What Is Cyclic Voltammetry? Theory, Typical Plots and Key Interpretation Steps for R&D Teams
AUG 21, 202511 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cyclic Voltammetry Fundamentals and Research Objectives
Cyclic voltammetry (CV) has evolved significantly since its inception in the early 20th century, becoming one of the most versatile and widely used electroanalytical techniques in modern electrochemistry. Initially developed as an extension of polarography, CV has transformed from a specialized research tool into an essential analytical method across multiple scientific disciplines. The technique's evolution has been marked by continuous refinements in instrumentation, methodology, and theoretical understanding, particularly in the latter half of the 20th century with the advent of digital computing and advanced electronics.
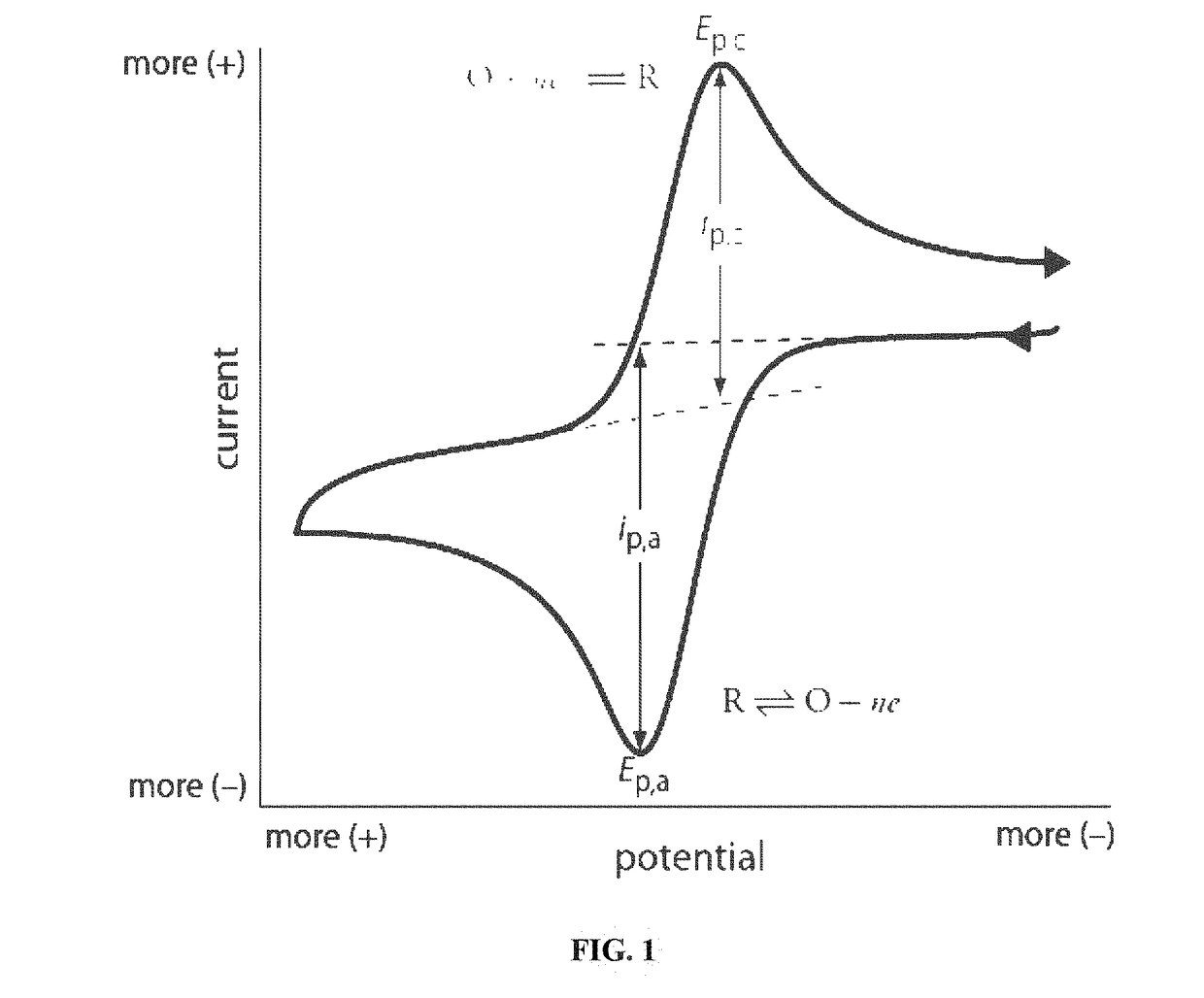
The fundamental principle of cyclic voltammetry involves applying a time-dependent potential to an electrochemical cell and measuring the resulting current. This creates a characteristic "duck-shaped" voltammogram that provides rich information about electron transfer kinetics, reaction mechanisms, and thermodynamic properties of redox-active species. The technique's power lies in its ability to rapidly provide qualitative information about electrochemical processes, including the thermodynamics of redox processes, the kinetics of heterogeneous electron-transfer reactions, and coupled chemical reactions or adsorption processes.
Recent technological advancements have significantly enhanced CV capabilities, including the development of ultramicroelectrodes enabling measurements in extremely small sample volumes, high-speed data acquisition systems allowing for investigation of rapid electron transfer processes, and integration with other analytical techniques such as spectroscopy for simultaneous multi-parameter analysis. These developments have expanded CV applications from traditional fields like inorganic chemistry to cutting-edge areas including energy storage, biosensing, and materials science.
