How to Design CV Experiments to Distinguish Surface vs Diffusion-controlled Processes — Protocols
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CV Experiment Design Background and Objectives
Cyclic voltammetry (CV) has evolved as a cornerstone analytical technique in electrochemistry since its development in the mid-20th century. This powerful method allows researchers to investigate electron transfer processes at electrode surfaces by measuring current response to potential changes in a cyclic manner. The technique's versatility has made it indispensable across multiple disciplines including materials science, analytical chemistry, and energy storage research.
The evolution of CV techniques has paralleled advancements in electrode materials, instrumentation precision, and theoretical frameworks. Early applications focused primarily on simple redox systems, while modern implementations address complex multi-electron processes, surface-confined reactions, and nanoscale phenomena. Recent years have witnessed significant refinements in both experimental protocols and data interpretation methodologies, particularly in distinguishing between surface-controlled and diffusion-controlled electrochemical processes.
Understanding the fundamental differences between surface and diffusion-controlled processes represents a critical challenge in electrochemical characterization. Surface-controlled processes involve electron transfer limited by adsorption/desorption kinetics at the electrode interface, while diffusion-controlled processes are governed by mass transport of electroactive species to and from the electrode surface. The ability to differentiate between these mechanisms provides essential insights into reaction pathways, material properties, and system optimization parameters.
The primary objective of this technical investigation is to establish robust experimental protocols for CV experiments specifically designed to distinguish between surface and diffusion-controlled electrochemical processes. This includes identifying diagnostic criteria, optimizing experimental parameters, and developing systematic approaches for data analysis and interpretation.
Secondary objectives encompass the standardization of CV experimental conditions to ensure reproducibility across different laboratory settings, the integration of complementary techniques to validate mechanistic assignments, and the exploration of advanced CV methodologies such as microelectrode techniques and fast-scan approaches that may offer enhanced discrimination capabilities.
The technological significance of this research extends beyond fundamental electrochemistry. Accurate mechanistic understanding directly impacts the development of next-generation energy storage systems, electrocatalysts, sensors, and electroanalytical methods. For instance, in battery research, distinguishing between surface-limited and diffusion-limited processes informs strategies for improving charge/discharge rates and cycle life. Similarly, in electrocatalysis, mechanistic insights guide rational catalyst design for enhanced activity and selectivity.
This investigation aims to bridge the gap between theoretical electrochemical principles and practical experimental implementation, providing researchers with clear guidelines for designing, conducting, and interpreting CV experiments focused on mechanistic differentiation. The resulting protocols will serve as valuable resources for both academic research and industrial applications in electrochemical technology development.
The evolution of CV techniques has paralleled advancements in electrode materials, instrumentation precision, and theoretical frameworks. Early applications focused primarily on simple redox systems, while modern implementations address complex multi-electron processes, surface-confined reactions, and nanoscale phenomena. Recent years have witnessed significant refinements in both experimental protocols and data interpretation methodologies, particularly in distinguishing between surface-controlled and diffusion-controlled electrochemical processes.
Understanding the fundamental differences between surface and diffusion-controlled processes represents a critical challenge in electrochemical characterization. Surface-controlled processes involve electron transfer limited by adsorption/desorption kinetics at the electrode interface, while diffusion-controlled processes are governed by mass transport of electroactive species to and from the electrode surface. The ability to differentiate between these mechanisms provides essential insights into reaction pathways, material properties, and system optimization parameters.
The primary objective of this technical investigation is to establish robust experimental protocols for CV experiments specifically designed to distinguish between surface and diffusion-controlled electrochemical processes. This includes identifying diagnostic criteria, optimizing experimental parameters, and developing systematic approaches for data analysis and interpretation.
Secondary objectives encompass the standardization of CV experimental conditions to ensure reproducibility across different laboratory settings, the integration of complementary techniques to validate mechanistic assignments, and the exploration of advanced CV methodologies such as microelectrode techniques and fast-scan approaches that may offer enhanced discrimination capabilities.
The technological significance of this research extends beyond fundamental electrochemistry. Accurate mechanistic understanding directly impacts the development of next-generation energy storage systems, electrocatalysts, sensors, and electroanalytical methods. For instance, in battery research, distinguishing between surface-limited and diffusion-limited processes informs strategies for improving charge/discharge rates and cycle life. Similarly, in electrocatalysis, mechanistic insights guide rational catalyst design for enhanced activity and selectivity.
This investigation aims to bridge the gap between theoretical electrochemical principles and practical experimental implementation, providing researchers with clear guidelines for designing, conducting, and interpreting CV experiments focused on mechanistic differentiation. The resulting protocols will serve as valuable resources for both academic research and industrial applications in electrochemical technology development.
Market Applications of CV in Electrochemical Analysis
Cyclic voltammetry (CV) has established itself as an indispensable analytical technique across various market sectors due to its ability to distinguish between surface and diffusion-controlled electrochemical processes. The pharmaceutical industry extensively utilizes CV for drug development and quality control, particularly in analyzing the electrochemical behavior of active pharmaceutical ingredients and detecting impurities that might affect drug efficacy or safety. The technique's ability to characterize reaction mechanisms provides crucial insights during formulation development.
