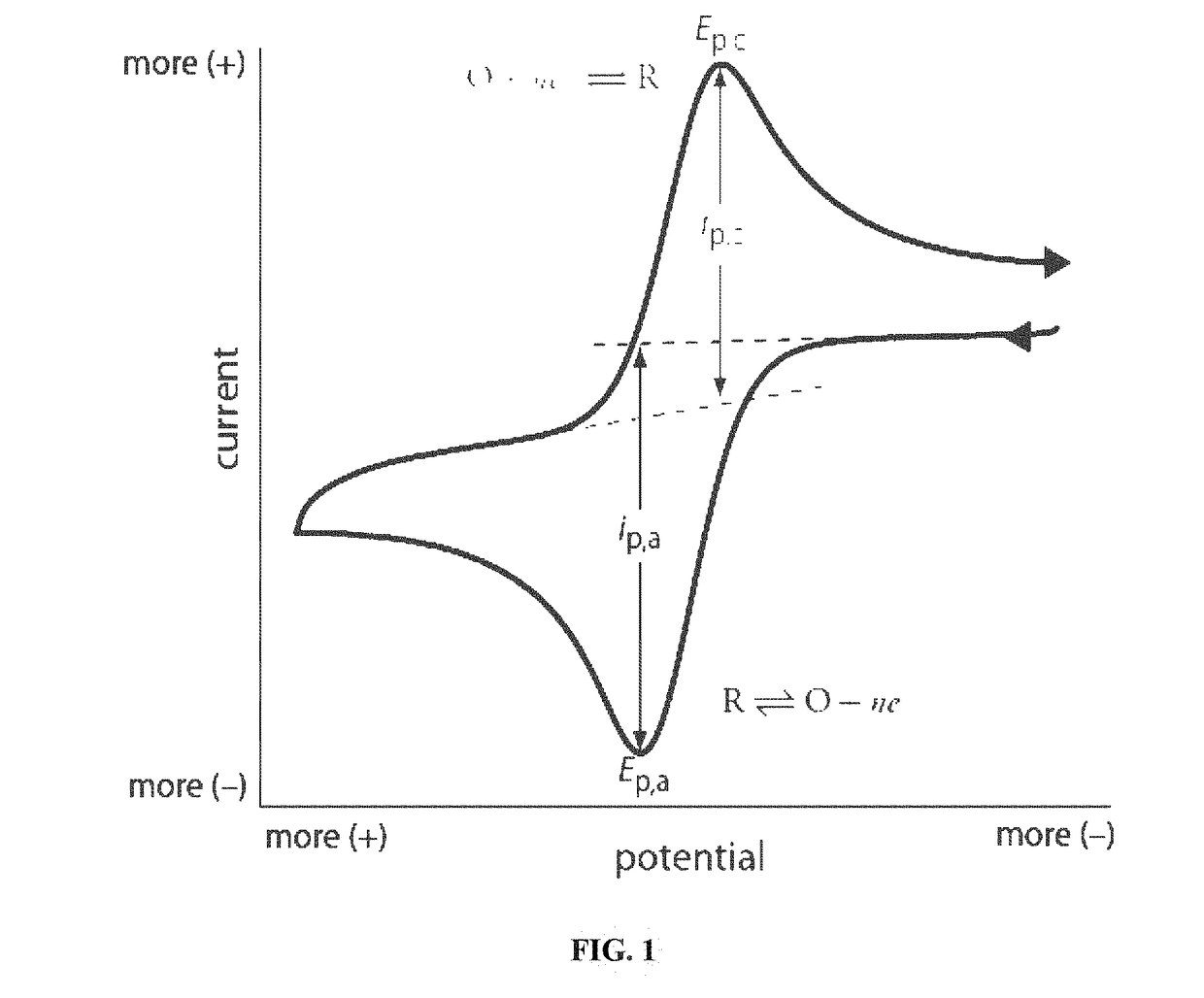
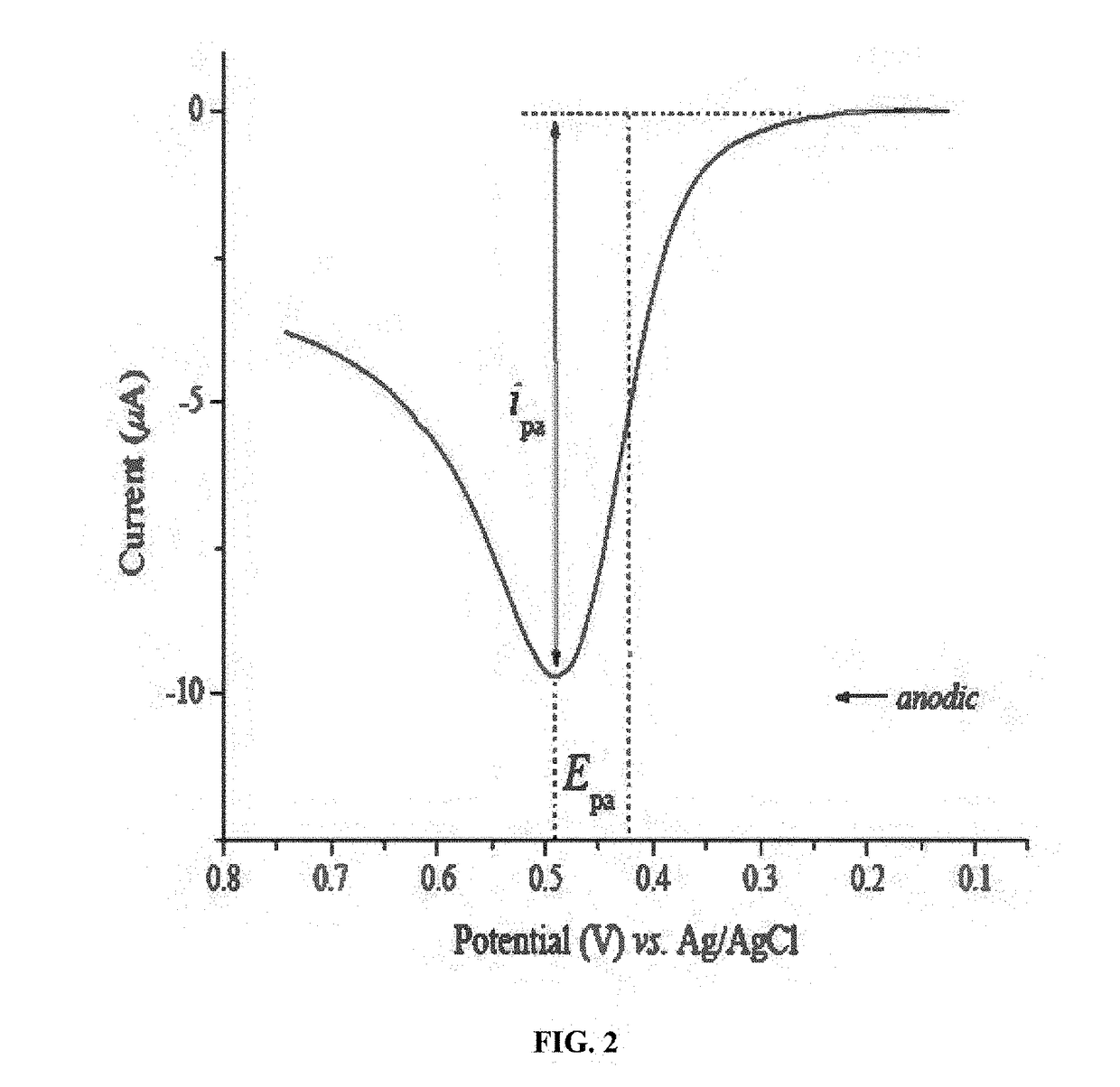
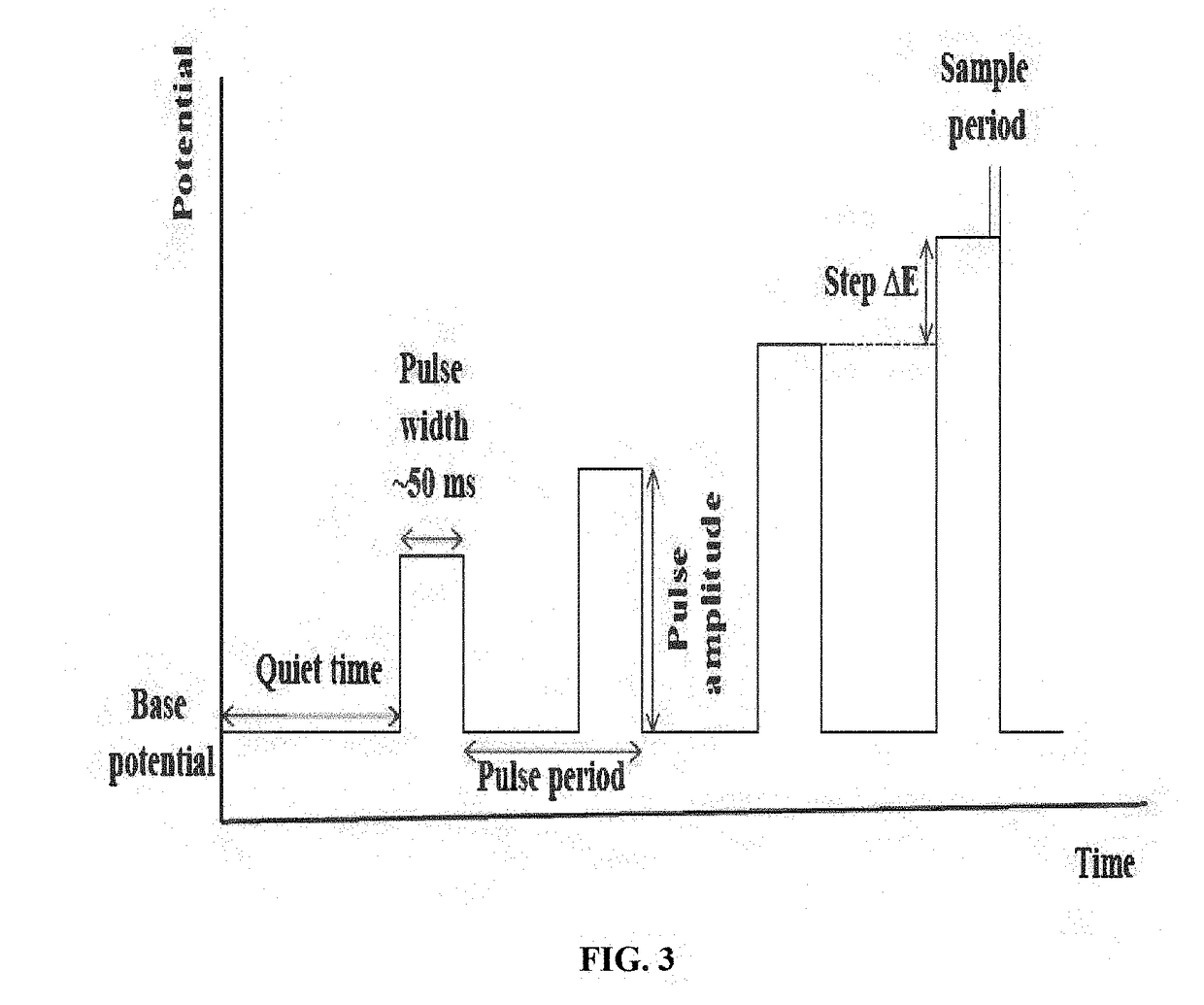
Cyclic Voltammetry Data Quality Checklist — Noise Sources, Reference Electrode Issues and Fixes
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Analysis Background and Objectives
Cyclic voltammetry (CV) has emerged as one of the most versatile and widely used electroanalytical techniques since its development in the early 1900s. The evolution of this technique has been marked by significant advancements in instrumentation, methodology, and theoretical understanding, transforming it from a rudimentary analytical tool to a sophisticated method capable of providing detailed insights into electrochemical processes.
The historical trajectory of CV began with basic polarographic methods and evolved through the introduction of triangular waveform potential sweeps in the 1950s. This innovation allowed researchers to observe both forward and reverse electrochemical reactions within a single experiment. The subsequent decades witnessed remarkable improvements in sensitivity, resolution, and data acquisition systems, culminating in today's highly precise digital potentiostats capable of detecting currents in the picoampere range.
Modern CV applications span numerous fields including energy storage, corrosion science, biosensing, materials characterization, and pharmaceutical analysis. The technique's ability to provide information about redox potentials, reaction mechanisms, kinetics, and mass transport phenomena makes it indispensable for both fundamental research and industrial applications.
