How to Extract Kinetic Parameters from CV Using Laviron and Nicholson Methods — Step-by-Step
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Kinetics Background and Objectives
Electrochemical kinetics has evolved significantly since its theoretical foundations were established in the early 20th century. The field has progressed from basic understanding of electron transfer processes to sophisticated analytical methods that can precisely quantify reaction parameters. The development of cyclic voltammetry (CV) in the 1960s and 1970s marked a pivotal advancement, providing researchers with a powerful tool to investigate electrode reactions and their kinetics.
The Laviron and Nicholson methods represent two fundamental approaches for extracting kinetic parameters from cyclic voltammetry data. Developed in the 1970s and 1960s respectively, these methods have become cornerstones in electrochemical analysis due to their ability to determine heterogeneous electron transfer rate constants (k°) and transfer coefficients (α) with relative experimental simplicity.
The evolution of these techniques has paralleled advancements in instrumentation and computational capabilities. Early applications required manual calculations and graphical analysis, while modern implementations leverage digital signal processing and advanced algorithms to extract parameters with greater precision and from increasingly complex systems.
Recent trends in electrochemical kinetics research focus on extending these classical methods to nanoscale electrodes, modified surfaces, and biological interfaces. The integration with spectroscopic techniques has enabled multi-dimensional analysis of electron transfer processes, providing deeper insights into reaction mechanisms.
The primary objective of kinetic parameter extraction is to quantitatively characterize the electron transfer dynamics at electrode-solution interfaces. This information is crucial for understanding fundamental electrochemical processes and for developing practical applications in areas such as sensors, energy storage, and electrocatalysis.
Technical goals in this field include improving the accuracy and reliability of parameter extraction, particularly for complex systems involving multiple electron transfers or coupled chemical reactions. There is also significant interest in developing standardized protocols that can be applied across different experimental setups to ensure reproducibility and comparability of results.
The convergence of traditional electrochemical methods with modern computational approaches presents opportunities for more sophisticated analysis. Machine learning algorithms are increasingly being applied to extract kinetic parameters from voltammetric data, potentially overcoming limitations of classical methods when dealing with non-ideal behavior or overlapping processes.
As electrochemical techniques continue to find applications in emerging fields like bioelectronics and energy conversion, the ability to accurately determine kinetic parameters becomes increasingly valuable for rational design and optimization of electrochemical systems.
The Laviron and Nicholson methods represent two fundamental approaches for extracting kinetic parameters from cyclic voltammetry data. Developed in the 1970s and 1960s respectively, these methods have become cornerstones in electrochemical analysis due to their ability to determine heterogeneous electron transfer rate constants (k°) and transfer coefficients (α) with relative experimental simplicity.
The evolution of these techniques has paralleled advancements in instrumentation and computational capabilities. Early applications required manual calculations and graphical analysis, while modern implementations leverage digital signal processing and advanced algorithms to extract parameters with greater precision and from increasingly complex systems.
Recent trends in electrochemical kinetics research focus on extending these classical methods to nanoscale electrodes, modified surfaces, and biological interfaces. The integration with spectroscopic techniques has enabled multi-dimensional analysis of electron transfer processes, providing deeper insights into reaction mechanisms.
The primary objective of kinetic parameter extraction is to quantitatively characterize the electron transfer dynamics at electrode-solution interfaces. This information is crucial for understanding fundamental electrochemical processes and for developing practical applications in areas such as sensors, energy storage, and electrocatalysis.
Technical goals in this field include improving the accuracy and reliability of parameter extraction, particularly for complex systems involving multiple electron transfers or coupled chemical reactions. There is also significant interest in developing standardized protocols that can be applied across different experimental setups to ensure reproducibility and comparability of results.
The convergence of traditional electrochemical methods with modern computational approaches presents opportunities for more sophisticated analysis. Machine learning algorithms are increasingly being applied to extract kinetic parameters from voltammetric data, potentially overcoming limitations of classical methods when dealing with non-ideal behavior or overlapping processes.