In environmental monitoring, CV has become a cornerstone technology for detecting heavy metals and organic pollutants in water samples. Commercial systems incorporating CV techniques offer rapid on-site analysis capabilities, allowing for real-time monitoring of environmental contaminants at concentrations as low as parts per billion. This market segment has seen significant growth as regulatory requirements for environmental testing become increasingly stringent worldwide.
The food and beverage industry employs CV for quality control and safety assurance, particularly in detecting additives, preservatives, and contaminants. The technique's ability to distinguish surface-adsorbed species from diffusion-controlled reactions makes it valuable for analyzing complex food matrices where multiple electroactive compounds may be present simultaneously.
In the energy sector, CV plays a critical role in battery research and development. The technique helps researchers understand electrode surface phenomena versus bulk diffusion processes, which is essential for improving battery performance, longevity, and safety. This application has gained tremendous market traction with the global push toward electric vehicles and renewable energy storage solutions.
The biosensor market represents one of the fastest-growing applications for CV technology. The technique enables the development of highly sensitive diagnostic devices for detecting biomarkers associated with various diseases. Companies are increasingly incorporating CV-based sensors into point-of-care diagnostic platforms, creating a market estimated to reach several billion dollars annually.
Industrial process control represents another significant market application, where CV is used for real-time monitoring of electrochemical processes in manufacturing. The ability to distinguish between surface and diffusion-controlled reactions allows for precise control of electroplating, corrosion protection, and electrochemical synthesis processes, resulting in improved product quality and reduced waste.
Academic and research institutions constitute a stable market segment for CV instrumentation, with continuous demand for increasingly sophisticated equipment that can perform advanced experimental protocols for distinguishing surface and diffusion-controlled processes with greater precision and under varied experimental conditions.
In environmental monitoring, CV has become a cornerstone technology for detecting heavy metals and organic pollutants in water samples. Commercial systems incorporating CV techniques offer rapid on-site analysis capabilities, allowing for real-time monitoring of environmental contaminants at concentrations as low as parts per billion. This market segment has seen significant growth as regulatory requirements for environmental testing become increasingly stringent worldwide.
The food and beverage industry employs CV for quality control and safety assurance, particularly in detecting additives, preservatives, and contaminants. The technique's ability to distinguish surface-adsorbed species from diffusion-controlled reactions makes it valuable for analyzing complex food matrices where multiple electroactive compounds may be present simultaneously.
In the energy sector, CV plays a critical role in battery research and development. The technique helps researchers understand electrode surface phenomena versus bulk diffusion processes, which is essential for improving battery performance, longevity, and safety. This application has gained tremendous market traction with the global push toward electric vehicles and renewable energy storage solutions.
The biosensor market represents one of the fastest-growing applications for CV technology. The technique enables the development of highly sensitive diagnostic devices for detecting biomarkers associated with various diseases. Companies are increasingly incorporating CV-based sensors into point-of-care diagnostic platforms, creating a market estimated to reach several billion dollars annually.
Industrial process control represents another significant market application, where CV is used for real-time monitoring of electrochemical processes in manufacturing. The ability to distinguish between surface and diffusion-controlled reactions allows for precise control of electroplating, corrosion protection, and electrochemical synthesis processes, resulting in improved product quality and reduced waste.
Academic and research institutions constitute a stable market segment for CV instrumentation, with continuous demand for increasingly sophisticated equipment that can perform advanced experimental protocols for distinguishing surface and diffusion-controlled processes with greater precision and under varied experimental conditions.
Technical Challenges in Surface vs Diffusion Process Differentiation
Distinguishing between surface-controlled and diffusion-controlled processes in cyclic voltammetry (CV) experiments presents significant technical challenges that researchers must overcome. The fundamental difficulty lies in the overlapping electrochemical signatures that both processes can produce, making definitive identification complex without carefully designed experimental protocols.
One primary challenge is the establishment of appropriate scan rate dependencies. While diffusion-controlled processes typically show peak currents proportional to the square root of scan rate (v^1/2), surface-controlled processes exhibit direct proportionality to scan rate (v). However, in real experimental conditions, mixed kinetics often occur, creating intermediate dependencies that are difficult to categorize definitively.
Electrode surface heterogeneity introduces another layer of complexity. Surface defects, varying active sites, and non-uniform distribution of electrocatalytic centers can create localized regions with different kinetic behaviors. This heterogeneity can mask the true nature of the underlying process, as measurements represent an average across the entire electrode surface rather than discrete mechanistic information.
Temperature effects further complicate the differentiation process. Diffusion coefficients and surface reaction rates respond differently to temperature changes, with diffusion processes generally showing higher temperature sensitivity. Maintaining precise temperature control throughout experiments is technically demanding but essential for accurate process identification.
Solution resistance and uncompensated resistance effects can distort voltammograms, particularly at higher scan rates, leading to peak potential shifts that may be misinterpreted as kinetic information. These effects are especially problematic when attempting to use peak separation as a diagnostic criterion for process identification.
The challenge of adsorption phenomena cannot be overlooked. Species may temporarily adsorb during what is predominantly a diffusion-controlled process, creating hybrid behaviors that do not cleanly fit either model. Similarly, surface-bound species may desorb during measurement, introducing diffusional components to otherwise surface-controlled reactions.