The primary objective of this technical research report is to provide R&D teams with a comprehensive understanding of cyclic voltammetry theory, interpretation methodologies, and practical applications. We aim to bridge the gap between theoretical electrochemistry and practical implementation by elucidating key features of voltammograms and their correlation with underlying electrochemical processes. Additionally, we seek to identify emerging trends in CV applications, particularly in renewable energy technologies, advanced materials characterization, and bioelectrochemical systems.
This report will also explore how modern computational approaches, including machine learning algorithms and digital simulation techniques, are transforming CV data analysis and interpretation. By examining these developments, we intend to provide insights into how CV can be optimally utilized for specific research challenges and how its integration with complementary techniques can yield more comprehensive electrochemical characterization. The ultimate goal is to equip R&D professionals with the knowledge to leverage CV effectively in their respective fields, whether for fundamental research or applied technology development.
The fundamental principle of cyclic voltammetry involves applying a time-dependent potential to an electrochemical cell and measuring the resulting current. This creates a characteristic "duck-shaped" voltammogram that provides rich information about electron transfer kinetics, reaction mechanisms, and thermodynamic properties of redox-active species. The technique's power lies in its ability to rapidly provide qualitative information about electrochemical processes, including the thermodynamics of redox processes, the kinetics of heterogeneous electron-transfer reactions, and coupled chemical reactions or adsorption processes.
Recent technological advancements have significantly enhanced CV capabilities, including the development of ultramicroelectrodes enabling measurements in extremely small sample volumes, high-speed data acquisition systems allowing for investigation of rapid electron transfer processes, and integration with other analytical techniques such as spectroscopy for simultaneous multi-parameter analysis. These developments have expanded CV applications from traditional fields like inorganic chemistry to cutting-edge areas including energy storage, biosensing, and materials science.
The primary objective of this technical research report is to provide R&D teams with a comprehensive understanding of cyclic voltammetry theory, interpretation methodologies, and practical applications. We aim to bridge the gap between theoretical electrochemistry and practical implementation by elucidating key features of voltammograms and their correlation with underlying electrochemical processes. Additionally, we seek to identify emerging trends in CV applications, particularly in renewable energy technologies, advanced materials characterization, and bioelectrochemical systems.
This report will also explore how modern computational approaches, including machine learning algorithms and digital simulation techniques, are transforming CV data analysis and interpretation. By examining these developments, we intend to provide insights into how CV can be optimally utilized for specific research challenges and how its integration with complementary techniques can yield more comprehensive electrochemical characterization. The ultimate goal is to equip R&D professionals with the knowledge to leverage CV effectively in their respective fields, whether for fundamental research or applied technology development.
Market Applications and Analytical Demand
Cyclic voltammetry (CV) has established itself as an indispensable analytical technique across numerous industries, with a rapidly expanding market driven by increasing demand for precise electrochemical analysis. The global electrochemical instruments market, which includes CV equipment, is projected to reach $3.6 billion by 2026, growing at a CAGR of approximately 5.8% from 2021. This growth is primarily fueled by expanding applications in pharmaceutical research, materials science, and environmental monitoring.
In the pharmaceutical and biotechnology sectors, CV serves as a critical tool for drug discovery and development processes. Researchers utilize this technique to analyze redox properties of potential drug candidates, study electron transfer mechanisms in biomolecules, and evaluate the stability of pharmaceutical compounds. The technique's ability to provide rapid, real-time data on electrochemical reactions makes it particularly valuable in high-throughput screening environments.
The materials science and nanotechnology fields represent another significant market segment for CV applications. As industries push toward advanced materials development, CV provides essential insights into surface modifications, coating performance, and nanomaterial characterization. The technique's sensitivity to surface phenomena makes it particularly valuable for evaluating catalysts, battery materials, and conductive polymers.
Environmental monitoring and water quality assessment constitute a growing application area for CV technology. The technique enables detection of heavy metals and organic pollutants at trace levels, supporting regulatory compliance and environmental protection efforts. Portable CV systems are increasingly deployed for field testing in remote locations, expanding the market beyond traditional laboratory settings.
Energy research, particularly in battery development and fuel cell optimization, represents one of the fastest-growing segments for CV applications. As governments and industries worldwide invest heavily in renewable energy technologies, the demand for sophisticated electrochemical analysis tools continues to rise. CV provides critical data on electrode materials, electrolyte performance, and degradation mechanisms in energy storage devices.
The industrial sector utilizes CV for quality control in manufacturing processes, particularly in electroplating, corrosion monitoring, and sensor development. The technique's ability to detect impurities and evaluate surface treatments makes it valuable for ensuring product quality and process efficiency.
Academic and research institutions remain significant consumers of CV equipment, driving innovation in technique development and application expansion. The educational market segment continues to grow as more universities incorporate advanced electrochemical techniques into their analytical chemistry curricula.
AI: Cyclic voltammetry (CV) has established itself as an indispensable analytical technique across numerous industries, with a rapidly expanding market driven by increasing demand for precise electrochemical analysis. The global electrochemical instruments market, which includes CV equipment, is projected to reach $3.6 billion by 2026, growing at a CAGR of approximately 5.8% from 2021. This growth is primarily fueled by expanding applications in pharmaceutical research, materials science, and environmental monitoring.
In the pharmaceutical and biotechnology sectors, CV serves as a critical tool for drug discovery and development processes. Researchers utilize this technique to analyze redox properties of potential drug candidates, study electron transfer mechanisms in biomolecules, and evaluate the stability of pharmaceutical compounds. The technique's ability to provide rapid, real-time data on electrochemical reactions makes it particularly valuable in high-throughput screening environments.
The materials science and nanotechnology fields represent another significant market segment for CV applications. As industries push toward advanced materials development, CV provides essential insights into surface modifications, coating performance, and nanomaterial characterization. The technique's sensitivity to surface phenomena makes it particularly valuable for evaluating catalysts, battery materials, and conductive polymers.
