How to Correctly Baseline and Subtract Capacitive Currents in CV — Practical Tips and Scripts
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CV Capacitive Current Fundamentals and Objectives
Cyclic voltammetry (CV) has evolved significantly since its introduction in the early 20th century, becoming one of the most versatile and widely used electroanalytical techniques in electrochemistry. The technique's development trajectory has been marked by continuous refinements in instrumentation, methodology, and data analysis approaches, particularly in addressing the challenge of capacitive current interference.
The fundamental principle of CV involves applying a triangular potential waveform to an electrode while measuring the resulting current. However, the measured current comprises both faradaic processes (electron transfer reactions) and non-faradaic processes (capacitive currents). This duality has presented persistent challenges in data interpretation since the technique's inception.
Capacitive currents arise from the electrical double layer formation at the electrode-electrolyte interface, acting essentially as a capacitor. As potential changes during CV scanning, this capacitive component can significantly mask the faradaic signals of interest, particularly at higher scan rates or when analyzing systems with low concentrations of electroactive species.
The evolution of CV analysis has seen several milestone developments, from simple graphical baseline subtraction methods in the 1960s to sophisticated digital filtering techniques in the 1980s and 1990s. Recent advances in computational methods have further enhanced our ability to separate these current components, with machine learning approaches emerging in the last decade.
The primary technical objective of this research is to establish robust, reproducible methodologies for accurate baseline determination and capacitive current subtraction in cyclic voltammetry. This includes developing algorithmic approaches that can adapt to various experimental conditions and electrode materials, while maintaining high fidelity to the underlying electrochemical processes.
Secondary objectives include quantifying the uncertainty introduced by different baseline correction methods, establishing standardized protocols applicable across diverse research environments, and creating open-source computational tools that implement these methods with appropriate validation metrics.
The significance of this technical challenge extends beyond mere data processing. Accurate capacitive current subtraction directly impacts the reliability of derived electrochemical parameters, including electron transfer kinetics, reaction mechanisms, and quantitative concentration determinations. These parameters are crucial in applications ranging from energy storage device characterization to bioelectrochemical sensing and fundamental studies of electron transfer processes.
As electrochemical methods continue to gain prominence in emerging fields like electrocatalysis, battery research, and biosensing, the need for standardized, reliable approaches to CV data processing becomes increasingly critical to ensure reproducibility and comparability across the scientific community.
The fundamental principle of CV involves applying a triangular potential waveform to an electrode while measuring the resulting current. However, the measured current comprises both faradaic processes (electron transfer reactions) and non-faradaic processes (capacitive currents). This duality has presented persistent challenges in data interpretation since the technique's inception.
Capacitive currents arise from the electrical double layer formation at the electrode-electrolyte interface, acting essentially as a capacitor. As potential changes during CV scanning, this capacitive component can significantly mask the faradaic signals of interest, particularly at higher scan rates or when analyzing systems with low concentrations of electroactive species.
The evolution of CV analysis has seen several milestone developments, from simple graphical baseline subtraction methods in the 1960s to sophisticated digital filtering techniques in the 1980s and 1990s. Recent advances in computational methods have further enhanced our ability to separate these current components, with machine learning approaches emerging in the last decade.
The primary technical objective of this research is to establish robust, reproducible methodologies for accurate baseline determination and capacitive current subtraction in cyclic voltammetry. This includes developing algorithmic approaches that can adapt to various experimental conditions and electrode materials, while maintaining high fidelity to the underlying electrochemical processes.
Secondary objectives include quantifying the uncertainty introduced by different baseline correction methods, establishing standardized protocols applicable across diverse research environments, and creating open-source computational tools that implement these methods with appropriate validation metrics.
The significance of this technical challenge extends beyond mere data processing. Accurate capacitive current subtraction directly impacts the reliability of derived electrochemical parameters, including electron transfer kinetics, reaction mechanisms, and quantitative concentration determinations. These parameters are crucial in applications ranging from energy storage device characterization to bioelectrochemical sensing and fundamental studies of electron transfer processes.
As electrochemical methods continue to gain prominence in emerging fields like electrocatalysis, battery research, and biosensing, the need for standardized, reliable approaches to CV data processing becomes increasingly critical to ensure reproducibility and comparability across the scientific community.