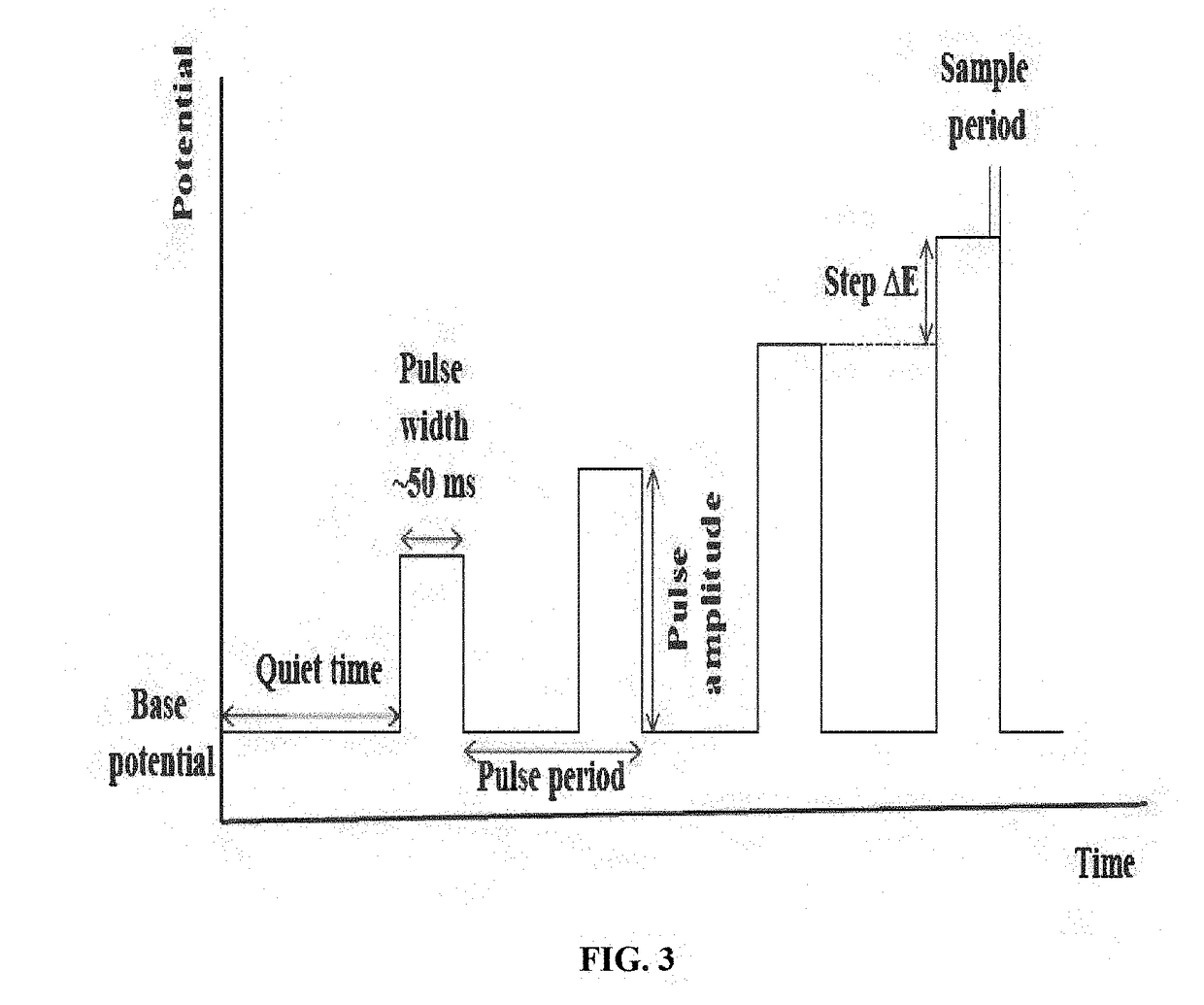
Instrumentation limitations also present technical barriers. High-quality potentiostats with fast response times and low noise characteristics are required for accurate measurements at the wide range of scan rates needed for comprehensive kinetic analysis. Signal-to-noise ratios become particularly problematic at very fast scan rates, which are often necessary for definitive process identification.
Data analysis and interpretation frameworks remain somewhat subjective, with different mathematical models yielding varying conclusions from the same experimental data. The lack of standardized analytical approaches across the field makes comparative studies challenging and contributes to inconsistent classifications of borderline cases.
One primary challenge is the establishment of appropriate scan rate dependencies. While diffusion-controlled processes typically show peak currents proportional to the square root of scan rate (v^1/2), surface-controlled processes exhibit direct proportionality to scan rate (v). However, in real experimental conditions, mixed kinetics often occur, creating intermediate dependencies that are difficult to categorize definitively.
Electrode surface heterogeneity introduces another layer of complexity. Surface defects, varying active sites, and non-uniform distribution of electrocatalytic centers can create localized regions with different kinetic behaviors. This heterogeneity can mask the true nature of the underlying process, as measurements represent an average across the entire electrode surface rather than discrete mechanistic information.