As electrochemical techniques continue to find applications in emerging fields like bioelectronics and energy conversion, the ability to accurately determine kinetic parameters becomes increasingly valuable for rational design and optimization of electrochemical systems.
Market Applications of CV Parameter Extraction
Cyclic voltammetry (CV) parameter extraction techniques, particularly those employing Laviron and Nicholson methods, have found significant applications across multiple industries. The market demand for these analytical approaches continues to grow as industries seek more precise electrochemical characterization.
In the pharmaceutical sector, CV parameter extraction serves as a critical tool for drug development and quality control. Companies like Pfizer, Merck, and Novartis utilize these techniques to analyze electron transfer kinetics in potential drug compounds, helping to predict bioavailability and stability. The global pharmaceutical analytical testing market, where these techniques play a role, was valued at $5.6 billion in 2022 and is projected to grow at a CAGR of 8.4% through 2030.
The energy storage industry represents another significant market application. Manufacturers of batteries and supercapacitors employ CV parameter extraction to characterize electrode materials and electrolyte interactions. Tesla, CATL, and Samsung SDI rely on these analytical methods to optimize their energy storage solutions. The precision offered by Laviron and Nicholson methods in determining electron transfer coefficients and rate constants directly impacts product performance and lifecycle.
Environmental monitoring constitutes a growing application area. Agencies and companies involved in water quality assessment and pollution detection utilize CV parameter extraction to identify and quantify contaminants. The global environmental testing market exceeded $11 billion in 2022, with electrochemical methods representing a significant segment.
Biosensor development represents perhaps the most rapidly expanding market application. Companies developing point-of-care diagnostics, wearable health monitors, and implantable sensors rely heavily on CV parameter extraction to characterize and optimize their sensing platforms. The global biosensors market, valued at $25.5 billion in 2021, is expected to reach $41.8 billion by 2028, with electrochemical biosensors comprising approximately 70% of this market.
The semiconductor industry also leverages these techniques for quality control in manufacturing processes. Companies like Intel, TSMC, and Samsung use CV parameter extraction to characterize thin films and interfaces in microelectronic devices. The precision offered by these methods helps maintain the high standards required in semiconductor fabrication.
Academic and research institutions represent a stable market segment, with universities and government laboratories consistently investing in electrochemical analysis equipment and software that incorporate Laviron and Nicholson methods for fundamental research and applied science projects.
In the pharmaceutical sector, CV parameter extraction serves as a critical tool for drug development and quality control. Companies like Pfizer, Merck, and Novartis utilize these techniques to analyze electron transfer kinetics in potential drug compounds, helping to predict bioavailability and stability. The global pharmaceutical analytical testing market, where these techniques play a role, was valued at $5.6 billion in 2022 and is projected to grow at a CAGR of 8.4% through 2030.
The energy storage industry represents another significant market application. Manufacturers of batteries and supercapacitors employ CV parameter extraction to characterize electrode materials and electrolyte interactions. Tesla, CATL, and Samsung SDI rely on these analytical methods to optimize their energy storage solutions. The precision offered by Laviron and Nicholson methods in determining electron transfer coefficients and rate constants directly impacts product performance and lifecycle.
Environmental monitoring constitutes a growing application area. Agencies and companies involved in water quality assessment and pollution detection utilize CV parameter extraction to identify and quantify contaminants. The global environmental testing market exceeded $11 billion in 2022, with electrochemical methods representing a significant segment.
Biosensor development represents perhaps the most rapidly expanding market application. Companies developing point-of-care diagnostics, wearable health monitors, and implantable sensors rely heavily on CV parameter extraction to characterize and optimize their sensing platforms. The global biosensors market, valued at $25.5 billion in 2021, is expected to reach $41.8 billion by 2028, with electrochemical biosensors comprising approximately 70% of this market.