Market Applications for Accurate CV Analysis
The market for accurate cyclic voltammetry (CV) analysis spans multiple high-value sectors where precise electrochemical measurements are critical. In the pharmaceutical industry, accurate CV analysis enables researchers to evaluate drug interactions with biological systems at the molecular level, significantly reducing development costs and accelerating time-to-market for new medications. Companies like Pfizer and Merck have integrated advanced CV analysis into their drug discovery pipelines, resulting in more efficient screening processes.
Energy storage represents another substantial market application, with the global lithium-ion battery market projected to reach $129.3 billion by 2027. Precise CV analysis is essential for battery material development, where even small improvements in electrode performance can translate to significant competitive advantages. Tesla, CATL, and Samsung SDI rely on accurate CV measurements to characterize new electrode materials and optimize battery formulations.
In semiconductor manufacturing, where quality control demands are exceptionally high, CV analysis helps identify impurities and defects in materials. The semiconductor industry's strict tolerance requirements make accurate baseline subtraction particularly valuable, as it enables detection of minute electrochemical signals that would otherwise be obscured by capacitive currents.
Environmental monitoring represents a growing application area, with accurate CV analysis enabling detection of heavy metals and organic pollutants at concentrations below parts per billion. Water quality monitoring systems increasingly incorporate electrochemical sensors that depend on precise CV measurements to maintain regulatory compliance and protect public health.
The biosensor market, valued at $21.2 billion in 2020, heavily utilizes CV techniques for developing point-of-care diagnostic devices. Companies like Abbott and Roche have commercialized glucose sensors and immunoassays that rely on accurate CV measurements to achieve clinical-grade diagnostic accuracy in portable formats.
Corrosion monitoring in infrastructure and aerospace applications represents another significant market, where CV analysis helps predict material failures before they occur. The cost savings from preventing a single critical failure in these sectors can justify substantial investment in advanced electrochemical analysis tools.
As these markets continue to expand, demand for automated CV analysis software with accurate baseline subtraction capabilities is growing rapidly, creating opportunities for specialized analytical instrument providers and software developers to serve these diverse but technically demanding sectors.
Energy storage represents another substantial market application, with the global lithium-ion battery market projected to reach $129.3 billion by 2027. Precise CV analysis is essential for battery material development, where even small improvements in electrode performance can translate to significant competitive advantages. Tesla, CATL, and Samsung SDI rely on accurate CV measurements to characterize new electrode materials and optimize battery formulations.
In semiconductor manufacturing, where quality control demands are exceptionally high, CV analysis helps identify impurities and defects in materials. The semiconductor industry's strict tolerance requirements make accurate baseline subtraction particularly valuable, as it enables detection of minute electrochemical signals that would otherwise be obscured by capacitive currents.
Environmental monitoring represents a growing application area, with accurate CV analysis enabling detection of heavy metals and organic pollutants at concentrations below parts per billion. Water quality monitoring systems increasingly incorporate electrochemical sensors that depend on precise CV measurements to maintain regulatory compliance and protect public health.
The biosensor market, valued at $21.2 billion in 2020, heavily utilizes CV techniques for developing point-of-care diagnostic devices. Companies like Abbott and Roche have commercialized glucose sensors and immunoassays that rely on accurate CV measurements to achieve clinical-grade diagnostic accuracy in portable formats.
Corrosion monitoring in infrastructure and aerospace applications represents another significant market, where CV analysis helps predict material failures before they occur. The cost savings from preventing a single critical failure in these sectors can justify substantial investment in advanced electrochemical analysis tools.
As these markets continue to expand, demand for automated CV analysis software with accurate baseline subtraction capabilities is growing rapidly, creating opportunities for specialized analytical instrument providers and software developers to serve these diverse but technically demanding sectors.
Technical Challenges in Capacitive Current Subtraction
The current landscape of capacitive current subtraction in cyclic voltammetry (CV) presents several significant technical challenges that impede accurate data analysis and interpretation. One primary obstacle is the inherent difficulty in distinguishing between non-faradaic capacitive currents and faradaic currents of interest. This separation becomes particularly problematic when analyzing systems with high surface area electrodes or when working with low concentrations of electroactive species.
Signal-to-noise ratio limitations represent another substantial challenge. Capacitive currents often overwhelm the faradaic response, especially at high scan rates or when using microelectrodes. This creates a fundamental detection limit that restricts the sensitivity of CV measurements and complicates the analysis of trace electroactive species.