Temperature effects further complicate the differentiation process. Diffusion coefficients and surface reaction rates respond differently to temperature changes, with diffusion processes generally showing higher temperature sensitivity. Maintaining precise temperature control throughout experiments is technically demanding but essential for accurate process identification.
Solution resistance and uncompensated resistance effects can distort voltammograms, particularly at higher scan rates, leading to peak potential shifts that may be misinterpreted as kinetic information. These effects are especially problematic when attempting to use peak separation as a diagnostic criterion for process identification.
The challenge of adsorption phenomena cannot be overlooked. Species may temporarily adsorb during what is predominantly a diffusion-controlled process, creating hybrid behaviors that do not cleanly fit either model. Similarly, surface-bound species may desorb during measurement, introducing diffusional components to otherwise surface-controlled reactions.
Instrumentation limitations also present technical barriers. High-quality potentiostats with fast response times and low noise characteristics are required for accurate measurements at the wide range of scan rates needed for comprehensive kinetic analysis. Signal-to-noise ratios become particularly problematic at very fast scan rates, which are often necessary for definitive process identification.
Data analysis and interpretation frameworks remain somewhat subjective, with different mathematical models yielding varying conclusions from the same experimental data. The lack of standardized analytical approaches across the field makes comparative studies challenging and contributes to inconsistent classifications of borderline cases.