The semiconductor industry also leverages these techniques for quality control in manufacturing processes. Companies like Intel, TSMC, and Samsung use CV parameter extraction to characterize thin films and interfaces in microelectronic devices. The precision offered by these methods helps maintain the high standards required in semiconductor fabrication.
Academic and research institutions represent a stable market segment, with universities and government laboratories consistently investing in electrochemical analysis equipment and software that incorporate Laviron and Nicholson methods for fundamental research and applied science projects.
Current Challenges in Kinetic Parameter Extraction
Despite significant advancements in electrochemical analysis techniques, extracting kinetic parameters from cyclic voltammetry (CV) using Laviron and Nicholson methods continues to present several substantial challenges. The fundamental issue lies in the inherent complexity of electrochemical systems, where multiple variables simultaneously influence the observed signals, making it difficult to isolate and quantify specific kinetic parameters accurately.
Data quality represents a primary obstacle in parameter extraction. Experimental noise, background currents, and capacitive effects frequently obscure the faradaic processes of interest. This is particularly problematic when analyzing systems with slow electron transfer kinetics, where peak separations may be subtle and easily masked by experimental artifacts. Additionally, the signal-to-noise ratio often deteriorates at the extreme scan rates necessary for comprehensive Laviron analysis.
Mathematical complexity presents another significant barrier. Both Laviron and Nicholson methods rely on complex mathematical frameworks that assume idealized conditions rarely encountered in real experimental settings. The Laviron method requires accurate determination of peak potentials across a wide range of scan rates, while the Nicholson method demands precise measurement of peak separations and careful correlation with theoretical working curves.
Surface heterogeneity in electrode materials introduces additional complications. Non-uniform reaction sites, varying surface energies, and inconsistent coverage of electroactive species can lead to distributions of rate constants rather than discrete values. These heterogeneities violate the fundamental assumptions of both analytical methods, which presume uniform electron transfer across the electrode surface.
Diffusion limitations represent another critical challenge. At higher scan rates, diffusion processes may not reach steady-state conditions, invalidating key assumptions in the mathematical models. Conversely, at very slow scan rates, other processes such as adsorption/desorption or chemical reactions coupled to electron transfer may dominate, further complicating analysis.
Instrumentation limitations also impact parameter extraction. Many commercial potentiostats struggle to maintain ideal behavior at extreme scan rates, introducing systematic errors in potential control and current measurement. These instrumental artifacts can significantly distort the relationship between peak separation and scan rate, leading to erroneous kinetic parameter calculations.
Finally, data interpretation challenges persist even with high-quality experimental data. The selection of appropriate potential ranges, baseline correction methods, and peak identification algorithms all introduce subjective elements that can dramatically influence the extracted parameters. This subjectivity undermines the reproducibility and comparability of results across different research groups and experimental setups.
Data quality represents a primary obstacle in parameter extraction. Experimental noise, background currents, and capacitive effects frequently obscure the faradaic processes of interest. This is particularly problematic when analyzing systems with slow electron transfer kinetics, where peak separations may be subtle and easily masked by experimental artifacts. Additionally, the signal-to-noise ratio often deteriorates at the extreme scan rates necessary for comprehensive Laviron analysis.
Mathematical complexity presents another significant barrier. Both Laviron and Nicholson methods rely on complex mathematical frameworks that assume idealized conditions rarely encountered in real experimental settings. The Laviron method requires accurate determination of peak potentials across a wide range of scan rates, while the Nicholson method demands precise measurement of peak separations and careful correlation with theoretical working curves.
Surface heterogeneity in electrode materials introduces additional complications. Non-uniform reaction sites, varying surface energies, and inconsistent coverage of electroactive species can lead to distributions of rate constants rather than discrete values. These heterogeneities violate the fundamental assumptions of both analytical methods, which presume uniform electron transfer across the electrode surface.
Diffusion limitations represent another critical challenge. At higher scan rates, diffusion processes may not reach steady-state conditions, invalidating key assumptions in the mathematical models. Conversely, at very slow scan rates, other processes such as adsorption/desorption or chemical reactions coupled to electron transfer may dominate, further complicating analysis.