The non-linear behavior of capacitive currents with respect to scan rate introduces additional complexity. While theoretical models suggest a linear relationship, real experimental systems frequently deviate from this ideality due to surface roughness, adsorption phenomena, and electrode heterogeneity. These deviations make mathematical modeling and subtraction procedures less straightforward than commonly assumed.
Electrode surface modifications and changes during measurement cycles present dynamic challenges. As experiments progress, electrode surfaces may undergo physical or chemical alterations that change their capacitive properties. This temporal evolution of capacitive behavior makes consistent baseline correction particularly difficult across multiple cycles or extended measurements.
Software limitations constitute a significant technical barrier. Many commercial electrochemical workstations provide only rudimentary baseline correction tools that fail to address the complexities of real experimental data. The lack of sophisticated, accessible software solutions forces researchers to develop custom analysis scripts, creating inconsistencies in data processing methodologies across the field.
Reference electrode stability issues further complicate accurate capacitive current subtraction. Drift in reference potential during measurements can be misinterpreted as changes in capacitive or faradaic currents, leading to systematic errors in baseline determination and subsequent analysis.
Temperature and environmental fluctuations introduce additional variables that affect capacitive currents. These external factors can cause baseline drift that is difficult to model mathematically, particularly in long-duration experiments or when precise temperature control is not feasible.
The integration of these multiple challenges creates a complex technical landscape that requires sophisticated approaches combining experimental design, mathematical modeling, and computational analysis to achieve reliable capacitive current subtraction in cyclic voltammetry.
Signal-to-noise ratio limitations represent another substantial challenge. Capacitive currents often overwhelm the faradaic response, especially at high scan rates or when using microelectrodes. This creates a fundamental detection limit that restricts the sensitivity of CV measurements and complicates the analysis of trace electroactive species.
The non-linear behavior of capacitive currents with respect to scan rate introduces additional complexity. While theoretical models suggest a linear relationship, real experimental systems frequently deviate from this ideality due to surface roughness, adsorption phenomena, and electrode heterogeneity. These deviations make mathematical modeling and subtraction procedures less straightforward than commonly assumed.
Electrode surface modifications and changes during measurement cycles present dynamic challenges. As experiments progress, electrode surfaces may undergo physical or chemical alterations that change their capacitive properties. This temporal evolution of capacitive behavior makes consistent baseline correction particularly difficult across multiple cycles or extended measurements.
Software limitations constitute a significant technical barrier. Many commercial electrochemical workstations provide only rudimentary baseline correction tools that fail to address the complexities of real experimental data. The lack of sophisticated, accessible software solutions forces researchers to develop custom analysis scripts, creating inconsistencies in data processing methodologies across the field.
Reference electrode stability issues further complicate accurate capacitive current subtraction. Drift in reference potential during measurements can be misinterpreted as changes in capacitive or faradaic currents, leading to systematic errors in baseline determination and subsequent analysis.
Temperature and environmental fluctuations introduce additional variables that affect capacitive currents. These external factors can cause baseline drift that is difficult to model mathematically, particularly in long-duration experiments or when precise temperature control is not feasible.
The integration of these multiple challenges creates a complex technical landscape that requires sophisticated approaches combining experimental design, mathematical modeling, and computational analysis to achieve reliable capacitive current subtraction in cyclic voltammetry.
Current Methodologies for CV Baseline Correction
01 Measurement and analysis of capacitive currents in CV
Cyclic voltammetry techniques are used to measure and analyze capacitive currents in electrochemical systems. These measurements help in understanding the double-layer capacitance at electrode interfaces and distinguishing between faradaic and non-faradaic processes. Advanced analysis methods can separate capacitive contributions from total current responses, providing insights into electrode surface properties and electrochemical behavior.- Capacitive current measurement and analysis in CV: Capacitive currents in cyclic voltammetry are measured and analyzed to characterize electrochemical systems. These measurements provide information about the double-layer capacitance at the electrode-electrolyte interface. The capacitive current component can be separated from faradaic currents through various analytical techniques, allowing for more accurate determination of electrochemical parameters. This approach is particularly useful for studying electrode materials and electrolyte interactions.