Environmental monitoring and water quality assessment constitute a growing application area for CV technology. The technique enables detection of heavy metals and organic pollutants at trace levels, supporting regulatory compliance and environmental protection efforts. Portable CV systems are increasingly deployed for field testing in remote locations, expanding the market beyond traditional laboratory settings.
Energy research, particularly in battery development and fuel cell optimization, represents one of the fastest-growing segments for CV applications. As governments and industries worldwide invest heavily in renewable energy technologies, the demand for sophisticated electrochemical analysis tools continues to rise. CV provides critical data on electrode materials, electrolyte performance, and degradation mechanisms in energy storage devices.
The industrial sector utilizes CV for quality control in manufacturing processes, particularly in electroplating, corrosion monitoring, and sensor development. The technique's ability to detect impurities and evaluate surface treatments makes it valuable for ensuring product quality and process efficiency.
Academic and research institutions remain significant consumers of CV equipment, driving innovation in technique development and application expansion. The educational market segment continues to grow as more universities incorporate advanced electrochemical techniques into their analytical chemistry curricula.
In the pharmaceutical and biotechnology sectors, CV serves as a critical tool for drug discovery and development processes. Researchers utilize this technique to analyze redox properties of potential drug candidates, study electron transfer mechanisms in biomolecules, and evaluate the stability of pharmaceutical compounds. The technique's ability to provide rapid, real-time data on electrochemical reactions makes it particularly valuable in high-throughput screening environments.
The materials science and nanotechnology fields represent another significant market segment for CV applications. As industries push toward advanced materials development, CV provides essential insights into surface modifications, coating performance, and nanomaterial characterization. The technique's sensitivity to surface phenomena makes it particularly valuable for evaluating catalysts, battery materials, and conductive polymers.
Environmental monitoring and water quality assessment constitute a growing application area for CV technology. The technique enables detection of heavy metals and organic pollutants at trace levels, supporting regulatory compliance and environmental protection efforts. Portable CV systems are increasingly deployed for field testing in remote locations, expanding the market beyond traditional laboratory settings.
Energy research, particularly in battery development and fuel cell optimization, represents one of the fastest-growing segments for CV applications. As governments and industries worldwide invest heavily in renewable energy technologies, the demand for sophisticated electrochemical analysis tools continues to rise. CV provides critical data on electrode materials, electrolyte performance, and degradation mechanisms in energy storage devices.
The industrial sector utilizes CV for quality control in manufacturing processes, particularly in electroplating, corrosion monitoring, and sensor development. The technique's ability to detect impurities and evaluate surface treatments makes it valuable for ensuring product quality and process efficiency.
Academic and research institutions remain significant consumers of CV equipment, driving innovation in technique development and application expansion. The educational market segment continues to grow as more universities incorporate advanced electrochemical techniques into their analytical chemistry curricula.
AI: Cyclic voltammetry (CV) has established itself as an indispensable analytical technique across numerous industries, with a rapidly expanding market driven by increasing demand for precise electrochemical analysis. The global electrochemical instruments market, which includes CV equipment, is projected to reach $3.6 billion by 2026, growing at a CAGR of approximately 5.8% from 2021. This growth is primarily fueled by expanding applications in pharmaceutical research, materials science, and environmental monitoring.
In the pharmaceutical and biotechnology sectors, CV serves as a critical tool for drug discovery and development processes. Researchers utilize this technique to analyze redox properties of potential drug candidates, study electron transfer mechanisms in biomolecules, and evaluate the stability of pharmaceutical compounds. The technique's ability to provide rapid, real-time data on electrochemical reactions makes it particularly valuable in high-throughput screening environments.
The materials science and nanotechnology fields represent another significant market segment for CV applications. As industries push toward advanced materials development, CV provides essential insights into surface modifications, coating performance, and nanomaterial characterization. The technique's sensitivity to surface phenomena makes it particularly valuable for evaluating catalysts, battery materials, and conductive polymers.
Environmental monitoring and water quality assessment constitute a growing application area for CV technology. The technique enables detection of heavy metals and organic pollutants at trace levels, supporting regulatory compliance and environmental protection efforts. Portable CV systems are increasingly deployed for field testing in remote locations, expanding the market beyond traditional laboratory settings.
Energy research, particularly in battery development and fuel cell optimization, represents one of the fastest-growing segments for CV applications. As governments and industries worldwide invest heavily in renewable energy technologies, the demand for sophisticated electrochemical analysis tools continues to rise. CV provides critical data on electrode materials, electrolyte performance, and degradation mechanisms in energy storage devices.
The industrial sector utilizes CV for quality control in manufacturing processes, particularly in electroplating, corrosion monitoring, and sensor development. The technique's ability to detect impurities and evaluate surface treatments makes it valuable for ensuring product quality and process efficiency.
Academic and research institutions remain significant consumers of CV equipment, driving innovation in technique development and application expansion. The educational market segment continues to grow as more universities incorporate advanced electrochemical techniques into their analytical chemistry curricula.
Current Challenges in Electrochemical Analysis
Despite significant advancements in electrochemical analysis techniques, cyclic voltammetry (CV) continues to face several critical challenges that limit its application and reliability in research and development environments. One of the most persistent issues is signal-to-noise ratio degradation, particularly when analyzing samples with low analyte concentrations or in complex matrices. This challenge becomes especially pronounced when attempting to detect trace compounds in environmental samples or biological fluids, where background interference can easily mask the electrochemical response of target analytes.