Instrumentation limitations also impact parameter extraction. Many commercial potentiostats struggle to maintain ideal behavior at extreme scan rates, introducing systematic errors in potential control and current measurement. These instrumental artifacts can significantly distort the relationship between peak separation and scan rate, leading to erroneous kinetic parameter calculations.
Finally, data interpretation challenges persist even with high-quality experimental data. The selection of appropriate potential ranges, baseline correction methods, and peak identification algorithms all introduce subjective elements that can dramatically influence the extracted parameters. This subjectivity undermines the reproducibility and comparability of results across different research groups and experimental setups.
Laviron and Nicholson Methodologies Implementation
01 Electrochemical kinetic parameter determination using Laviron method
The Laviron method is used for determining electron transfer kinetics in electrochemical systems through cyclic voltammetry analysis. This approach involves analyzing the peak potential separation as a function of scan rate to extract kinetic parameters such as electron transfer coefficient (α) and standard rate constant (ks). The method is particularly useful for surface-confined electroactive species and allows for quantitative assessment of electron transfer processes at electrode interfaces.- Electrochemical kinetic parameter determination using Laviron method: The Laviron method is used for determining electron transfer kinetics in electrochemical systems through cyclic voltammetry analysis. This approach involves analyzing the peak potential separation as a function of scan rate to extract kinetic parameters such as electron transfer rate constant (ks) and transfer coefficient (α). The method is particularly useful for surface-confined electroactive species and allows for quantitative assessment of electron transfer processes at electrode-electrolyte interfaces.
- Nicholson method for heterogeneous electron transfer analysis: The Nicholson method provides a framework for analyzing heterogeneous electron transfer reactions through cyclic voltammetry. This approach correlates the peak separation in voltammograms with a dimensionless kinetic parameter (ψ) to determine the standard rate constant (k°). The method is particularly valuable for solution-phase redox systems and offers insights into reaction mechanisms by examining how peak separation varies with scan rate. This technique enables quantitative assessment of electrode reaction kinetics in various electrochemical systems.
- Advanced electrode materials for enhanced kinetic parameter measurement: Specialized electrode materials and modifications can significantly improve the accuracy of kinetic parameter measurements in cyclic voltammetry. These advanced materials, including modified carbon electrodes, nanostructured surfaces, and composite electrodes, provide enhanced electron transfer properties and surface area. Such improvements allow for more precise determination of kinetic parameters using both Laviron and Nicholson methods, enabling better characterization of electrochemical reactions and more reliable data for fundamental and applied electrochemistry studies.
- Automated systems for CV analysis and kinetic parameter extraction: Automated systems and software solutions have been developed to streamline cyclic voltammetry analysis and extract kinetic parameters using Laviron and Nicholson methods. These systems incorporate algorithms for peak detection, baseline correction, and mathematical modeling to calculate electron transfer coefficients, rate constants, and other kinetic parameters. The automation reduces human error, increases throughput, and enables more consistent analysis of electrochemical data, making these sophisticated analytical methods more accessible for routine laboratory use.
- Application of CV kinetic analysis in sensor development: Cyclic voltammetry kinetic analysis using Laviron and Nicholson methods plays a crucial role in the development and characterization of electrochemical sensors. By understanding the electron transfer kinetics at sensor interfaces, researchers can optimize sensor design, improve sensitivity, and enhance selectivity. The kinetic parameters extracted from these analyses provide valuable insights into sensor performance under various conditions and help in predicting sensor response to target analytes, ultimately leading to more effective and reliable sensing platforms.