Current CV Protocol Solutions and Implementations
01 Peak characteristics in CV for process identification
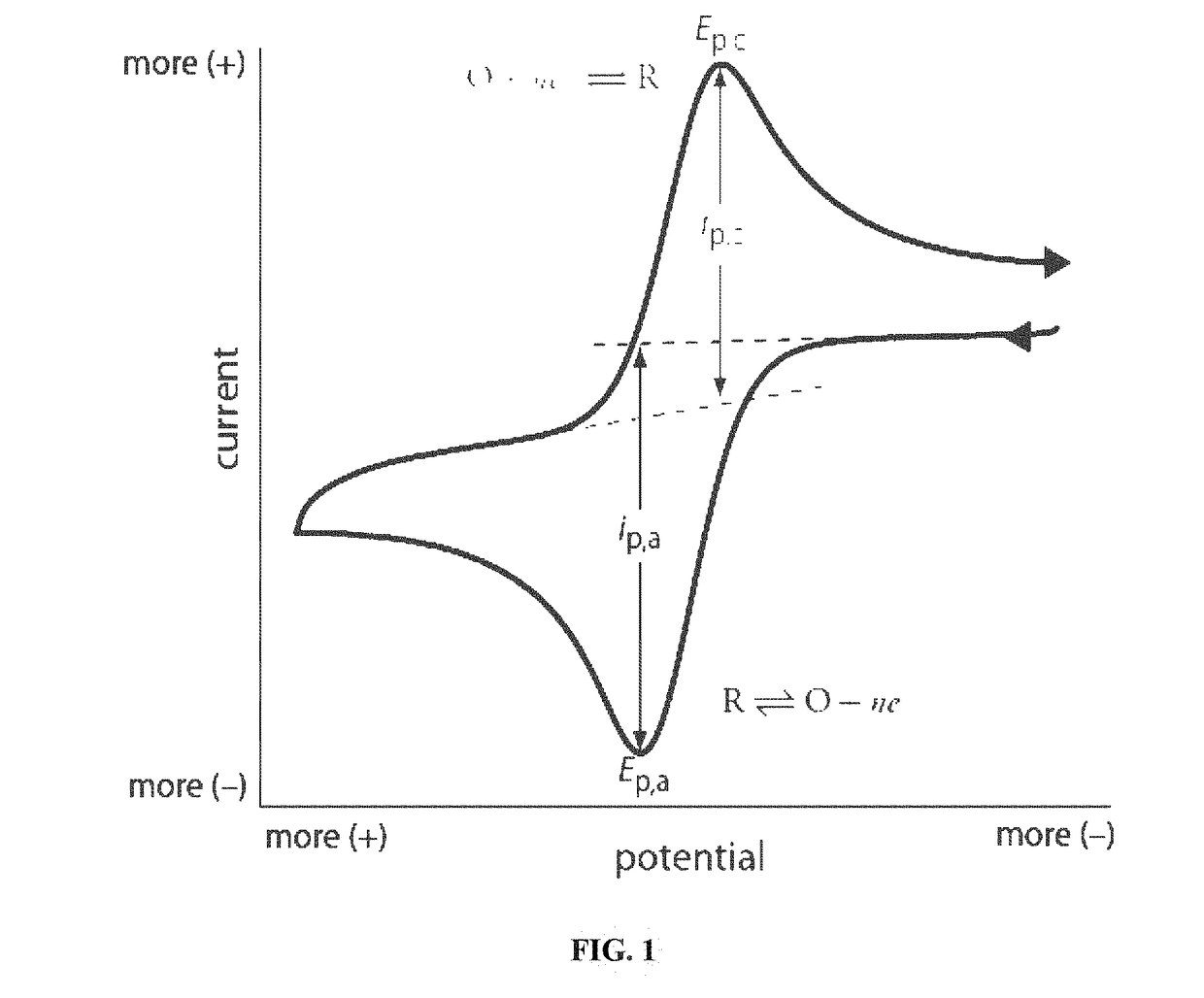
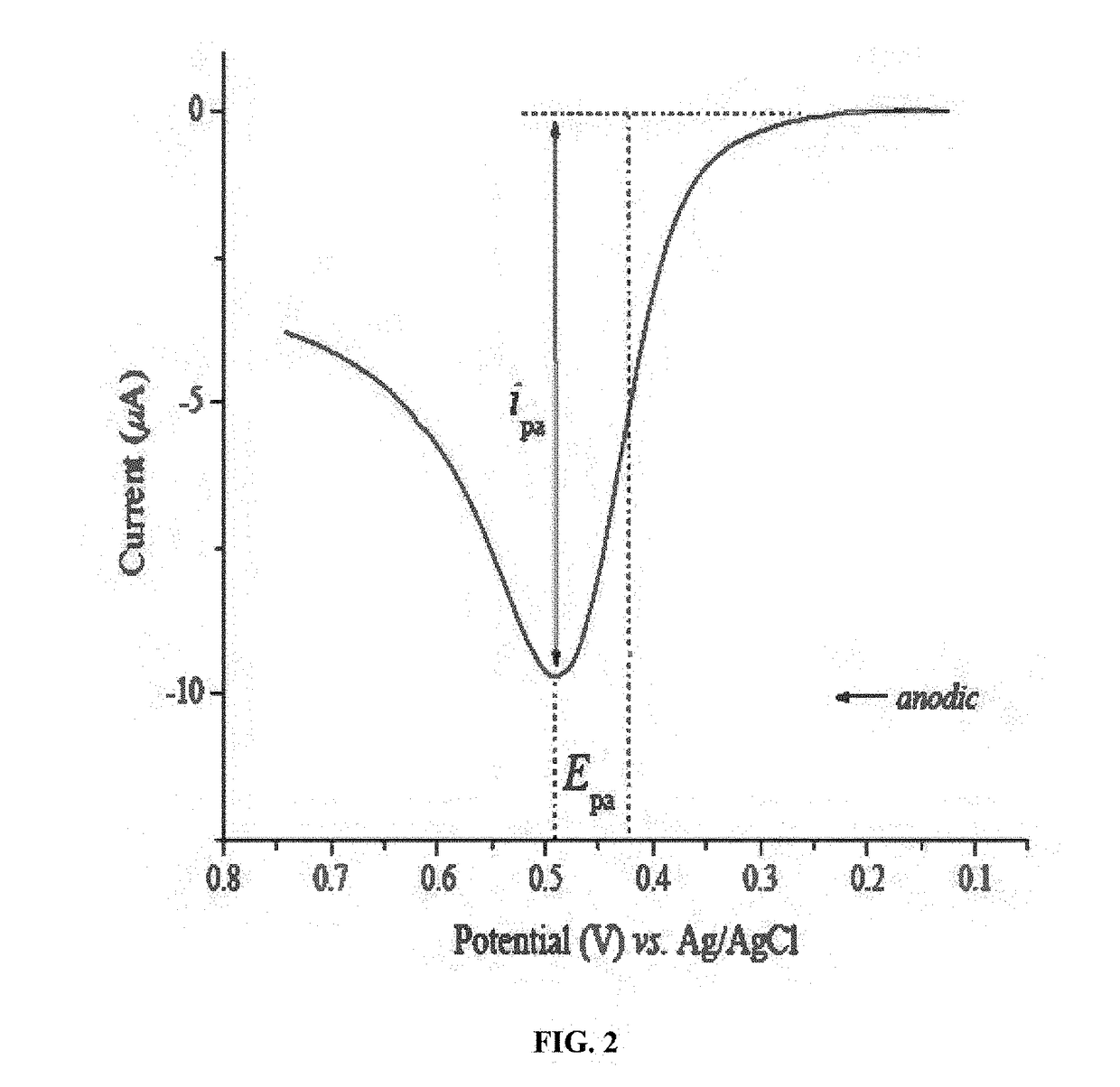
Cyclic voltammetry can distinguish between surface-controlled and diffusion-controlled processes by analyzing peak characteristics. In surface-controlled processes, the peak current is directly proportional to scan rate, while in diffusion-controlled processes, it's proportional to the square root of scan rate. Additionally, surface processes typically show symmetric peaks with minimal peak separation, whereas diffusion-controlled processes exhibit asymmetric peaks with larger peak-to-peak separation that increases with scan rate.- Peak characteristics in CV for process identification: Cyclic voltammetry experiments can distinguish between surface-controlled and diffusion-controlled processes by analyzing peak characteristics. In surface-controlled processes, peak currents are directly proportional to scan rate, while in diffusion-controlled processes, peak currents are proportional to the square root of scan rate. Additionally, peak separation and shape differ significantly between these processes, with surface processes showing symmetric peaks and minimal peak separation compared to diffusion-controlled reactions.
- Electrode modification techniques for process control: Modifying electrode surfaces can shift reactions from diffusion-controlled to surface-controlled processes or vice versa. Techniques include coating electrodes with nanomaterials, polymers, or specific catalysts that can either facilitate surface adsorption of reactants or create a layer that requires diffusion. These modifications allow researchers to optimize electrochemical systems for specific applications by controlling the dominant electron transfer mechanism.