Surface fouling represents another major obstacle in CV applications. The accumulation of reaction products or sample components on electrode surfaces progressively alters the electrochemical response during measurements, leading to signal drift and reduced reproducibility. This phenomenon is particularly problematic in continuous monitoring applications and when analyzing biological samples containing proteins that readily adsorb onto electrode surfaces.
Reproducibility and standardization remain significant hurdles across different laboratories and experimental setups. Variations in electrode preparation, reference electrode stability, and cell design can lead to substantial differences in voltammetric responses, making direct comparison of results challenging. The lack of universally accepted protocols for electrode preparation and measurement conditions further exacerbates this issue.
The interpretation of complex voltammograms presents a formidable challenge, especially for multi-component systems where overlapping peaks and coupled reactions occur. Current data analysis approaches often rely heavily on operator expertise and experience, introducing subjectivity into the analytical process. While computational methods have been developed to address this challenge, they frequently require sophisticated algorithms and significant computational resources.
Temporal resolution limitations restrict the application of CV in studying fast reaction kinetics. Traditional CV techniques struggle to capture electrochemical events occurring on microsecond or nanosecond timescales, which are critical for understanding rapid electron transfer processes in catalysis and energy storage applications.
Miniaturization and integration with other analytical techniques represent ongoing challenges for expanding CV applications. While microelectrode arrays and lab-on-chip devices have shown promise, issues related to fabrication reproducibility, electrode stability, and signal processing in miniaturized systems continue to limit widespread adoption of these approaches in routine analytical workflows.
Surface fouling represents another major obstacle in CV applications. The accumulation of reaction products or sample components on electrode surfaces progressively alters the electrochemical response during measurements, leading to signal drift and reduced reproducibility. This phenomenon is particularly problematic in continuous monitoring applications and when analyzing biological samples containing proteins that readily adsorb onto electrode surfaces.
Reproducibility and standardization remain significant hurdles across different laboratories and experimental setups. Variations in electrode preparation, reference electrode stability, and cell design can lead to substantial differences in voltammetric responses, making direct comparison of results challenging. The lack of universally accepted protocols for electrode preparation and measurement conditions further exacerbates this issue.