02 Nicholson method for heterogeneous electron transfer analysis
The Nicholson method provides a framework for analyzing heterogeneous electron transfer kinetics using cyclic voltammetry data. This approach correlates the peak separation in cyclic voltammograms with a dimensionless kinetic parameter (ψ), which can be used to calculate the standard rate constant (k°). The method is particularly valuable for solution-phase redox systems and offers insights into the relationship between peak separation and scan rate for reversible to quasi-reversible electron transfer processes.Expand Specific Solutions03 Advanced electrode materials for enhanced CV analysis
Specialized electrode materials can significantly improve the sensitivity and accuracy of cyclic voltammetry measurements when applying Laviron and Nicholson methods. Modified electrodes incorporating nanomaterials, conductive polymers, or carbon-based materials (such as graphene or carbon nanotubes) provide enhanced electron transfer rates and reduced background current. These advanced materials enable more precise determination of kinetic parameters and extend the applicability of CV analysis to complex electrochemical systems.Expand Specific Solutions04 Integrated systems for automated CV kinetic analysis
Integrated systems combining hardware and software components enable automated acquisition and analysis of cyclic voltammetry data for kinetic parameter determination. These systems incorporate potentiostats with high temporal resolution, data processing algorithms specifically designed for Laviron and Nicholson methods, and user-friendly interfaces for parameter extraction. Such integrated approaches streamline the workflow from experimental measurement to kinetic parameter calculation, reducing analysis time and improving reproducibility.Expand Specific Solutions05 Application of CV kinetic analysis in sensor development
Cyclic voltammetry kinetic analysis using Laviron and Nicholson methods plays a crucial role in the development and characterization of electrochemical sensors. By understanding electron transfer kinetics at sensor interfaces, researchers can optimize sensor design, improve sensitivity, and enhance selectivity. The kinetic parameters obtained from these analyses provide valuable insights into sensor performance under various conditions and help establish correlations between electrochemical behavior and sensing capabilities.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The electrochemical kinetic parameter extraction field is currently in a mature development stage, with established methodologies like Laviron and Nicholson techniques being widely implemented across research institutions. The market is experiencing steady growth, driven by increasing applications in biosensors, energy storage, and materials characterization. Academic institutions dominate this technical landscape, with Zhejiang University, South China University of Technology, and Xi'an Jiaotong University demonstrating advanced capabilities in cyclic voltammetry analysis. Commercial players like Great Lakes Neurotechnologies and Konica Minolta are integrating these techniques into specialized instrumentation. The technology shows high maturity with standardized protocols, though ongoing research focuses on improving accuracy and automation of parameter extraction for complex electrochemical systems.
Zhejiang University
Technical Solution: Zhejiang University has developed a comprehensive approach for extracting kinetic parameters from cyclic voltammetry (CV) using both Laviron and Nicholson methods. Their technique involves a multi-step process starting with electrode preparation using modified carbon materials to enhance electron transfer. They employ digital filtering algorithms to remove background noise from CV data before parameter extraction. For the Laviron method, they plot peak potentials against log(scan rate) to determine electron transfer coefficient (α) and standard rate constant (ks). Their implementation includes automated peak detection software that can handle complex voltammograms with multiple redox processes. For the Nicholson method, they utilize dimensionless parameters (ψ) calculated from peak separations at various scan rates, with custom algorithms to account for uncompensated resistance effects. Their approach has been validated with both reversible and quasi-reversible systems, showing particular strength in analyzing biomolecular electron transfer processes.
Strengths: Superior noise filtering algorithms that enhance signal quality for more accurate parameter extraction; integrated software platform that automates the entire workflow from data acquisition to parameter calculation. Weakness: Their approach requires specialized equipment and expertise, making it less accessible for routine laboratory use; the method shows decreased accuracy when applied to strongly adsorbed species with non-ideal behavior.