Despite its widespread utility, CV data quality remains a persistent challenge. Noise interference, reference electrode instability, and various experimental artifacts can significantly compromise data integrity and lead to erroneous interpretations. These issues become particularly critical in applications requiring high precision, such as trace analysis or studies of fast electron transfer processes.
The primary technical objectives of addressing CV data quality issues include: establishing standardized protocols for noise identification and mitigation; developing robust solutions for reference electrode stability across diverse experimental conditions; creating comprehensive validation methodologies to ensure data reliability; and formulating clear guidelines for distinguishing between genuine electrochemical phenomena and instrumental artifacts.
Recent technological trends in this domain focus on automated data processing algorithms, advanced filtering techniques, and innovative cell designs that minimize external interference. Additionally, there is growing interest in machine learning approaches for real-time data quality assessment and correction, potentially revolutionizing how electrochemists ensure experimental validity.
By systematically addressing these challenges, researchers aim to enhance the reproducibility and reliability of CV measurements, ultimately expanding the technique's applicability to more complex systems and enabling more accurate quantitative analysis in fields ranging from energy research to biomedical diagnostics.
The historical trajectory of CV began with basic polarographic methods and evolved through the introduction of triangular waveform potential sweeps in the 1950s. This innovation allowed researchers to observe both forward and reverse electrochemical reactions within a single experiment. The subsequent decades witnessed remarkable improvements in sensitivity, resolution, and data acquisition systems, culminating in today's highly precise digital potentiostats capable of detecting currents in the picoampere range.
Modern CV applications span numerous fields including energy storage, corrosion science, biosensing, materials characterization, and pharmaceutical analysis. The technique's ability to provide information about redox potentials, reaction mechanisms, kinetics, and mass transport phenomena makes it indispensable for both fundamental research and industrial applications.
Despite its widespread utility, CV data quality remains a persistent challenge. Noise interference, reference electrode instability, and various experimental artifacts can significantly compromise data integrity and lead to erroneous interpretations. These issues become particularly critical in applications requiring high precision, such as trace analysis or studies of fast electron transfer processes.
The primary technical objectives of addressing CV data quality issues include: establishing standardized protocols for noise identification and mitigation; developing robust solutions for reference electrode stability across diverse experimental conditions; creating comprehensive validation methodologies to ensure data reliability; and formulating clear guidelines for distinguishing between genuine electrochemical phenomena and instrumental artifacts.
Recent technological trends in this domain focus on automated data processing algorithms, advanced filtering techniques, and innovative cell designs that minimize external interference. Additionally, there is growing interest in machine learning approaches for real-time data quality assessment and correction, potentially revolutionizing how electrochemists ensure experimental validity.