The interpretation of complex voltammograms presents a formidable challenge, especially for multi-component systems where overlapping peaks and coupled reactions occur. Current data analysis approaches often rely heavily on operator expertise and experience, introducing subjectivity into the analytical process. While computational methods have been developed to address this challenge, they frequently require sophisticated algorithms and significant computational resources.
Temporal resolution limitations restrict the application of CV in studying fast reaction kinetics. Traditional CV techniques struggle to capture electrochemical events occurring on microsecond or nanosecond timescales, which are critical for understanding rapid electron transfer processes in catalysis and energy storage applications.
Miniaturization and integration with other analytical techniques represent ongoing challenges for expanding CV applications. While microelectrode arrays and lab-on-chip devices have shown promise, issues related to fabrication reproducibility, electrode stability, and signal processing in miniaturized systems continue to limit widespread adoption of these approaches in routine analytical workflows.
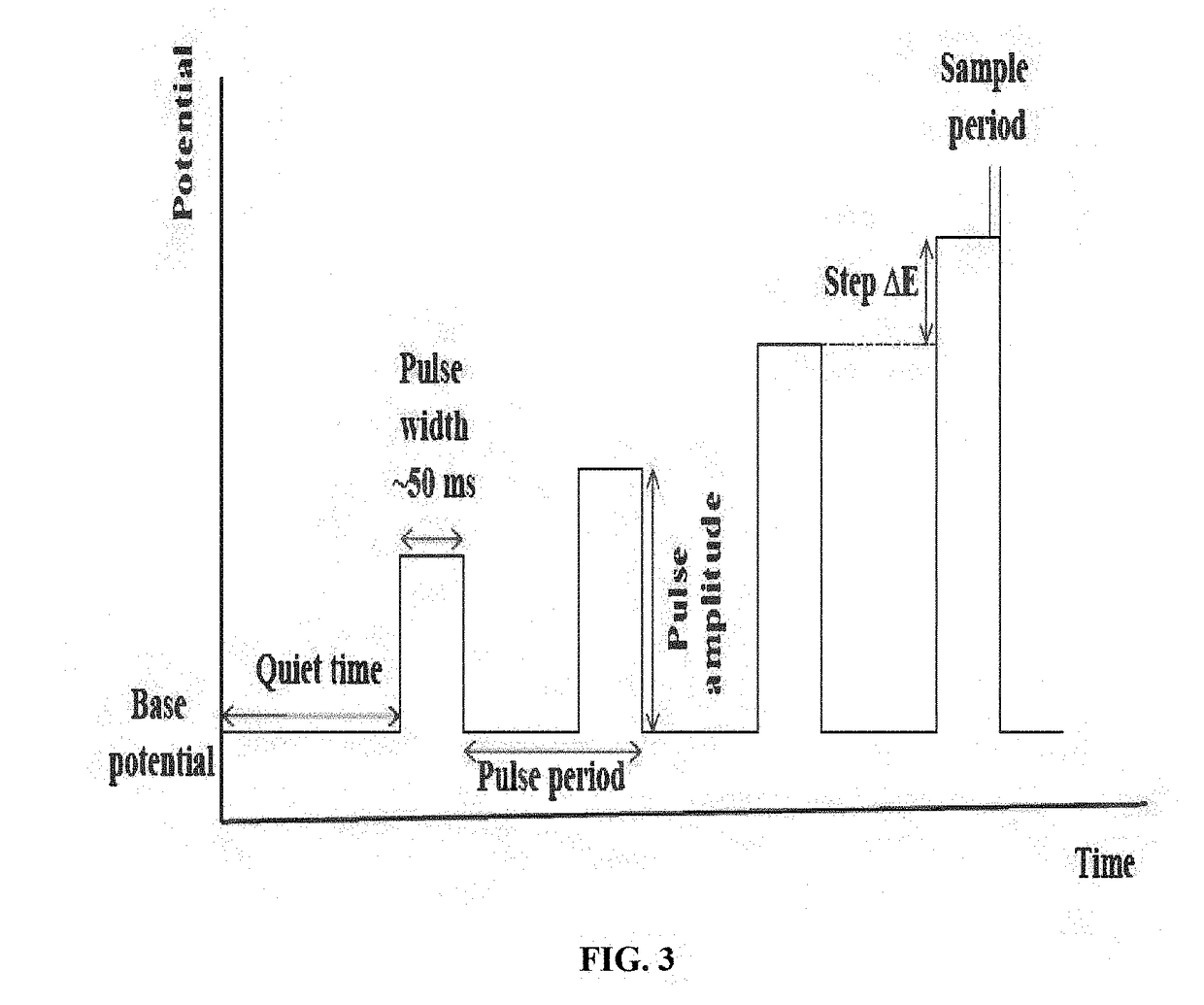
Modern CV Instrumentation and Methodologies
01 Cyclic voltammetry techniques for electrode material analysis
Cyclic voltammetry is widely used to analyze electrode materials for batteries and supercapacitors. The technique helps evaluate electrochemical performance by measuring redox reactions, charge transfer kinetics, and cycling stability. CV plots typically show characteristic peaks that indicate oxidation and reduction processes, with the peak position, height, and separation providing information about reaction reversibility and electrode efficiency. This analysis is crucial for developing high-performance energy storage devices.- Cyclic voltammetry techniques for electrode material analysis: Cyclic voltammetry is widely used to analyze the electrochemical properties of electrode materials. This technique involves applying a varying potential to an electrode and measuring the resulting current. The shape, peak positions, and symmetry of the CV plots provide information about the redox reactions, electron transfer kinetics, and electrochemical reversibility of the electrode materials. These analyses are crucial for developing high-performance electrodes for batteries, supercapacitors, and other energy storage devices.
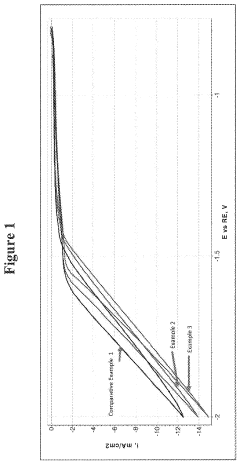
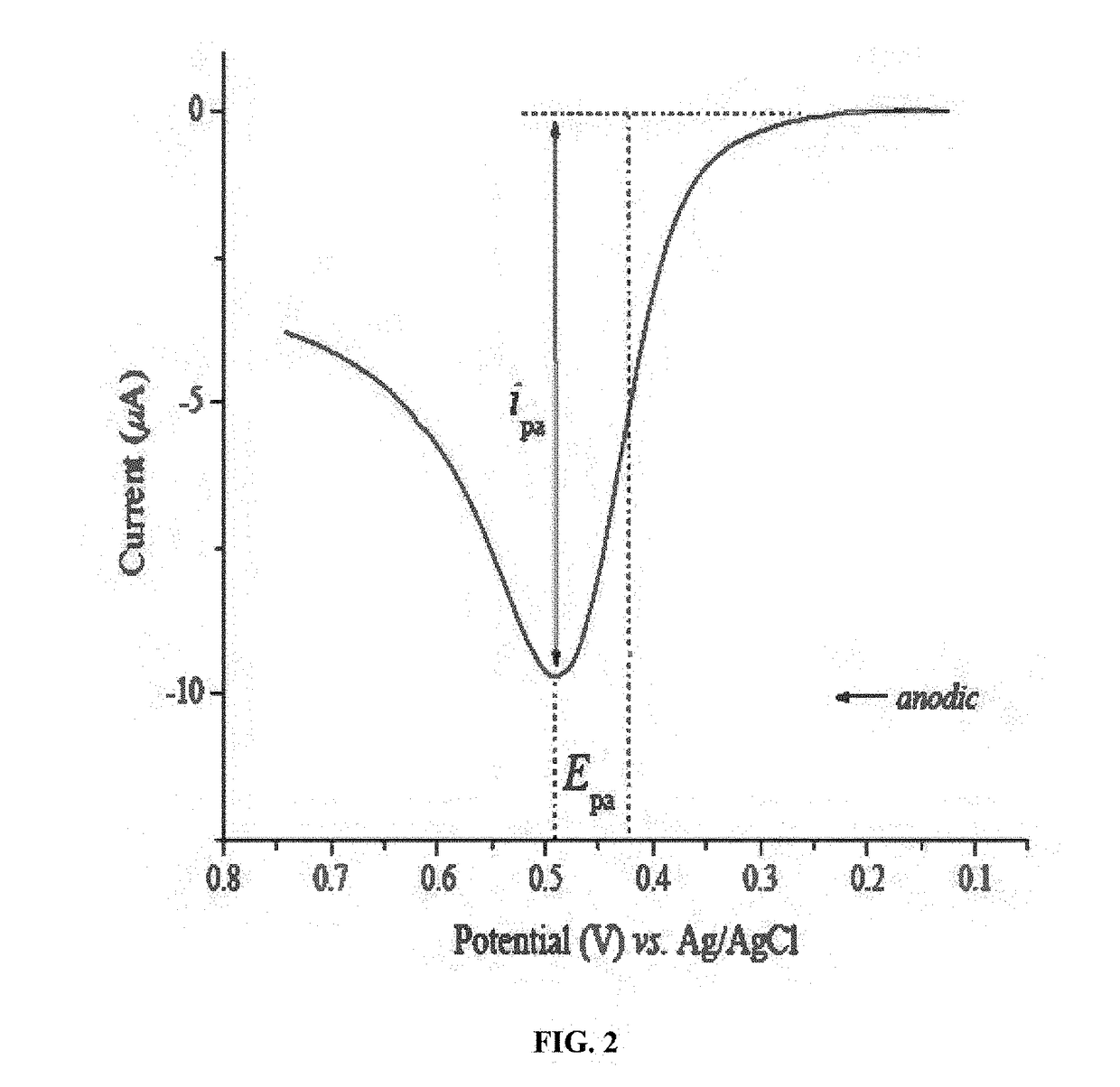
- Interpretation of redox peaks in cyclic voltammograms: The interpretation of redox peaks in cyclic voltammograms provides essential information about electrochemical reactions. The position, height, and separation of anodic and cathodic peaks indicate the redox potential, reaction rate, and reversibility of the electrochemical process. A small peak separation suggests a reversible reaction, while larger separations indicate quasi-reversible or irreversible processes. The peak current ratio can also reveal the stability of reaction products and the presence of coupled chemical reactions.