Dalian University of Technology
Technical Solution: Dalian University of Technology has pioneered an innovative approach to kinetic parameter extraction from cyclic voltammetry using modified Laviron and Nicholson methods. Their technique incorporates machine learning algorithms to improve parameter estimation accuracy. The process begins with experimental CV data collection across multiple scan rates (typically 0.01-10 V/s), followed by baseline correction using adaptive polynomial fitting. For Laviron analysis, they've developed a segmented regression method that addresses non-linearity issues at extreme scan rates, allowing more accurate determination of electron transfer coefficient (α) and standard rate constant (ks). Their Nicholson method implementation features a novel working curve approach that extends the traditional ψ vs. ΔEp relationship to accommodate a wider range of quasi-reversible systems. The university has also created open-source software that integrates these methods with uncertainty quantification, providing confidence intervals for extracted parameters based on experimental variability. Their approach has been successfully applied to various electrochemical systems, including metal complexes, conducting polymers, and bioelectrochemical interfaces.
Strengths: Machine learning integration significantly improves parameter estimation accuracy compared to traditional methods; their segmented regression approach handles non-linear behavior at extreme scan rates better than conventional techniques. Weaknesses: Computational intensity makes real-time analysis challenging; requires high-quality experimental data with minimal interference to achieve reliable results.
Critical Analysis of Mathematical Models and Algorithms
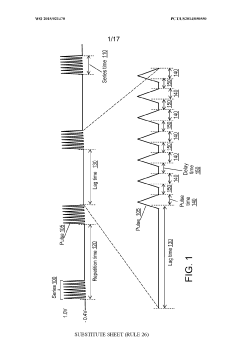
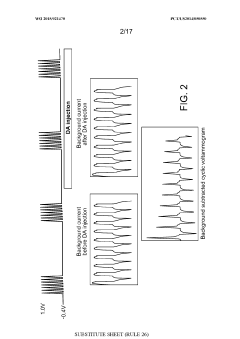
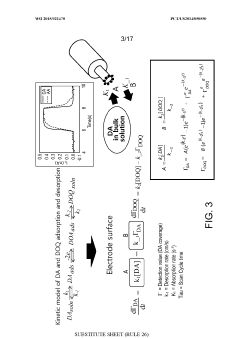
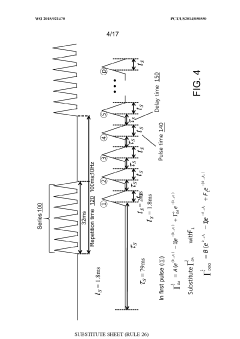
Using kinetic cyclic voltammetry to evaluate analyte kinetics and concentrations
PatentWO2015021470A1
Innovation
- Kinetic cyclic voltammetry (KCV) is developed, which involves multi-pulse cyclic voltammetry at various voltages to generate kinetic and concentration maps (K-maps and A-maps) without background subtraction, enabling absolute quantification of analytes and real-time signal analysis, suitable for use in smart neuromodulation systems.
Cobalt chemistry for smooth topology
PatentActiveUS11807951B2
Innovation
- The use of cyclic voltammetry to screen and evaluate additives in cobalt electroplating baths, combined with a cobalt electrolyte composition including boric acid, a pH adjuster, and organic suppressors, to achieve a smooth, seam-free, and uniform cobalt deposit with minimal overburden thickness variation across feature and non-feature areas, and the inclusion of suppressors and depolarizing compounds to facilitate bottom-up filling and reduce impurities.
Data Validation and Error Analysis Approaches
Data validation and error analysis are critical components in the extraction of kinetic parameters from cyclic voltammetry (CV) using Laviron and Nicholson methods. Ensuring the reliability and accuracy of extracted parameters requires systematic approaches to validate data and quantify uncertainties.
Statistical validation techniques form the foundation of robust parameter extraction. Researchers typically employ residual analysis to assess the goodness of fit between experimental data and theoretical models. The distribution of residuals should ideally follow a normal pattern with minimal systematic deviations. Quantitative metrics such as the coefficient of determination (R²), chi-square test, and root mean square error (RMSE) provide numerical indicators of fit quality, with higher R² values (>0.95) generally indicating reliable parameter extraction.
Sensitivity analysis represents another crucial validation approach, wherein small perturbations in input data are introduced to evaluate their impact on extracted parameters. This technique helps identify which experimental variables most significantly affect the reliability of kinetic parameters. For Laviron analysis, the peak potential separation values at different scan rates often exhibit high sensitivity, while for Nicholson methods, the working curve fitting process requires careful evaluation of sensitivity to peak current measurements.