- Supercapacitor characterization using CV: Cyclic voltammetry is employed to characterize supercapacitors by analyzing capacitive currents. The technique helps evaluate the capacitance, energy density, and power density of supercapacitor materials. The shape of CV curves provides insights into the charge storage mechanisms, distinguishing between double-layer capacitance and pseudocapacitance. This method is crucial for developing high-performance energy storage devices with enhanced capacitive properties.
- Electrode material optimization for capacitive performance: Various electrode materials are optimized to enhance capacitive performance as measured by cyclic voltammetry. Materials such as carbon-based electrodes, metal oxides, and conductive polymers are developed to increase specific capacitance and reduce capacitive current decay. Surface modifications and nanostructuring techniques are employed to maximize the effective surface area and improve charge transfer kinetics, resulting in superior capacitive behavior during CV measurements.
- Capacitive current reduction techniques in CV: Various techniques are developed to reduce unwanted capacitive currents in cyclic voltammetry measurements to improve signal-to-noise ratio. These include electrode surface treatments, specialized cell designs, and advanced measurement protocols. Background subtraction methods and mathematical algorithms are employed to separate faradaic processes from capacitive contributions. These approaches enhance the sensitivity and accuracy of CV measurements, particularly for detecting low concentrations of electroactive species.
- Biosensor applications utilizing capacitive currents: Capacitive currents in cyclic voltammetry are utilized for biosensing applications. Changes in the double-layer capacitance at modified electrodes can indicate biomolecular binding events or enzymatic reactions. This approach enables label-free detection of various analytes including proteins, nucleic acids, and small molecules. The sensitivity of capacitive current measurements allows for the development of highly responsive biosensors with potential applications in medical diagnostics, environmental monitoring, and food safety.
02 Electrode materials and structures for optimizing capacitive performance
Various electrode materials and structures are designed to enhance or control capacitive currents in cyclic voltammetry applications. These include carbon-based materials, metal oxides, and composite structures with specific surface characteristics. The electrode design affects double-layer formation, charge storage capabilities, and overall electrochemical performance, allowing for tailored capacitive responses in energy storage and sensing applications.Expand Specific Solutions03 Supercapacitor development and characterization using CV
Cyclic voltammetry is extensively used in the development and characterization of supercapacitors. The technique helps evaluate capacitive behavior, energy storage capacity, and cycling stability of supercapacitor materials. By analyzing the shape, area, and symmetry of CV curves, researchers can determine specific capacitance, rate capability, and degradation mechanisms, guiding the optimization of supercapacitor performance for various applications.Expand Specific Solutions04 Correction methods for capacitive current interference
Various correction methods are developed to address capacitive current interference in cyclic voltammetry measurements. These techniques include background subtraction, mathematical modeling, and specialized scanning protocols that help isolate faradaic processes from capacitive contributions. Advanced algorithms and data processing approaches enable more accurate analysis of electrochemical reactions by minimizing the impact of capacitive currents on measurement results.Expand Specific Solutions05 Applications of capacitive current analysis in sensors and diagnostics
Capacitive current analysis in cyclic voltammetry is applied in developing sensors and diagnostic tools. By monitoring changes in capacitive behavior, these systems can detect specific analytes, biomolecules, or environmental contaminants. The technique enables label-free detection methods with high sensitivity and selectivity, finding applications in medical diagnostics, environmental monitoring, and quality control processes across various industries.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The cyclic voltammetry (CV) capacitive current baseline correction market is currently in a growth phase, with increasing demand driven by expanding applications in electrochemical analysis across multiple industries. The global market for electrochemical instruments, including CV technologies, is estimated at approximately $1.5 billion, with steady annual growth of 5-7%. Technologically, the field is moderately mature but continues to evolve, with companies like Analog Devices and Synaptics leading in signal processing innovations, while established players such as IBM and Samsung Electronics contribute significant intellectual property in electrochemical measurement techniques. Academic institutions including University of Electronic Science & Technology of China and Rutgers University are advancing fundamental research, while specialized companies like Elite Semiconductor and BCD Micro-Electronics are developing application-specific integrated circuits optimized for precise current measurement and baseline correction in electrochemical applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has integrated advanced cyclic voltammetry baseline correction capabilities into their semiconductor characterization systems, focusing on high-throughput and automated analysis. Their approach combines hardware innovations with sophisticated signal processing to address capacitive current challenges in both research and production environments. Samsung's technology employs a multi-frequency excitation technique that allows simultaneous measurement of faradaic and capacitive components, enabling real-time separation without the need for separate blank scans. Their system incorporates a digital filtering architecture that processes the raw current signal through a series of adaptive filters calibrated to the specific electrochemical cell characteristics. A key innovation is their implementation of predictive capacitive modeling that accounts for scan rate dependencies and potential-dependent capacitance changes. The platform includes automated calibration routines that characterize the measurement system's intrinsic capacitance and compensate for it during analysis, ensuring consistent results across different measurement setups and environmental conditions.