- Mathematical models and algorithms for process differentiation: Advanced mathematical models and algorithms have been developed to quantitatively distinguish between surface and diffusion-controlled processes in cyclic voltammetry data. These include Randles-Sevcik equation for diffusion processes, Laviron analysis for surface-confined processes, and digital simulation techniques. By applying these models to experimental data, researchers can determine kinetic parameters and identify the dominant electron transfer mechanism.
- In-situ monitoring techniques for process transition: In-situ monitoring techniques allow researchers to observe transitions between surface and diffusion-controlled processes during electrochemical reactions. These techniques include combining cyclic voltammetry with spectroscopic methods, quartz crystal microbalance, or scanning probe microscopy. Such combined approaches provide real-time information about surface coverage, adsorption/desorption kinetics, and mass transport phenomena, enabling more accurate process identification.
- Microelectrode and array systems for enhanced discrimination: Microelectrode and electrode array systems offer enhanced capabilities for distinguishing between surface and diffusion-controlled processes. These systems provide improved signal-to-noise ratios, reduced capacitive currents, and faster response times compared to conventional electrodes. The unique diffusion profiles at microelectrodes (hemispherical diffusion) create distinct voltammetric responses that make process identification more straightforward, particularly for complex systems with mixed kinetics.
02 Modified electrodes for enhanced surface processes
Modified electrodes with specific surface treatments or coatings can enhance surface-controlled electrochemical processes and help distinguish them from diffusion-controlled reactions. These modifications include nanomaterial coatings, conductive polymers, or specific functional groups that facilitate electron transfer at the electrode surface. CV experiments using these modified electrodes show characteristic responses that clearly differentiate between reactions occurring at the electrode surface versus those limited by diffusion from the bulk solution.Expand Specific Solutions03 Mathematical analysis and modeling of CV data
Mathematical analysis and modeling techniques applied to cyclic voltammetry data provide quantitative methods to distinguish between surface and diffusion-controlled processes. These include Randles-Sevcik equation for diffusion-controlled processes, Laviron analysis for surface-confined processes, and digital simulation techniques. By analyzing the relationship between peak current and scan rate through logarithmic plots, researchers can determine the dominant electron transfer mechanism and calculate key parameters such as diffusion coefficients or surface coverage.Expand Specific Solutions04 In-situ and operando CV techniques
Advanced in-situ and operando cyclic voltammetry techniques allow real-time observation of electrochemical processes at the electrode-electrolyte interface. These methods combine CV with spectroscopic or microscopic techniques to simultaneously monitor surface changes and bulk solution behavior. By correlating electrochemical signals with surface structural changes, researchers can definitively identify surface-confined processes versus diffusion-limited reactions, particularly important for catalyst characterization and battery electrode materials.Expand Specific Solutions05 Microelectrodes and specialized cell designs
Microelectrodes and specialized electrochemical cell designs offer enhanced capabilities for distinguishing surface and diffusion-controlled processes in cyclic voltammetry experiments. Microelectrodes exhibit unique mass transport properties that emphasize radial diffusion, resulting in sigmoidal voltammograms for diffusion-controlled processes rather than the peak-shaped responses seen at conventional electrodes. Specialized cell configurations can control mass transport conditions, allowing researchers to isolate and characterize surface processes by minimizing diffusion effects or enhancing surface interactions.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The cyclic voltammetry (CV) experimental design field for distinguishing surface vs diffusion-controlled processes is currently in a mature development stage, with established protocols driving research across multiple industries. The global market for electrochemical analysis equipment is estimated at $3-5 billion, growing steadily with semiconductor and materials science applications. Technologically, companies demonstrate varying maturity levels: Applied Materials, Micron Technology, and ASM International lead with advanced CV methodologies integrated into semiconductor manufacturing; AIXTRON and Tokyo Electron offer specialized equipment with sophisticated analytical capabilities; while emerging players like Intermolecular and Beneq focus on high-throughput combinatorial approaches. Academic institutions including Xi'an Jiaotong University and Harbin Engineering University contribute fundamental research advancing theoretical frameworks for interpreting complex electrochemical signatures.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed sophisticated CV experimental protocols for semiconductor and thin film applications that specifically target the differentiation between surface and diffusion-controlled processes. Their approach utilizes high-precision in-situ electrochemical measurement systems integrated with their deposition tools. The protocol employs multi-sweep voltammetry at various scan rates (typically ranging from 1-500 mV/s) combined with advanced mathematical modeling to extract kinetic parameters. Their methodology includes specialized electrode configurations that minimize solution resistance and capacitive effects, allowing for more accurate determination of electron transfer kinetics. The company's protocols incorporate temperature-controlled experiments to calculate activation energies, which further aids in distinguishing between surface-limited and diffusion-limited processes. Applied Materials' systems also feature real-time data processing algorithms that analyze peak shapes, positions, and dependencies on scan rate to automatically classify processes according to their rate-determining steps.
Strengths: Seamless integration with semiconductor manufacturing equipment allowing for in-line process monitoring; highly reproducible measurements with automated analysis capabilities. Weaknesses: Systems primarily optimized for semiconductor applications rather than general electrochemistry; significant capital investment required; complex setup procedures that require specialized training.