By systematically addressing these challenges, researchers aim to enhance the reproducibility and reliability of CV measurements, ultimately expanding the technique's applicability to more complex systems and enabling more accurate quantitative analysis in fields ranging from energy research to biomedical diagnostics.
Market Applications for High-Quality CV Analysis
Cyclic voltammetry (CV) analysis with high data quality has established itself as a cornerstone technology across multiple industries, driving innovation and enabling precise electrochemical characterization. The pharmaceutical sector represents one of the largest markets for advanced CV analysis, where it facilitates drug development through electrochemical screening of compounds, assessment of redox properties, and evaluation of drug-target interactions at the molecular level. Pharmaceutical companies increasingly rely on noise-free CV data to accelerate drug discovery processes and reduce development costs.
In the energy storage sector, high-quality CV analysis has become indispensable for battery research and development. Companies developing next-generation lithium-ion, solid-state, and flow batteries utilize precise CV measurements to characterize electrode materials, investigate degradation mechanisms, and optimize electrolyte compositions. The global battery market, projected to grow substantially due to electric vehicle adoption and renewable energy integration, represents a significant application area for advanced CV techniques.
Environmental monitoring constitutes another expanding market for high-quality CV analysis. Regulatory agencies and environmental service companies employ CV techniques for detecting heavy metals and organic pollutants in water, soil, and air samples. The ability to eliminate reference electrode drift and minimize noise enables detection at increasingly lower concentrations, meeting stringent environmental standards worldwide.
The semiconductor industry leverages precise CV analysis for quality control in manufacturing processes and materials characterization. As device dimensions continue to shrink, the demand for electrochemical techniques capable of characterizing nanoscale features with minimal noise interference has grown substantially. Semiconductor manufacturers utilize CV to evaluate thin films, interfaces, and surface modifications critical to device performance.
Biosensing and medical diagnostics represent rapidly expanding markets for high-quality CV analysis. The development of point-of-care devices, implantable sensors, and lab-on-chip platforms relies on electrochemical detection methods where signal-to-noise ratio directly impacts diagnostic accuracy. Companies in this space are particularly sensitive to reference electrode stability issues, as their products often operate in complex biological matrices.
Academic and government research institutions form a substantial market segment, requiring advanced CV instrumentation for fundamental research across chemistry, materials science, and biology. These institutions often pioneer new applications and analytical methods that subsequently transfer to industrial settings, creating a continuous innovation pipeline for CV technology.
The food and beverage industry has adopted CV analysis for quality control, authenticity verification, and contaminant detection. Manufacturers utilize electrochemical techniques to assess antioxidant content, detect adulterants, and monitor fermentation processes, with data quality directly impacting production decisions and regulatory compliance.
In the energy storage sector, high-quality CV analysis has become indispensable for battery research and development. Companies developing next-generation lithium-ion, solid-state, and flow batteries utilize precise CV measurements to characterize electrode materials, investigate degradation mechanisms, and optimize electrolyte compositions. The global battery market, projected to grow substantially due to electric vehicle adoption and renewable energy integration, represents a significant application area for advanced CV techniques.
Environmental monitoring constitutes another expanding market for high-quality CV analysis. Regulatory agencies and environmental service companies employ CV techniques for detecting heavy metals and organic pollutants in water, soil, and air samples. The ability to eliminate reference electrode drift and minimize noise enables detection at increasingly lower concentrations, meeting stringent environmental standards worldwide.
The semiconductor industry leverages precise CV analysis for quality control in manufacturing processes and materials characterization. As device dimensions continue to shrink, the demand for electrochemical techniques capable of characterizing nanoscale features with minimal noise interference has grown substantially. Semiconductor manufacturers utilize CV to evaluate thin films, interfaces, and surface modifications critical to device performance.
Biosensing and medical diagnostics represent rapidly expanding markets for high-quality CV analysis. The development of point-of-care devices, implantable sensors, and lab-on-chip platforms relies on electrochemical detection methods where signal-to-noise ratio directly impacts diagnostic accuracy. Companies in this space are particularly sensitive to reference electrode stability issues, as their products often operate in complex biological matrices.
Academic and government research institutions form a substantial market segment, requiring advanced CV instrumentation for fundamental research across chemistry, materials science, and biology. These institutions often pioneer new applications and analytical methods that subsequently transfer to industrial settings, creating a continuous innovation pipeline for CV technology.
The food and beverage industry has adopted CV analysis for quality control, authenticity verification, and contaminant detection. Manufacturers utilize electrochemical techniques to assess antioxidant content, detect adulterants, and monitor fermentation processes, with data quality directly impacting production decisions and regulatory compliance.
Current Challenges in CV Data Acquisition
Cyclic voltammetry (CV) data acquisition faces numerous challenges that significantly impact data quality and interpretation reliability. Electrical noise represents one of the most pervasive issues, manifesting as random fluctuations that obscure genuine electrochemical signals. This noise can originate from various sources including electromagnetic interference from nearby electronic equipment, power line fluctuations (50/60 Hz), and radio frequency interference. Laboratory environments with multiple instruments operating simultaneously often create complex noise profiles that prove difficult to isolate and eliminate.