Strengths: Exceptional integration with semiconductor manufacturing processes enables high-throughput analysis with minimal operator intervention. The system's automation and reproducibility make it ideal for quality control applications. Weaknesses: The technology is optimized for specific semiconductor applications and may require significant adaptation for other electrochemical systems or research contexts.
Analog Devices International Unlimited Co.
Technical Solution: Analog Devices has developed advanced potentiostat circuits specifically designed for precise cyclic voltammetry measurements with integrated capacitive current compensation. Their technology employs a dual-feedback loop architecture that actively measures and subtracts capacitive currents in real-time during CV scans. The system utilizes proprietary algorithms that model the double-layer capacitance at electrode interfaces and automatically adjusts compensation parameters based on scan rate and electrolyte conditions. Their latest solutions incorporate digital signal processing techniques that perform frequency domain analysis to separate faradaic and non-faradaic components of the measured current, enabling more accurate baseline correction even in complex electrochemical systems with varying capacitive behaviors across potential ranges. The technology includes adaptive filtering that accounts for potential-dependent capacitance changes during measurement cycles.
Strengths: Superior noise rejection capabilities and high precision analog front-end design provide industry-leading sensitivity for detecting small faradaic currents in the presence of large capacitive backgrounds. Their integrated approach reduces the need for post-processing. Weaknesses: The sophisticated compensation algorithms require significant computational resources and specialized hardware, making implementations relatively expensive compared to software-only solutions.
Key Algorithms and Software Solutions for CV Data Processing
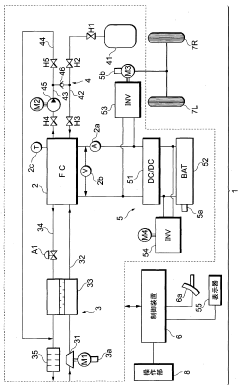
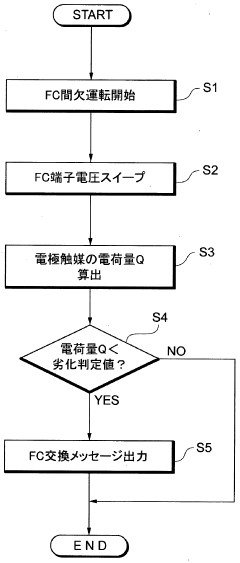
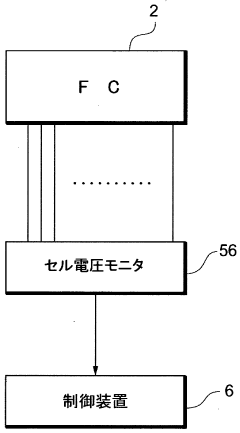
A fuel cell system, electrode catalyst degradation judgment method, and moving body
PatentWO2008108451A1
Innovation
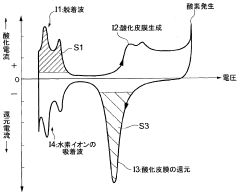
- The use of cyclic voltammetry (CV) characteristics to determine the state of the electrode catalyst by measuring changes in current and voltage while keeping voltage constant, allowing for precise calculation of the effective area and charge associated with catalyst deterioration.