Cross-validation methodologies enhance confidence in extracted parameters. By dividing experimental datasets into training and validation subsets, researchers can test the predictive capability of their parameter extraction models. K-fold cross-validation is particularly valuable when working with limited experimental data, as is often the case in electrochemical studies.
Error propagation analysis quantifies how measurement uncertainties translate into parameter uncertainties. For both Laviron and Nicholson methods, this involves calculating standard errors and confidence intervals for extracted parameters such as electron transfer rate constants (k°) and transfer coefficients (α). Monte Carlo simulations offer a powerful approach to error propagation by generating synthetic datasets based on experimental uncertainties and analyzing the resulting distribution of extracted parameters.
Benchmarking against standard redox systems with well-established kinetic parameters provides an external validation mechanism. Common reference systems include ferrocene/ferrocenium couples and ruthenium hexamine complexes, which serve as calibration standards for evaluating the accuracy of extraction methodologies.
Digital filtering techniques help minimize noise interference in raw CV data before parameter extraction. Savitzky-Golay filters and wavelet transforms are particularly effective for electrochemical data, preserving peak shapes while reducing high-frequency noise that could otherwise lead to parameter extraction errors.
Statistical validation techniques form the foundation of robust parameter extraction. Researchers typically employ residual analysis to assess the goodness of fit between experimental data and theoretical models. The distribution of residuals should ideally follow a normal pattern with minimal systematic deviations. Quantitative metrics such as the coefficient of determination (R²), chi-square test, and root mean square error (RMSE) provide numerical indicators of fit quality, with higher R² values (>0.95) generally indicating reliable parameter extraction.
Sensitivity analysis represents another crucial validation approach, wherein small perturbations in input data are introduced to evaluate their impact on extracted parameters. This technique helps identify which experimental variables most significantly affect the reliability of kinetic parameters. For Laviron analysis, the peak potential separation values at different scan rates often exhibit high sensitivity, while for Nicholson methods, the working curve fitting process requires careful evaluation of sensitivity to peak current measurements.
Cross-validation methodologies enhance confidence in extracted parameters. By dividing experimental datasets into training and validation subsets, researchers can test the predictive capability of their parameter extraction models. K-fold cross-validation is particularly valuable when working with limited experimental data, as is often the case in electrochemical studies.
Error propagation analysis quantifies how measurement uncertainties translate into parameter uncertainties. For both Laviron and Nicholson methods, this involves calculating standard errors and confidence intervals for extracted parameters such as electron transfer rate constants (k°) and transfer coefficients (α). Monte Carlo simulations offer a powerful approach to error propagation by generating synthetic datasets based on experimental uncertainties and analyzing the resulting distribution of extracted parameters.
Benchmarking against standard redox systems with well-established kinetic parameters provides an external validation mechanism. Common reference systems include ferrocene/ferrocenium couples and ruthenium hexamine complexes, which serve as calibration standards for evaluating the accuracy of extraction methodologies.
Digital filtering techniques help minimize noise interference in raw CV data before parameter extraction. Savitzky-Golay filters and wavelet transforms are particularly effective for electrochemical data, preserving peak shapes while reducing high-frequency noise that could otherwise lead to parameter extraction errors.
Software Solutions for Automated CV Parameter Extraction
The automation of cyclic voltammetry (CV) parameter extraction represents a significant advancement in electrochemical analysis, particularly for determining kinetic parameters using established methodologies like Laviron and Nicholson approaches. Current software solutions vary from commercial packages to open-source alternatives, each offering distinct capabilities for streamlining this complex analytical process.