ASM International NV
Technical Solution: ASM International has developed sophisticated CV experimental protocols specifically designed to differentiate between surface and diffusion-controlled processes in atomic layer deposition (ALD) and chemical vapor deposition (CVD) applications. Their methodology employs a multi-parameter approach combining variable scan rate analysis with specialized electrode configurations. The protocol systematically varies scan rates from 5-200 mV/s while monitoring peak current relationships - linear relationships indicating surface-controlled processes and square root relationships suggesting diffusion control. ASM's approach incorporates rotating disk electrode techniques to create well-defined mass transport conditions, allowing precise control over diffusion layer thickness. Their systems feature temperature-controlled cells enabling activation energy determination through Arrhenius analysis, providing additional mechanistic insights. The company has also developed specialized thin-film electrodes with precisely controlled surface areas that enable quantitative surface coverage calculations. Their data analysis platform includes advanced peak deconvolution algorithms to separate overlapping processes and digital simulation capabilities to fit experimental data to theoretical models.
Strengths: Highly specialized for thin film and semiconductor applications; excellent reproducibility through precise environmental control; comprehensive software for mechanism identification. Weaknesses: Systems optimized primarily for semiconductor materials rather than general electrochemistry; complex setup and calibration procedures; significant capital investment required.
Key Analytical Techniques for Process Mechanism Identification
Rare earth metal incorporated zeolite modified electrodes for detection and quantification of heavy metal ions in aqueous solution
PatentInactiveUS20170315079A1
Innovation
- Development of rare earth metal impregnated zeolite modified carbon paste electrodes, specifically lanthanum or cerium impregnated mordenite electrodes, for use in square wave anodic stripping voltammetry, enhancing electroactive surface area and detection limits.
Cobalt chemistry for smooth topology
PatentWO2021118972A1
Innovation
- The use of cyclic voltammetry to screen and evaluate additives in cobalt electroplating baths, combined with a cobalt electrolyte composition including boric acid, organic suppressors, and optional additives, to achieve a seamless, seam-free, and void-free cobalt deposit with controlled overburden thickness.
Standardization and Validation Frameworks
To ensure the reliability and reproducibility of cyclic voltammetry (CV) experiments distinguishing between surface and diffusion-controlled processes, standardized frameworks and validation protocols are essential. The electrochemical community has developed several standardization approaches that serve as benchmarks for experimental design and data interpretation. These frameworks typically include reference materials with well-characterized electrochemical behaviors, such as ferrocene/ferrocenium for surface-controlled processes and ruthenium hexamine for diffusion-controlled systems.
A comprehensive standardization framework begins with electrode preparation protocols, as surface conditions significantly impact electrochemical responses. Standardized polishing procedures for different electrode materials (glassy carbon, gold, platinum) must be meticulously documented, including abrasive materials, polishing duration, and post-polishing cleaning steps. Electrochemical preconditioning protocols, such as potential cycling in specific electrolytes, further ensure reproducible electrode surfaces.
Validation methodologies incorporate statistical analysis of key CV parameters across multiple experiments. For surface-controlled processes, peak potential separation (ΔEp) should approach the theoretical 59/n mV at 25°C, while peak current ratios (ipa/ipc) should approximate unity. Diffusion-controlled processes require validation through Randles-Sevcik analysis, confirming the linear relationship between peak current and square root of scan rate. Deviation metrics from these theoretical behaviors provide quantitative measures of experimental quality.
Interlaboratory comparison studies represent another critical component of validation frameworks. Round-robin testing across multiple research facilities using identical protocols helps identify systematic errors and establish reproducibility limits. These collaborative efforts have led to the development of standardized reporting formats that specify minimum required experimental parameters, including reference electrode details, solution resistance compensation methods, and temperature control specifications.
Digital data validation tools have emerged to automate the assessment of experimental quality. These software solutions analyze raw CV data against theoretical models, flagging potential experimental artifacts such as uncompensated resistance effects or electrode fouling. Machine learning algorithms are increasingly being integrated into these validation frameworks to identify subtle patterns indicative of mixed kinetic regimes or adsorption effects that might otherwise be overlooked in manual analysis.
Uncertainty quantification represents the frontier of CV validation frameworks. Modern approaches incorporate Monte Carlo simulations to propagate experimental uncertainties through data analysis pipelines, providing confidence intervals for derived kinetic parameters. This statistical rigor is particularly important when distinguishing between surface and diffusion control in complex systems where both mechanisms may contribute to the observed electrochemical response.
A comprehensive standardization framework begins with electrode preparation protocols, as surface conditions significantly impact electrochemical responses. Standardized polishing procedures for different electrode materials (glassy carbon, gold, platinum) must be meticulously documented, including abrasive materials, polishing duration, and post-polishing cleaning steps. Electrochemical preconditioning protocols, such as potential cycling in specific electrolytes, further ensure reproducible electrode surfaces.