- Cyclic voltammetry for sensor development and characterization: Cyclic voltammetry plays a crucial role in the development and characterization of electrochemical sensors. The technique helps evaluate sensor sensitivity, selectivity, and detection limits by analyzing the current response to target analytes. CV plots can reveal interference effects, electrode fouling, and signal stability over time. By interpreting these plots, researchers can optimize sensor designs, modify electrode surfaces, and enhance detection capabilities for various applications including environmental monitoring, biomedical diagnostics, and industrial quality control.
- Advanced data analysis methods for cyclic voltammetry: Advanced data analysis methods enhance the interpretation of cyclic voltammetry results. These include digital filtering to reduce noise, baseline correction techniques, peak deconvolution for overlapping signals, and mathematical modeling to extract kinetic parameters. Machine learning and artificial intelligence approaches are increasingly applied to analyze complex CV data, identify patterns, and predict electrochemical behavior. These advanced methods improve the accuracy and reliability of information obtained from cyclic voltammograms, particularly for complex electrochemical systems.
- Cyclic voltammetry for battery and energy storage research: Cyclic voltammetry is an essential technique in battery and energy storage research. It helps characterize electrode materials, electrolytes, and interfaces in various energy storage systems. CV plots reveal information about charge storage mechanisms, intercalation processes, capacitive behaviors, and degradation pathways. By analyzing the evolution of CV curves during cycling, researchers can assess the stability and lifetime of energy storage devices. This technique guides the development of improved materials and designs for batteries, supercapacitors, and other electrochemical energy storage technologies.
02 Interpretation of CV curves for battery materials
CV curves for battery materials reveal critical information about electrochemical behavior. The shape, symmetry, and area of CV curves indicate the capacitive properties and energy storage mechanisms. Redox peak positions show the potential at which oxidation and reduction reactions occur, while peak separation indicates reaction reversibility. The stability of these peaks over multiple cycles demonstrates the material's durability. Quantitative analysis of CV data helps calculate specific capacitance, rate capability, and diffusion coefficients essential for battery development.Expand Specific Solutions03 CV analysis for sensor development and electrochemical detection
Cyclic voltammetry serves as a powerful tool for developing electrochemical sensors and detection methods. The technique enables the characterization of sensor surfaces, evaluation of analyte-electrode interactions, and determination of detection limits. CV plots for sensors typically show distinctive peaks corresponding to specific analytes, with peak current proportional to analyte concentration. Analysis of these plots helps optimize sensor design by identifying interference effects, response time, and sensitivity parameters. This approach is particularly valuable for biosensors, environmental monitoring, and medical diagnostic applications.Expand Specific Solutions04 Advanced data processing and interpretation algorithms for CV
Advanced algorithms and computational methods enhance the interpretation of cyclic voltammetry data. These approaches include peak deconvolution techniques to separate overlapping signals, machine learning algorithms for pattern recognition in complex CV plots, and simulation models that predict electrochemical behavior. Digital filtering methods improve signal-to-noise ratios, while mathematical transformations convert raw data into more interpretable formats. These computational tools enable more accurate quantification of kinetic parameters, diffusion coefficients, and reaction mechanisms from CV measurements.Expand Specific Solutions05 CV characterization of novel nanomaterials and composites
Cyclic voltammetry provides crucial insights into the electrochemical properties of novel nanomaterials and composites. The technique reveals how nanostructuring affects electron transfer rates, active surface area, and catalytic activity. CV plots of nanomaterials often show enhanced current responses and shifted peak potentials compared to bulk materials, indicating improved electrochemical performance. Analysis of these plots helps understand structure-property relationships, synergistic effects in composite materials, and surface modification impacts. This information guides the rational design of advanced materials for energy, catalysis, and sensing applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Cyclic Voltammetry (CV) technology is currently in a mature growth phase, with an expanding market driven by increasing applications in pharmaceutical research, materials science, and electrochemical analysis. The global market for electrochemical instruments, including CV equipment, is estimated at approximately $1.5-2 billion annually with steady growth projections. Leading players represent diverse sectors: established analytical instrument manufacturers (Beckman Coulter, Sartorius BioAnalytical), specialized electrochemical companies (Dionex, APE GmbH), and research institutions (Oxford University Innovation, Zhejiang University). Major corporations like IBM, Toyota, and Bristol Myers Squibb are investing in CV applications for energy storage, materials development, and drug discovery. The technology demonstrates high maturity with standardized methodologies, though innovations continue in miniaturization, automation, and integration with other analytical techniques, particularly for biomedical and nanomaterial applications.