Reference electrode stability presents another critical challenge in CV measurements. Drift in reference potential during experiments can lead to misinterpretation of peak positions and potentials, compromising the accuracy of thermodynamic and kinetic parameters derived from CV data. This instability may result from contamination of the reference electrode, depletion of internal filling solutions, or junction potential variations at the liquid-liquid interface of the reference electrode.
Cell design inadequacies frequently contribute to poor data quality. Improper electrode positioning can create non-uniform current distributions, while insufficient solution resistance compensation introduces distortions in voltammograms. These distortions manifest as peak potential shifts, broadening of peaks, and reduction in peak currents, all of which complicate quantitative analysis.
Sample preparation issues represent another significant challenge. Impurities in electrolyte solutions can introduce unexpected redox signals or alter the electrochemical behavior of target analytes. Inadequate degassing procedures often leave dissolved oxygen in solution, which produces its own reduction signals that may overlap with analyte responses in the negative potential region.
Electrode surface conditions dramatically affect CV measurements. Surface contamination, oxide formation, and adsorption of organic species can block active sites, alter electron transfer kinetics, and introduce capacitive currents that mask faradaic processes. These surface phenomena are particularly problematic in studies involving biomolecules or complex matrices where adsorption is prevalent.
Data acquisition hardware limitations also impact CV data quality. Insufficient sampling rates may fail to capture fast electron transfer processes, while analog-to-digital converter resolution constraints can limit the detection of small current signals. Many commercial potentiostats struggle with compensation for uncompensated resistance, particularly in low-conductivity media.
Temperature fluctuations during measurements represent an often-overlooked challenge. Even minor temperature variations can significantly alter diffusion coefficients, reaction kinetics, and equilibrium potentials, introducing variability in repeated measurements and complicating kinetic analyses.
Reference electrode stability presents another critical challenge in CV measurements. Drift in reference potential during experiments can lead to misinterpretation of peak positions and potentials, compromising the accuracy of thermodynamic and kinetic parameters derived from CV data. This instability may result from contamination of the reference electrode, depletion of internal filling solutions, or junction potential variations at the liquid-liquid interface of the reference electrode.
Cell design inadequacies frequently contribute to poor data quality. Improper electrode positioning can create non-uniform current distributions, while insufficient solution resistance compensation introduces distortions in voltammograms. These distortions manifest as peak potential shifts, broadening of peaks, and reduction in peak currents, all of which complicate quantitative analysis.
Sample preparation issues represent another significant challenge. Impurities in electrolyte solutions can introduce unexpected redox signals or alter the electrochemical behavior of target analytes. Inadequate degassing procedures often leave dissolved oxygen in solution, which produces its own reduction signals that may overlap with analyte responses in the negative potential region.
Electrode surface conditions dramatically affect CV measurements. Surface contamination, oxide formation, and adsorption of organic species can block active sites, alter electron transfer kinetics, and introduce capacitive currents that mask faradaic processes. These surface phenomena are particularly problematic in studies involving biomolecules or complex matrices where adsorption is prevalent.
Data acquisition hardware limitations also impact CV data quality. Insufficient sampling rates may fail to capture fast electron transfer processes, while analog-to-digital converter resolution constraints can limit the detection of small current signals. Many commercial potentiostats struggle with compensation for uncompensated resistance, particularly in low-conductivity media.
Temperature fluctuations during measurements represent an often-overlooked challenge. Even minor temperature variations can significantly alter diffusion coefficients, reaction kinetics, and equilibrium potentials, introducing variability in repeated measurements and complicating kinetic analyses.
Established Methods for CV Noise Reduction
01 Electrode materials and modifications for improved CV data quality
The quality of cyclic voltammetry data can be significantly improved by optimizing electrode materials and surface modifications. Various materials such as carbon-based electrodes, metal nanoparticles, and modified surfaces with specific functional groups can enhance sensitivity, reduce background noise, and improve reproducibility. These modifications can lead to better peak resolution, lower detection limits, and more reliable quantitative analysis in electrochemical measurements.- Noise reduction and signal processing techniques: Various signal processing methods can be applied to improve cyclic voltammetry data quality by reducing noise and enhancing signal clarity. These techniques include digital filtering, wavelet transformation, and advanced algorithms that can separate meaningful electrochemical signals from background noise. Implementing these processing techniques allows for more accurate peak identification and quantification, leading to more reliable analytical results in electrochemical measurements.
- Electrode surface preparation and modification: The quality of cyclic voltammetry data is significantly influenced by electrode surface conditions. Proper preparation techniques such as polishing, cleaning, and chemical modification of electrodes can enhance signal reproducibility and sensitivity. Surface modifications with nanomaterials, polymers, or specific functional groups can improve electron transfer kinetics and reduce fouling effects, resulting in clearer voltammetric responses and more reliable analytical measurements.
- Reference electrode stability and calibration: Maintaining stable reference electrodes is crucial for high-quality cyclic voltammetry data. Regular calibration procedures and proper maintenance of reference electrodes ensure accurate potential measurements. Methods for monitoring reference electrode drift and implementing correction factors can significantly improve data reliability. The use of internal standards and reference systems helps maintain consistency across multiple measurements and different experimental conditions.