Validation methodologies incorporate statistical analysis of key CV parameters across multiple experiments. For surface-controlled processes, peak potential separation (ΔEp) should approach the theoretical 59/n mV at 25°C, while peak current ratios (ipa/ipc) should approximate unity. Diffusion-controlled processes require validation through Randles-Sevcik analysis, confirming the linear relationship between peak current and square root of scan rate. Deviation metrics from these theoretical behaviors provide quantitative measures of experimental quality.
Interlaboratory comparison studies represent another critical component of validation frameworks. Round-robin testing across multiple research facilities using identical protocols helps identify systematic errors and establish reproducibility limits. These collaborative efforts have led to the development of standardized reporting formats that specify minimum required experimental parameters, including reference electrode details, solution resistance compensation methods, and temperature control specifications.
Digital data validation tools have emerged to automate the assessment of experimental quality. These software solutions analyze raw CV data against theoretical models, flagging potential experimental artifacts such as uncompensated resistance effects or electrode fouling. Machine learning algorithms are increasingly being integrated into these validation frameworks to identify subtle patterns indicative of mixed kinetic regimes or adsorption effects that might otherwise be overlooked in manual analysis.
Uncertainty quantification represents the frontier of CV validation frameworks. Modern approaches incorporate Monte Carlo simulations to propagate experimental uncertainties through data analysis pipelines, providing confidence intervals for derived kinetic parameters. This statistical rigor is particularly important when distinguishing between surface and diffusion control in complex systems where both mechanisms may contribute to the observed electrochemical response.
Interdisciplinary Applications and Future Trends
Cyclic voltammetry (CV) techniques for distinguishing surface versus diffusion-controlled processes are increasingly finding applications beyond traditional electrochemistry domains. The integration of these analytical methods into fields such as biomedical engineering has revolutionized biosensor development, enabling more precise detection of surface-bound biomarkers versus diffusion-limited analytes in complex biological matrices. This distinction has proven critical for developing next-generation point-of-care diagnostic devices with enhanced sensitivity and specificity.
In environmental monitoring, CV experimental protocols are being adapted to characterize pollutant behavior at environmental interfaces. By differentiating between contaminants that adsorb to surfaces versus those that remain mobile in solution, researchers can better predict environmental fate and develop more effective remediation strategies. These applications have particular relevance for understanding microplastic-pollutant interactions and nanomaterial behavior in aquatic systems.
The materials science sector has embraced these electrochemical techniques for smart material development. By understanding surface versus bulk diffusion processes, researchers can fine-tune properties of energy storage materials, catalysts, and responsive surfaces. This has led to breakthroughs in battery technology where electrode-electrolyte interface phenomena critically influence performance and longevity.
Looking toward future trends, artificial intelligence integration with CV experimental design represents a significant frontier. Machine learning algorithms are being developed to automatically optimize experimental parameters, identify process signatures, and extract mechanistic insights from complex voltammetric data. This computational approach promises to accelerate discovery by reducing the iterative experimental cycles traditionally required.
Miniaturization and high-throughput screening methodologies are emerging as another important trend. Microfluidic platforms incorporating arrays of microelectrodes enable parallel CV experiments with minimal sample volumes, particularly valuable for pharmaceutical development and toxicological screening. These systems can simultaneously evaluate multiple compounds or conditions, dramatically accelerating the characterization of surface versus diffusion-controlled processes.
The convergence of CV techniques with in situ spectroscopic methods represents perhaps the most transformative future direction. Combined electrochemical-spectroscopic approaches provide real-time molecular-level insights into interfacial processes during potential cycling. This multi-modal characterization offers unprecedented mechanistic understanding, bridging the gap between macroscopic electrochemical signals and molecular-scale phenomena at interfaces.
In environmental monitoring, CV experimental protocols are being adapted to characterize pollutant behavior at environmental interfaces. By differentiating between contaminants that adsorb to surfaces versus those that remain mobile in solution, researchers can better predict environmental fate and develop more effective remediation strategies. These applications have particular relevance for understanding microplastic-pollutant interactions and nanomaterial behavior in aquatic systems.
The materials science sector has embraced these electrochemical techniques for smart material development. By understanding surface versus bulk diffusion processes, researchers can fine-tune properties of energy storage materials, catalysts, and responsive surfaces. This has led to breakthroughs in battery technology where electrode-electrolyte interface phenomena critically influence performance and longevity.
Looking toward future trends, artificial intelligence integration with CV experimental design represents a significant frontier. Machine learning algorithms are being developed to automatically optimize experimental parameters, identify process signatures, and extract mechanistic insights from complex voltammetric data. This computational approach promises to accelerate discovery by reducing the iterative experimental cycles traditionally required.
Miniaturization and high-throughput screening methodologies are emerging as another important trend. Microfluidic platforms incorporating arrays of microelectrodes enable parallel CV experiments with minimal sample volumes, particularly valuable for pharmaceutical development and toxicological screening. These systems can simultaneously evaluate multiple compounds or conditions, dramatically accelerating the characterization of surface versus diffusion-controlled processes.
The convergence of CV techniques with in situ spectroscopic methods represents perhaps the most transformative future direction. Combined electrochemical-spectroscopic approaches provide real-time molecular-level insights into interfacial processes during potential cycling. This multi-modal characterization offers unprecedented mechanistic understanding, bridging the gap between macroscopic electrochemical signals and molecular-scale phenomena at interfaces.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!