Commercial software packages such as CHI Electrochemical Analyzer, NOVA (Metrohm Autolab), and EC-Lab (BioLogic) incorporate built-in functionalities for CV data processing and parameter extraction. These platforms typically feature user-friendly interfaces with automated peak detection, baseline correction, and integrated algorithms specifically designed for Laviron and Nicholson analyses. Their primary advantage lies in comprehensive technical support and validated analytical protocols, though they often come with substantial licensing costs.
Open-source alternatives have emerged as viable options for researchers with budget constraints. Python-based libraries such as PyCV, Electrochemistry Toolkit, and specialized packages built on NumPy and SciPy frameworks offer customizable solutions for CV parameter extraction. These tools provide flexibility for implementing both Laviron and Nicholson methodologies through scriptable workflows, enabling batch processing capabilities that commercial solutions may lack.
Recent developments in machine learning integration represent the cutting edge of automated CV analysis. Several research groups have developed neural network models capable of recognizing electrochemical patterns and extracting kinetic parameters with minimal human intervention. These AI-enhanced solutions demonstrate particular promise for complex systems where traditional analytical approaches may struggle with peak separation or background subtraction.
Cloud-based platforms have also emerged, offering web interfaces for CV analysis that eliminate the need for local software installation. Services like ElectroChem Cloud and CV Analyzer provide scalable computing resources for handling large datasets, with collaborative features allowing multiple researchers to access and analyze the same electrochemical data simultaneously.
The integration capabilities of these software solutions with laboratory information management systems (LIMS) vary considerably. Enterprise-level commercial packages typically offer robust API connections for seamless data transfer, while open-source solutions may require custom integration scripts. This connectivity aspect becomes increasingly important as laboratories adopt more comprehensive digital transformation strategies.
Performance benchmarking across these software solutions reveals trade-offs between processing speed, accuracy, and ease of use. Commercial packages generally excel in user experience but may lack the computational efficiency of specialized open-source tools optimized for specific analytical workflows. The selection of appropriate software ultimately depends on the specific research requirements, available expertise, and budgetary constraints.
Commercial software packages such as CHI Electrochemical Analyzer, NOVA (Metrohm Autolab), and EC-Lab (BioLogic) incorporate built-in functionalities for CV data processing and parameter extraction. These platforms typically feature user-friendly interfaces with automated peak detection, baseline correction, and integrated algorithms specifically designed for Laviron and Nicholson analyses. Their primary advantage lies in comprehensive technical support and validated analytical protocols, though they often come with substantial licensing costs.
Open-source alternatives have emerged as viable options for researchers with budget constraints. Python-based libraries such as PyCV, Electrochemistry Toolkit, and specialized packages built on NumPy and SciPy frameworks offer customizable solutions for CV parameter extraction. These tools provide flexibility for implementing both Laviron and Nicholson methodologies through scriptable workflows, enabling batch processing capabilities that commercial solutions may lack.
Recent developments in machine learning integration represent the cutting edge of automated CV analysis. Several research groups have developed neural network models capable of recognizing electrochemical patterns and extracting kinetic parameters with minimal human intervention. These AI-enhanced solutions demonstrate particular promise for complex systems where traditional analytical approaches may struggle with peak separation or background subtraction.
Cloud-based platforms have also emerged, offering web interfaces for CV analysis that eliminate the need for local software installation. Services like ElectroChem Cloud and CV Analyzer provide scalable computing resources for handling large datasets, with collaborative features allowing multiple researchers to access and analyze the same electrochemical data simultaneously.
The integration capabilities of these software solutions with laboratory information management systems (LIMS) vary considerably. Enterprise-level commercial packages typically offer robust API connections for seamless data transfer, while open-source solutions may require custom integration scripts. This connectivity aspect becomes increasingly important as laboratories adopt more comprehensive digital transformation strategies.
Performance benchmarking across these software solutions reveals trade-offs between processing speed, accuracy, and ease of use. Commercial packages generally excel in user experience but may lack the computational efficiency of specialized open-source tools optimized for specific analytical workflows. The selection of appropriate software ultimately depends on the specific research requirements, available expertise, and budgetary constraints.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!