- Electrolyte composition and purity control: The composition and purity of the electrolyte solution directly impact cyclic voltammetry data quality. Controlling factors such as ionic strength, pH, and buffer capacity ensures reproducible measurements. Techniques for removing impurities, dissolved oxygen, and other electroactive species that might interfere with the target analyte signals are essential. Proper electrolyte preparation protocols and storage conditions help maintain consistent experimental environments for reliable electrochemical analysis.
- Scan rate optimization and experimental parameter control: Optimizing scan rates and other experimental parameters is essential for obtaining high-quality cyclic voltammetry data. The selection of appropriate scan rates based on the kinetics of the electrochemical system under study affects peak resolution and current response. Controlling temperature, convection, and other environmental factors during measurements reduces variability. Automated systems for parameter optimization and experimental design help identify ideal conditions for specific analytical applications.
02 Signal processing and noise reduction techniques
Advanced signal processing algorithms and noise reduction techniques are essential for enhancing cyclic voltammetry data quality. These methods include digital filtering, baseline correction, wavelet transformation, and machine learning approaches that can effectively separate meaningful signals from background noise. Implementation of these techniques allows for more accurate peak identification, better quantification of electroactive species, and improved overall data reliability even in complex sample matrices.Expand Specific Solutions03 Calibration and standardization protocols
Establishing robust calibration and standardization protocols is crucial for ensuring high-quality cyclic voltammetry data. This includes the use of reference materials, standard addition methods, and internal standards to validate measurement accuracy. Proper calibration procedures help compensate for instrumental drift, electrode aging effects, and environmental variations, leading to more consistent and comparable results across different experimental sessions and laboratory settings.Expand Specific Solutions04 Environmental and experimental condition control
Controlling environmental and experimental conditions is vital for obtaining high-quality cyclic voltammetry data. Factors such as temperature, humidity, oxygen levels, solution pH, and electrolyte composition can significantly impact measurement outcomes. Advanced systems that provide precise control over these parameters, including temperature-controlled cells, oxygen-free environments, and automated solution handling, help minimize variability and enhance reproducibility of electrochemical measurements.Expand Specific Solutions05 Data validation and quality assessment methods
Implementing systematic data validation and quality assessment methods ensures reliable cyclic voltammetry results. These approaches include statistical analysis of replicate measurements, comparison with complementary analytical techniques, and application of quality control metrics specific to electrochemical data. Advanced software tools can automatically evaluate data quality parameters such as peak symmetry, reversibility criteria, and diffusion characteristics to flag potentially problematic measurements and guide researchers in optimizing their experimental protocols.Expand Specific Solutions
Leading Manufacturers and Research Groups in Electrochemistry
Cyclic Voltammetry data quality analysis is currently in a growth phase, with the market expanding as electrochemical analysis becomes increasingly critical in energy storage, materials science, and biomedical applications. The global market for electrochemical instruments is projected to reach several billion dollars by 2025, driven by research and industrial applications. Technologically, the field is maturing with companies like IBM, Toshiba, and Qualcomm developing advanced noise reduction algorithms and reference electrode technologies. Silicon Laboratories and Texas Instruments are focusing on high-precision analog components for improved signal processing, while Meta and Google are exploring AI-based data analysis tools for automated quality assessment. Universities like Huazhong University of Science & Technology are contributing fundamental research to address reference electrode stability issues and noise source identification.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed comprehensive solutions for cyclic voltammetry data quality enhancement through their industrial sensing and control expertise. Their approach integrates precision measurement hardware with advanced signal processing techniques specifically designed for electrochemical applications. Honeywell's systems employ specialized reference electrode designs with temperature compensation circuits that maintain stable potentials across varying environmental conditions. Their data acquisition systems feature high-resolution analog-to-digital converters with programmable sampling rates optimized for different CV scan speeds. Honeywell has implemented advanced digital filtering techniques including moving average filters and Savitzky-Golay smoothing algorithms that preserve critical peak information while reducing random noise. Their systems incorporate automated reference electrode diagnostics that can detect contamination, aging effects, or improper connections before they significantly impact measurement quality. Additionally, Honeywell has developed specialized electromagnetic shielding techniques for their measurement cells that effectively isolate sensitive electrochemical signals from external interference sources.
Strengths: Exceptional integration with industrial control systems; robust design suitable for continuous operation in manufacturing environments; comprehensive diagnostic capabilities for preventive maintenance. Weaknesses: Systems sometimes optimized for process control rather than research applications; higher implementation complexity requiring specialized integration knowledge; premium pricing reflecting industrial-grade reliability requirements.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced electrochemical measurement systems with specific focus on cyclic voltammetry data quality for medical diagnostic applications. Their approach integrates hardware and software solutions to address multiple noise sources simultaneously. Philips' systems employ high-precision analog-to-digital converters with 24-bit resolution, allowing for detection of small signals even in noisy environments. Their reference electrode technology incorporates specialized non-polarizable materials with minimal temperature sensitivity, addressing drift issues common in clinical settings. Philips has implemented digital signal processing techniques including wavelet transformation for noise identification and removal without distorting the underlying CV signals. Their systems feature automatic reference electrode diagnostics that can detect degradation or contamination before it significantly impacts measurement quality. Additionally, Philips has developed specialized shielding techniques for their measurement cells that minimize electromagnetic interference from external sources such as nearby electronic equipment in laboratory or clinical environments.