Sartorius BioAnalytical Instruments, Inc.
Technical Solution: Sartorius BioAnalytical Instruments has pioneered cyclic voltammetry applications in bioprocess monitoring and biopharmaceutical development. Their technology integrates cyclic voltammetry with spectroscopic techniques to provide real-time analysis of biological reactions. Their systems employ specialized biocompatible electrode materials and microfluidic platforms that enable CV measurements in small sample volumes (as low as 50 μL). Sartorius has developed proprietary algorithms for interpreting CV data from complex biological matrices, allowing for the detection of specific biomolecules and reaction intermediates in the presence of interfering substances. Their instrumentation features temperature-controlled measurement cells (4-60°C range) to maintain biological sample integrity during analysis, and their software includes specialized tools for kinetic analysis of enzyme-catalyzed reactions through CV peak evolution monitoring over time.
Strengths: Specialized expertise in biological applications of CV provides unique capabilities for biopharmaceutical and bioprocess industries. Their microfluidic platforms enable analysis with minimal sample consumption. Weaknesses: Their systems are highly specialized for biological applications and may have limited flexibility for traditional materials science or general electrochemical applications.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has developed comprehensive cyclic voltammetry solutions focused on high-throughput applications in pharmaceutical research and clinical diagnostics. Their systems feature multi-channel potentiostats capable of running simultaneous CV experiments across 8, 16, or 96 samples, dramatically increasing analytical throughput. They've pioneered miniaturized electrode arrays that maintain analytical performance while reducing sample volume requirements to under 20 μL per measurement. Beckman's technology incorporates automated electrode cleaning and conditioning protocols that ensure reproducibility across large sample sets. Their data management systems are designed for pharmaceutical compliance, with full audit trails and data integrity features that meet FDA 21 CFR Part 11 requirements. The company has also developed specialized CV methods for protein-drug interaction studies, utilizing modified electrode surfaces to capture specific binding events through changes in electrochemical response patterns.
Strengths: Industry-leading throughput capabilities make their systems ideal for screening applications and large-scale studies. Their compliance-ready software simplifies regulatory documentation. Weaknesses: The focus on throughput sometimes comes at the expense of ultimate sensitivity compared to more specialized single-channel systems, and their systems typically command premium pricing in the market.
Critical Parameters and Data Interpretation Frameworks
Cobalt chemistry for smooth topology
PatentActiveUS11807951B2
Innovation
- The use of cyclic voltammetry to screen and evaluate additives in cobalt electroplating baths, combined with a cobalt electrolyte composition including boric acid, a pH adjuster, and organic suppressors, to achieve a smooth, seam-free, and uniform cobalt deposit with minimal overburden thickness variation across feature and non-feature areas, and the inclusion of suppressors and depolarizing compounds to facilitate bottom-up filling and reduce impurities.
Rare earth metal incorporated zeolite modified electrodes for detection and quantification of heavy metal ions in aqueous solution
PatentInactiveUS20170315079A1
Innovation
- Development of rare earth metal impregnated zeolite modified carbon paste electrodes, specifically lanthanum or cerium impregnated mordenite electrodes, for use in square wave anodic stripping voltammetry, enhancing electroactive surface area and detection limits.
Standardization and Quality Control Protocols
To ensure reliable and reproducible cyclic voltammetry (CV) results across different laboratories and research settings, standardization and quality control protocols are essential. These protocols establish a framework for consistent measurement practices, data validation, and instrument calibration that significantly enhance the credibility of electrochemical analyses.
Electrode preparation represents a critical first step in standardization. Surfaces must undergo consistent cleaning procedures, including mechanical polishing with alumina or diamond slurries of specified particle sizes, followed by sonication in appropriate solvents. For carbon-based electrodes, additional activation steps such as electrochemical pretreatment at defined potentials may be necessary to achieve reproducible electron transfer kinetics.
Reference electrode calibration demands regular verification against known standards such as ferrocene/ferrocenium (Fc/Fc+) couples. This practice ensures potential measurements remain accurate over time and comparable between different experimental setups. Similarly, counter electrodes require verification of proper surface area and positioning relative to working electrodes to maintain uniform current distribution.
Cell design standardization encompasses consistent electrode spacing, solution volumes, and temperature control (±0.1°C). These parameters significantly impact diffusion processes and reaction kinetics. Standard operating procedures should specify degassing protocols, typically involving argon or nitrogen purging for precise durations to eliminate oxygen interference.
Quality control measures must include regular testing with well-characterized redox couples such as potassium ferricyanide or ruthenium hexamine. These standard systems provide benchmark values for peak separation, current magnitude, and scan rate dependencies that verify proper instrument function. Statistical process control charts tracking these parameters over time can identify instrument drift or degradation before it compromises research outcomes.
Validation protocols should specify acceptance criteria for key CV parameters. For example, peak potential separations for reversible systems should approach theoretical values (59/n mV at 25°C), while peak current ratios should remain near unity. Deviations beyond established thresholds trigger troubleshooting procedures or instrument recalibration.
Data processing standardization is equally important, with clear guidelines for baseline correction, peak identification algorithms, and integration methods. Reporting standards should mandate inclusion of all experimental parameters—scan rates, concentration values, temperature, reference electrode details, and solvent properties—to enable proper interpretation and reproduction by other researchers.
Interlaboratory comparison studies provide the ultimate validation of standardization efforts, with multiple facilities analyzing identical samples using harmonized protocols to establish reproducibility limits and identify sources of systematic variation.