Using kinetic cyclic voltammetry to evaluate analyte kinetics and concentrations
PatentWO2015021470A1
Innovation
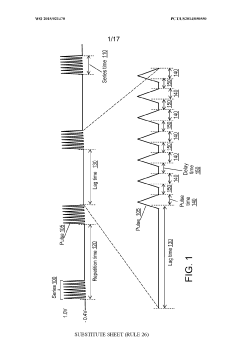
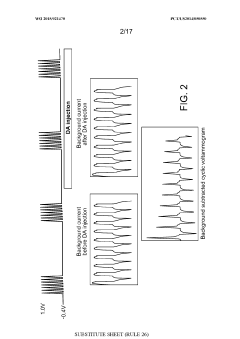
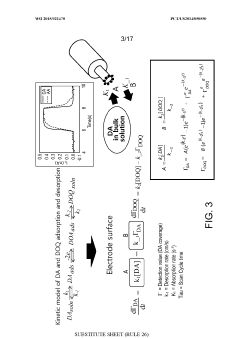
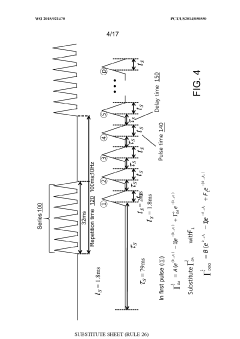
- Kinetic cyclic voltammetry (KCV) is developed, which involves multi-pulse cyclic voltammetry at various voltages to generate kinetic and concentration maps (K-maps and A-maps) without background subtraction, enabling absolute quantification of analytes and real-time signal analysis, suitable for use in smart neuromodulation systems.
Validation Protocols for CV Baseline Correction
Validation protocols for CV baseline correction must be established to ensure the accuracy and reliability of electrochemical measurements. These protocols should include systematic approaches to verify that baseline subtraction methods effectively isolate faradaic currents from capacitive contributions without introducing artifacts or distortions.
A comprehensive validation protocol begins with standard reference systems that exhibit well-characterized electrochemical behavior. Ferrocene/ferrocenium couples, ruthenium hexamine complexes, and other reversible redox systems with established theoretical responses serve as excellent benchmarks. By comparing baseline-corrected data with theoretical models, researchers can quantitatively assess correction accuracy.
Statistical validation methods should be incorporated to evaluate the reproducibility and consistency of baseline correction techniques. This includes performing multiple measurements under identical conditions and calculating standard deviations of key parameters such as peak potentials, peak currents, and peak separations. Lower variability in these metrics indicates more reliable baseline correction.
Residual analysis provides another critical validation approach. After baseline subtraction, the residual capacitive current should be minimal and randomly distributed around zero. Systematic deviations in residuals suggest inadequate correction methods or inappropriate model selection. Plotting residuals against potential can reveal patterns that indicate specific correction deficiencies.
Cross-validation between different baseline correction methods offers additional verification. When multiple independent approaches (polynomial fitting, moving average, wavelet transform) yield consistent results for the same dataset, confidence in the correction increases. Significant discrepancies between methods warrant further investigation into the underlying assumptions of each approach.
Digital simulation tools can generate synthetic CV data with precisely known capacitive and faradaic components. These simulations enable quantitative assessment of correction algorithms by comparing recovered faradaic currents with the original input parameters. This approach is particularly valuable for evaluating algorithm performance across diverse electrochemical systems and experimental conditions.
Documentation standards form the final component of validation protocols. Researchers should maintain detailed records of all correction parameters, algorithms, and validation metrics. This documentation ensures transparency, reproducibility, and facilitates method refinement over time. Standardized reporting formats enable meaningful comparison between different studies and laboratories.
A comprehensive validation protocol begins with standard reference systems that exhibit well-characterized electrochemical behavior. Ferrocene/ferrocenium couples, ruthenium hexamine complexes, and other reversible redox systems with established theoretical responses serve as excellent benchmarks. By comparing baseline-corrected data with theoretical models, researchers can quantitatively assess correction accuracy.
Statistical validation methods should be incorporated to evaluate the reproducibility and consistency of baseline correction techniques. This includes performing multiple measurements under identical conditions and calculating standard deviations of key parameters such as peak potentials, peak currents, and peak separations. Lower variability in these metrics indicates more reliable baseline correction.
Residual analysis provides another critical validation approach. After baseline subtraction, the residual capacitive current should be minimal and randomly distributed around zero. Systematic deviations in residuals suggest inadequate correction methods or inappropriate model selection. Plotting residuals against potential can reveal patterns that indicate specific correction deficiencies.
Cross-validation between different baseline correction methods offers additional verification. When multiple independent approaches (polynomial fitting, moving average, wavelet transform) yield consistent results for the same dataset, confidence in the correction increases. Significant discrepancies between methods warrant further investigation into the underlying assumptions of each approach.
Digital simulation tools can generate synthetic CV data with precisely known capacitive and faradaic components. These simulations enable quantitative assessment of correction algorithms by comparing recovered faradaic currents with the original input parameters. This approach is particularly valuable for evaluating algorithm performance across diverse electrochemical systems and experimental conditions.