Strengths: Exceptional integration with clinical workflows; comprehensive validation protocols ensuring data reliability; advanced software tools for automated quality assessment. Weaknesses: Systems often optimized for specific clinical applications rather than general research; premium pricing reflecting medical-grade quality requirements; some solutions require specialized training for optimal operation.
Critical Reference Electrode Technologies and Innovations
Rare earth metal incorporated zeolite modified electrodes for detection and quantification of heavy metal ions in aqueous solution
PatentInactiveUS20170315079A1
Innovation
- Development of rare earth metal impregnated zeolite modified carbon paste electrodes, specifically lanthanum or cerium impregnated mordenite electrodes, for use in square wave anodic stripping voltammetry, enhancing electroactive surface area and detection limits.
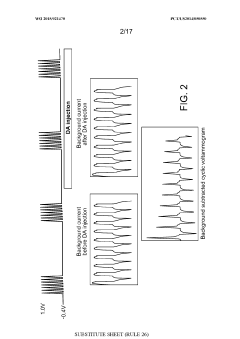
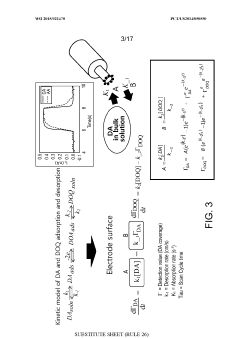
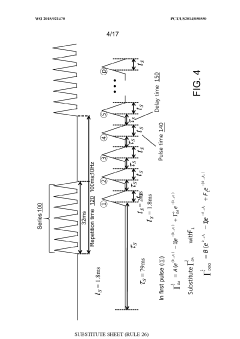
Using kinetic cyclic voltammetry to evaluate analyte kinetics and concentrations
PatentWO2015021470A1
Innovation
- Kinetic cyclic voltammetry (KCV) is developed, which involves multi-pulse cyclic voltammetry at various voltages to generate kinetic and concentration maps (K-maps and A-maps) without background subtraction, enabling absolute quantification of analytes and real-time signal analysis, suitable for use in smart neuromodulation systems.
Standardization and Quality Control Protocols
To ensure consistent and reliable cyclic voltammetry (CV) measurements, standardized protocols must be established across laboratories and research institutions. These protocols should address the various factors that can compromise data quality, including noise sources and reference electrode issues. A comprehensive quality control framework begins with instrument calibration using certified standard redox couples such as ferrocene/ferrocenium or potassium ferricyanide/ferrocyanide, which should be performed regularly to verify system performance.
Environmental controls represent another critical aspect of standardization. Temperature fluctuations can significantly alter electrochemical responses, necessitating temperature-controlled cells maintained within ±0.5°C. Similarly, oxygen interference must be addressed through standardized degassing procedures, typically involving high-purity inert gas purging for a minimum of 10 minutes before measurements.
Reference electrode stability verification should be incorporated into routine quality control procedures. This includes regular potential checks against known standards and implementation of double-junction designs to minimize contamination risks. The protocols should specify acceptable drift limits (typically <2 mV/hour) and replacement criteria when these limits are exceeded.
Signal processing standardization is equally important, with clear guidelines for data filtering that preserve genuine electrochemical features while removing noise. This includes specifications for appropriate filter types (e.g., Savitzky-Golay), parameters, and validation methods to ensure filtering does not introduce artifacts or distort peak characteristics.
Documentation requirements constitute a fundamental component of quality control protocols. Each CV experiment should be accompanied by comprehensive metadata including cell configuration, reference electrode type and maintenance history, solution composition, degassing method, temperature, scan rate, and all post-processing steps applied to the raw data.
Interlaboratory validation programs should be established to verify protocol effectiveness across different research environments. These programs would involve distributing identical samples and standardized procedures to multiple laboratories, with subsequent statistical analysis of the results to identify systematic errors and refine protocols accordingly.
Implementation of these standardization and quality control protocols would significantly enhance data reliability and reproducibility in cyclic voltammetry research, facilitating more meaningful comparisons between studies and accelerating progress in electrochemical science and technology development.
Environmental controls represent another critical aspect of standardization. Temperature fluctuations can significantly alter electrochemical responses, necessitating temperature-controlled cells maintained within ±0.5°C. Similarly, oxygen interference must be addressed through standardized degassing procedures, typically involving high-purity inert gas purging for a minimum of 10 minutes before measurements.
Reference electrode stability verification should be incorporated into routine quality control procedures. This includes regular potential checks against known standards and implementation of double-junction designs to minimize contamination risks. The protocols should specify acceptable drift limits (typically <2 mV/hour) and replacement criteria when these limits are exceeded.
Signal processing standardization is equally important, with clear guidelines for data filtering that preserve genuine electrochemical features while removing noise. This includes specifications for appropriate filter types (e.g., Savitzky-Golay), parameters, and validation methods to ensure filtering does not introduce artifacts or distort peak characteristics.
Documentation requirements constitute a fundamental component of quality control protocols. Each CV experiment should be accompanied by comprehensive metadata including cell configuration, reference electrode type and maintenance history, solution composition, degassing method, temperature, scan rate, and all post-processing steps applied to the raw data.