Electrode preparation represents a critical first step in standardization. Surfaces must undergo consistent cleaning procedures, including mechanical polishing with alumina or diamond slurries of specified particle sizes, followed by sonication in appropriate solvents. For carbon-based electrodes, additional activation steps such as electrochemical pretreatment at defined potentials may be necessary to achieve reproducible electron transfer kinetics.
Reference electrode calibration demands regular verification against known standards such as ferrocene/ferrocenium (Fc/Fc+) couples. This practice ensures potential measurements remain accurate over time and comparable between different experimental setups. Similarly, counter electrodes require verification of proper surface area and positioning relative to working electrodes to maintain uniform current distribution.
Cell design standardization encompasses consistent electrode spacing, solution volumes, and temperature control (±0.1°C). These parameters significantly impact diffusion processes and reaction kinetics. Standard operating procedures should specify degassing protocols, typically involving argon or nitrogen purging for precise durations to eliminate oxygen interference.
Quality control measures must include regular testing with well-characterized redox couples such as potassium ferricyanide or ruthenium hexamine. These standard systems provide benchmark values for peak separation, current magnitude, and scan rate dependencies that verify proper instrument function. Statistical process control charts tracking these parameters over time can identify instrument drift or degradation before it compromises research outcomes.
Validation protocols should specify acceptance criteria for key CV parameters. For example, peak potential separations for reversible systems should approach theoretical values (59/n mV at 25°C), while peak current ratios should remain near unity. Deviations beyond established thresholds trigger troubleshooting procedures or instrument recalibration.
Data processing standardization is equally important, with clear guidelines for baseline correction, peak identification algorithms, and integration methods. Reporting standards should mandate inclusion of all experimental parameters—scan rates, concentration values, temperature, reference electrode details, and solvent properties—to enable proper interpretation and reproduction by other researchers.
Interlaboratory comparison studies provide the ultimate validation of standardization efforts, with multiple facilities analyzing identical samples using harmonized protocols to establish reproducibility limits and identify sources of systematic variation.
Integration with Complementary Analytical Techniques
Cyclic voltammetry (CV) achieves its maximum analytical potential when integrated with complementary analytical techniques. This integration creates a comprehensive analytical framework that overcomes the inherent limitations of CV as a standalone method. Spectroscopic techniques such as UV-visible spectroscopy and infrared spectroscopy provide valuable molecular structure information that complements the electrochemical data from CV, enabling researchers to correlate electronic transitions with redox processes.
Mass spectrometry, when coupled with CV, allows for the identification of reaction intermediates and products formed during electrochemical processes. This combination is particularly valuable in mechanistic studies where understanding the identity of transient species is crucial. The integration can be achieved through specialized setups like electrochemical mass spectrometry (EC-MS) or by analyzing aliquots taken at different points during CV experiments.
X-ray techniques, including X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), provide structural and surface composition information that complements CV data. These techniques help researchers understand how crystal structure and surface chemistry influence electrochemical behavior, which is particularly important in materials science applications such as battery and catalyst development.
Microscopy techniques like scanning electron microscopy (SEM) and atomic force microscopy (AFM) offer morphological insights that can be correlated with CV data. The surface structure and topography often significantly impact electrochemical performance, making these visual techniques valuable companions to CV analysis.
Computational methods represent another powerful complementary approach. Density functional theory (DFT) calculations can predict redox potentials and reaction mechanisms, which can then be verified experimentally using CV. This integration of theoretical and experimental approaches enhances the interpretative power of CV data and provides deeper insights into reaction mechanisms.
In practical implementation, multi-technique analytical platforms that combine CV with spectroscopic or microscopic techniques in situ or operando configurations are becoming increasingly common in advanced research settings. These setups allow for real-time correlation between electrochemical events and spectroscopic or structural changes, providing unprecedented insights into complex electrochemical systems.
The strategic integration of CV with these complementary techniques ultimately leads to more robust and comprehensive understanding of electrochemical systems, enabling R&D teams to make more informed decisions in areas ranging from materials development to drug discovery and environmental monitoring.
Mass spectrometry, when coupled with CV, allows for the identification of reaction intermediates and products formed during electrochemical processes. This combination is particularly valuable in mechanistic studies where understanding the identity of transient species is crucial. The integration can be achieved through specialized setups like electrochemical mass spectrometry (EC-MS) or by analyzing aliquots taken at different points during CV experiments.
X-ray techniques, including X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), provide structural and surface composition information that complements CV data. These techniques help researchers understand how crystal structure and surface chemistry influence electrochemical behavior, which is particularly important in materials science applications such as battery and catalyst development.
Microscopy techniques like scanning electron microscopy (SEM) and atomic force microscopy (AFM) offer morphological insights that can be correlated with CV data. The surface structure and topography often significantly impact electrochemical performance, making these visual techniques valuable companions to CV analysis.
Computational methods represent another powerful complementary approach. Density functional theory (DFT) calculations can predict redox potentials and reaction mechanisms, which can then be verified experimentally using CV. This integration of theoretical and experimental approaches enhances the interpretative power of CV data and provides deeper insights into reaction mechanisms.
In practical implementation, multi-technique analytical platforms that combine CV with spectroscopic or microscopic techniques in situ or operando configurations are becoming increasingly common in advanced research settings. These setups allow for real-time correlation between electrochemical events and spectroscopic or structural changes, providing unprecedented insights into complex electrochemical systems.
The strategic integration of CV with these complementary techniques ultimately leads to more robust and comprehensive understanding of electrochemical systems, enabling R&D teams to make more informed decisions in areas ranging from materials development to drug discovery and environmental monitoring.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!