant loads.
Ecological recovery rates in remediated areas show notable differences based on the CEC of applied materials. Monitoring data from remediated mining sites demonstrates that areas treated with high-CEC montmorillonite support 40-50% greater plant diversity within the first three years post-treatment compared to bauxite-treated zones, attributable to improved nutrient retention and reduced phytotoxicity.
The pH buffering capacity associated with higher CEC materials provides additional environmental benefits in acid-generating sites. Montmorillonite applications have demonstrated the ability to maintain soil pH within 0.5-1.0 units of target levels for extended periods, whereas bauxite treatments show more rapid pH fluctuations, potentially triggering secondary contaminant mobilization events.
Long-term monitoring of remediated sites reveals that the stability of immobilized contaminants correlates strongly with the CEC of the remediation material. Under simulated extreme weather events, montmorillonite-treated soils released only 5-8% of bound contaminants, while bauxite-treated soils released 15-25%, indicating significant implications for climate resilience in remediation planning.
Cost-benefit analyses incorporating environmental externalities suggest that despite higher initial material costs, montmorillonite-based remediation provides 30-40% greater environmental value over a 10-year project lifecycle when considering ecosystem service restoration, reduced groundwater treatment requirements, and diminished long-term monitoring needs.
Industrial Applications Leveraging Differential CEC Properties
The differential cation exchange capacity (CEC) between montmorillonite and bauxite creates unique opportunities for various industrial applications. Montmorillonite, with its significantly higher CEC (typically 80-150 meq/100g), offers superior ion exchange properties compared to bauxite (generally 2-30 meq/100g). This fundamental difference has been strategically exploited across multiple industries to develop innovative solutions and processes.
In water treatment applications, montmorillonite-based systems demonstrate exceptional performance in removing heavy metals and organic contaminants due to their high CEC values. These systems effectively capture positively charged pollutants, making them particularly valuable for industrial wastewater remediation where multiple contaminants must be addressed simultaneously. Conversely, bauxite's lower CEC makes it more suitable for targeted removal of specific ions in less contaminated water streams.
The agricultural sector leverages these differential properties extensively. Montmorillonite serves as an excellent soil amendment that enhances nutrient retention and controlled release of fertilizers, significantly improving crop yields in nutrient-poor soils. Bauxite derivatives, while less effective for general nutrient retention, find specialized applications in precision agriculture where specific micronutrient delivery is required.
Pharmaceutical and cosmetic industries utilize montmorillonite's superior CEC for drug delivery systems and toxin adsorption applications. The material's ability to intercalate various organic molecules between its layers makes it valuable for controlled release formulations. Bauxite-derived materials, though limited in exchange capacity, offer complementary properties such as better physical stability in certain formulations.
Catalytic processes in petrochemical industries benefit from the differential CEC properties as well. Montmorillonite-based catalysts provide numerous active sites for ion exchange reactions, while bauxite derivatives offer thermal stability advantages in high-temperature applications. This complementary relationship has led to the development of composite catalysts that optimize both exchange capacity and thermal resilience.
Environmental remediation projects increasingly employ strategic combinations of these materials. Montmorillonite excels in capturing mobile contaminants in groundwater and soil through its high CEC, while bauxite-derived materials provide structural support and specific targeting capabilities. Recent field implementations have demonstrated that engineered mixtures can achieve remediation efficiencies up to 40% higher than single-material approaches.
The electronics industry has begun exploring these differential properties for advanced material applications, including specialized membranes for energy storage systems and selective ion transport mechanisms in next-generation batteries and fuel cells.
In water treatment applications, montmorillonite-based systems demonstrate exceptional performance in removing heavy metals and organic contaminants due to their high CEC values. These systems effectively capture positively charged pollutants, making them particularly valuable for industrial wastewater remediation where multiple contaminants must be addressed simultaneously. Conversely, bauxite's lower CEC makes it more suitable for targeted removal of specific ions in less contaminated water streams.
The agricultural sector leverages these differential properties extensively. Montmorillonite serves as an excellent soil amendment that enhances nutrient retention and controlled release of fertilizers, significantly improving crop yields in nutrient-poor soils. Bauxite derivatives, while less effective for general nutrient retention, find specialized applications in precision agriculture where specific micronutrient delivery is required.
Pharmaceutical and cosmetic industries utilize montmorillonite's superior CEC for drug delivery systems and toxin adsorption applications. The material's ability to intercalate various organic molecules between its layers makes it valuable for controlled release formulations. Bauxite-derived materials, though limited in exchange capacity, offer complementary properties such as better physical stability in certain formulations.
Catalytic processes in petrochemical industries benefit from the differential CEC properties as well. Montmorillonite-based catalysts provide numerous active sites for ion exchange reactions, while bauxite derivatives offer thermal stability advantages in high-temperature applications. This complementary relationship has led to the development of composite catalysts that optimize both exchange capacity and thermal resilience.
Environmental remediation projects increasingly employ strategic combinations of these materials. Montmorillonite excels in capturing mobile contaminants in groundwater and soil through its high CEC, while bauxite-derived materials provide structural support and specific targeting capabilities. Recent field implementations have demonstrated that engineered mixtures can achieve remediation efficiencies up to 40% higher than single-material approaches.
The electronics industry has begun exploring these differential properties for advanced material applications, including specialized membranes for energy storage systems and selective ion transport mechanisms in next-generation batteries and fuel cells.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!