Documentation standards form the final component of validation protocols. Researchers should maintain detailed records of all correction parameters, algorithms, and validation metrics. This documentation ensures transparency, reproducibility, and facilitates method refinement over time. Standardized reporting formats enable meaningful comparison between different studies and laboratories.
Data Reproducibility and Standardization in Electrochemistry
Data reproducibility and standardization represent critical challenges in electrochemistry, particularly when dealing with cyclic voltammetry (CV) measurements. The scientific community has long struggled with inconsistencies in data reporting, processing, and analysis methodologies, which hinder meaningful comparison of results across different research groups and laboratories.
The baseline correction and capacitive current subtraction in CV measurements exemplify these standardization challenges. Currently, researchers employ various approaches, from simple linear background subtraction to more complex polynomial fitting methods, with little consensus on best practices. This methodological diversity creates significant barriers to reproducibility and complicates meta-analysis of published data.
Several international electrochemical societies have attempted to establish standardized protocols for CV data processing. The International Union of Pure and Applied Chemistry (IUPAC) has published recommendations for reporting electrochemical data, while the International Society of Electrochemistry (ISE) has formed working groups dedicated to standardization issues. Despite these efforts, adoption remains inconsistent across the field.
Digital data formats present another dimension of the standardization challenge. The proliferation of proprietary file formats from instrument manufacturers creates interoperability issues when researchers attempt to share raw data or implement universal analysis scripts. Open-source initiatives like the Chemical Markup Language for Electrochemistry (CML-EC) offer promising solutions but have yet to achieve widespread adoption.
Automation tools and software packages have emerged to address reproducibility concerns. Programs like EC-Lab, NOVA, and open-source alternatives such as pyCV provide standardized approaches to baseline correction and capacitive current subtraction. These tools implement algorithms ranging from traditional Savitzky-Golay filtering to machine learning approaches that can identify and remove non-faradaic contributions to the measured current.
The development of shared data repositories represents a significant advancement in electrochemical data standardization. Platforms like ElectroChemical Data Infrastructure (ECDI) and the Materials Project enable researchers to upload raw CV data alongside standardized processing scripts, fostering transparency and enabling direct comparison of results across different studies.
Looking forward, the integration of automated data processing pipelines with standardized reporting formats offers the most promising path toward improved reproducibility. Machine-readable data standards coupled with transparent processing algorithms would enable more rigorous peer review and facilitate meta-analyses that could accelerate discovery in electrochemical research and applications.
The baseline correction and capacitive current subtraction in CV measurements exemplify these standardization challenges. Currently, researchers employ various approaches, from simple linear background subtraction to more complex polynomial fitting methods, with little consensus on best practices. This methodological diversity creates significant barriers to reproducibility and complicates meta-analysis of published data.
Several international electrochemical societies have attempted to establish standardized protocols for CV data processing. The International Union of Pure and Applied Chemistry (IUPAC) has published recommendations for reporting electrochemical data, while the International Society of Electrochemistry (ISE) has formed working groups dedicated to standardization issues. Despite these efforts, adoption remains inconsistent across the field.
Digital data formats present another dimension of the standardization challenge. The proliferation of proprietary file formats from instrument manufacturers creates interoperability issues when researchers attempt to share raw data or implement universal analysis scripts. Open-source initiatives like the Chemical Markup Language for Electrochemistry (CML-EC) offer promising solutions but have yet to achieve widespread adoption.
Automation tools and software packages have emerged to address reproducibility concerns. Programs like EC-Lab, NOVA, and open-source alternatives such as pyCV provide standardized approaches to baseline correction and capacitive current subtraction. These tools implement algorithms ranging from traditional Savitzky-Golay filtering to machine learning approaches that can identify and remove non-faradaic contributions to the measured current.
The development of shared data repositories represents a significant advancement in electrochemical data standardization. Platforms like ElectroChemical Data Infrastructure (ECDI) and the Materials Project enable researchers to upload raw CV data alongside standardized processing scripts, fostering transparency and enabling direct comparison of results across different studies.
Looking forward, the integration of automated data processing pipelines with standardized reporting formats offers the most promising path toward improved reproducibility. Machine-readable data standards coupled with transparent processing algorithms would enable more rigorous peer review and facilitate meta-analyses that could accelerate discovery in electrochemical research and applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!