Interlaboratory validation programs should be established to verify protocol effectiveness across different research environments. These programs would involve distributing identical samples and standardized procedures to multiple laboratories, with subsequent statistical analysis of the results to identify systematic errors and refine protocols accordingly.
Implementation of these standardization and quality control protocols would significantly enhance data reliability and reproducibility in cyclic voltammetry research, facilitating more meaningful comparisons between studies and accelerating progress in electrochemical science and technology development.
Environmental Factors Affecting CV Measurements
Environmental factors play a crucial role in the quality and reliability of cyclic voltammetry (CV) measurements. Temperature fluctuations represent one of the most significant environmental variables affecting CV data. Even minor temperature changes can alter reaction kinetics, diffusion rates, and electrode surface properties, potentially leading to inconsistent peak potentials and current magnitudes. For precise measurements, temperature control systems maintaining stability within ±0.1°C are often necessary, particularly for quantitative analyses or when studying temperature-dependent electrochemical processes.
Electromagnetic interference (EMI) constitutes another major environmental challenge for CV measurements. Laboratory equipment such as motors, transformers, and nearby electronic devices generate electromagnetic fields that can introduce noise into the electrochemical signal. This interference typically manifests as periodic oscillations or random spikes in the voltammogram, obscuring important electrochemical features. Proper Faraday cage shielding, strategic equipment placement, and high-quality shielded cables can significantly mitigate these effects.
Vibration disturbances, though often overlooked, can substantially impact CV data quality. Mechanical vibrations from HVAC systems, nearby construction, or even foot traffic can cause physical movement of electrodes or solution agitation, resulting in convection effects that disrupt the diffusion-controlled conditions essential for reliable CV measurements. Anti-vibration tables, isolation platforms, and strategic laboratory placement away from mechanical equipment can help minimize these effects.
Ambient lighting conditions may also influence CV measurements, particularly when working with photosensitive materials or redox species. Direct sunlight or intense laboratory lighting can induce photochemical reactions or heating effects that alter the electrochemical behavior of the system under investigation. Conducting experiments in controlled lighting environments or using light-blocking enclosures around the electrochemical cell can prevent these light-induced artifacts.
Atmospheric composition represents another critical environmental factor, as exposure to oxygen, carbon dioxide, or other atmospheric gases can significantly alter electrochemical responses. Oxygen, being electroactive, can contribute to background currents and interfere with target analyte signals. Similarly, carbon dioxide absorption can change solution pH, affecting proton-coupled electron transfer reactions. Implementing inert gas purging systems (typically using nitrogen or argon) and maintaining sealed electrochemical cells are standard practices to control atmospheric exposure during sensitive CV measurements.
Humidity variations can affect both the electrode surfaces and reference electrode stability. High humidity environments may accelerate component corrosion or cause condensation on electrical connections, introducing resistance fluctuations and signal drift. Conversely, extremely dry conditions can lead to static electricity buildup, generating random noise spikes in the voltammetric data. Humidity-controlled environments or desiccant systems are often employed when conducting precision electrochemical measurements in variable climate conditions.
Electromagnetic interference (EMI) constitutes another major environmental challenge for CV measurements. Laboratory equipment such as motors, transformers, and nearby electronic devices generate electromagnetic fields that can introduce noise into the electrochemical signal. This interference typically manifests as periodic oscillations or random spikes in the voltammogram, obscuring important electrochemical features. Proper Faraday cage shielding, strategic equipment placement, and high-quality shielded cables can significantly mitigate these effects.
Vibration disturbances, though often overlooked, can substantially impact CV data quality. Mechanical vibrations from HVAC systems, nearby construction, or even foot traffic can cause physical movement of electrodes or solution agitation, resulting in convection effects that disrupt the diffusion-controlled conditions essential for reliable CV measurements. Anti-vibration tables, isolation platforms, and strategic laboratory placement away from mechanical equipment can help minimize these effects.
Ambient lighting conditions may also influence CV measurements, particularly when working with photosensitive materials or redox species. Direct sunlight or intense laboratory lighting can induce photochemical reactions or heating effects that alter the electrochemical behavior of the system under investigation. Conducting experiments in controlled lighting environments or using light-blocking enclosures around the electrochemical cell can prevent these light-induced artifacts.
Atmospheric composition represents another critical environmental factor, as exposure to oxygen, carbon dioxide, or other atmospheric gases can significantly alter electrochemical responses. Oxygen, being electroactive, can contribute to background currents and interfere with target analyte signals. Similarly, carbon dioxide absorption can change solution pH, affecting proton-coupled electron transfer reactions. Implementing inert gas purging systems (typically using nitrogen or argon) and maintaining sealed electrochemical cells are standard practices to control atmospheric exposure during sensitive CV measurements.
Humidity variations can affect both the electrode surfaces and reference electrode stability. High humidity environments may accelerate component corrosion or cause condensation on electrical connections, introducing resistance fluctuations and signal drift. Conversely, extremely dry conditions can lead to static electricity buildup, generating random noise spikes in the voltammetric data. Humidity-controlled environments or desiccant systems are often employed when conducting precision electrochemical measurements in variable climate conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!