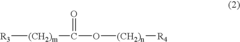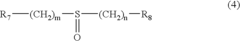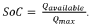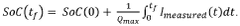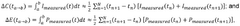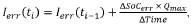Cyclic Voltammetry for Battery Materials: Interpreting Peaks Across SOC and Cycling
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery CV Analysis Background and Objectives
Cyclic voltammetry (CV) has emerged as a cornerstone analytical technique in electrochemistry since its development in the early 1900s. Initially utilized for studying redox reactions in solution, CV has evolved significantly over the past century to become an indispensable tool for battery material characterization. The technique's ability to provide insights into electron transfer kinetics, reaction mechanisms, and electrochemical reversibility makes it particularly valuable for understanding battery performance and degradation mechanisms.
The evolution of CV techniques has paralleled advancements in battery technology, with significant methodological refinements occurring during the lithium-ion battery revolution of the 1990s. Modern CV applications benefit from enhanced instrumentation precision, computational analysis capabilities, and integration with complementary characterization methods, enabling more nuanced interpretation of electrochemical processes across varying states of charge (SOC) and cycling conditions.
Current research trends indicate growing interest in applying CV for next-generation battery chemistries, including solid-state, sodium-ion, and multivalent systems. The technique's versatility in probing interfacial phenomena and reaction kinetics positions it as a critical tool for accelerating battery innovation. However, challenges remain in standardizing interpretation methodologies, particularly for complex multi-peak responses that evolve during cycling.
This technical research aims to establish a comprehensive framework for interpreting CV peak behaviors across different SOC levels and cycling stages. Specifically, we seek to correlate peak position shifts, intensity variations, and emergence/disappearance patterns with specific physicochemical processes occurring within battery materials. By developing systematic analytical approaches, we intend to enhance the diagnostic value of CV for battery performance evaluation.
The objectives of this investigation include: developing quantitative metrics for peak analysis that correlate with battery health indicators; establishing standardized protocols for CV data acquisition across varying SOC conditions; creating computational tools for automated peak identification and classification; and validating interpretation frameworks across multiple battery chemistries to ensure broad applicability.
Additionally, this research aims to bridge the gap between fundamental electrochemical theory and practical battery engineering by translating CV signatures into actionable insights for material optimization and cell design. By correlating specific CV features with performance parameters such as capacity retention, rate capability, and cycle life, we seek to position CV as not merely an analytical technique but a predictive tool for battery development.
Understanding the relationship between CV peak characteristics and battery aging mechanisms represents a particularly valuable objective, as it could enable early detection of degradation processes before they manifest as capacity loss or impedance increase in conventional testing protocols. This preventive diagnostic capability would significantly accelerate battery material screening and validation processes.
The evolution of CV techniques has paralleled advancements in battery technology, with significant methodological refinements occurring during the lithium-ion battery revolution of the 1990s. Modern CV applications benefit from enhanced instrumentation precision, computational analysis capabilities, and integration with complementary characterization methods, enabling more nuanced interpretation of electrochemical processes across varying states of charge (SOC) and cycling conditions.
Current research trends indicate growing interest in applying CV for next-generation battery chemistries, including solid-state, sodium-ion, and multivalent systems. The technique's versatility in probing interfacial phenomena and reaction kinetics positions it as a critical tool for accelerating battery innovation. However, challenges remain in standardizing interpretation methodologies, particularly for complex multi-peak responses that evolve during cycling.
This technical research aims to establish a comprehensive framework for interpreting CV peak behaviors across different SOC levels and cycling stages. Specifically, we seek to correlate peak position shifts, intensity variations, and emergence/disappearance patterns with specific physicochemical processes occurring within battery materials. By developing systematic analytical approaches, we intend to enhance the diagnostic value of CV for battery performance evaluation.
The objectives of this investigation include: developing quantitative metrics for peak analysis that correlate with battery health indicators; establishing standardized protocols for CV data acquisition across varying SOC conditions; creating computational tools for automated peak identification and classification; and validating interpretation frameworks across multiple battery chemistries to ensure broad applicability.
Additionally, this research aims to bridge the gap between fundamental electrochemical theory and practical battery engineering by translating CV signatures into actionable insights for material optimization and cell design. By correlating specific CV features with performance parameters such as capacity retention, rate capability, and cycle life, we seek to position CV as not merely an analytical technique but a predictive tool for battery development.
Understanding the relationship between CV peak characteristics and battery aging mechanisms represents a particularly valuable objective, as it could enable early detection of degradation processes before they manifest as capacity loss or impedance increase in conventional testing protocols. This preventive diagnostic capability would significantly accelerate battery material screening and validation processes.
Market Demand for Advanced Battery Characterization
The global battery market is experiencing unprecedented growth, driven by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. This growth has intensified the demand for advanced battery characterization techniques, particularly Cyclic Voltammetry (CV), which provides critical insights into battery material performance across different states of charge (SOC) and cycling conditions.
Market research indicates that the global battery testing equipment market is projected to reach $2.5 billion by 2027, growing at a CAGR of approximately 6.8% from 2022. Within this segment, electrochemical characterization tools like CV systems represent a significant and rapidly expanding portion, with estimated annual growth rates exceeding 8%.
The EV sector stands as the primary driver for advanced battery characterization demand. With major automotive manufacturers committing to electrification targets, the need for precise battery material evaluation has become critical for improving energy density, cycle life, and safety profiles. Tesla, Volkswagen, and BYD have all established dedicated battery research facilities with substantial investments in electrochemical characterization equipment.
Energy storage system (ESS) applications represent another substantial market segment. Grid-scale storage projects have increased by 62% year-over-year, creating demand for battery technologies that can be thoroughly characterized across various operational conditions. Utility companies and renewable energy developers require detailed electrochemical analysis to ensure battery performance under fluctuating charge-discharge cycles.
Consumer electronics manufacturers constitute the third major market segment, with companies like Apple, Samsung, and Xiaomi investing heavily in battery research to extend device runtime while reducing form factors. These companies rely on advanced CV techniques to evaluate new battery materials and optimize existing formulations.
Geographically, Asia-Pacific dominates the market demand, accounting for approximately 45% of global battery characterization equipment sales. This concentration aligns with the region's dominance in battery manufacturing, with China, Japan, and South Korea leading production capacity. North America and Europe follow with growing demand driven by domestic battery production initiatives and research activities.
Industry surveys reveal that 78% of battery researchers consider advanced characterization techniques essential for accelerating material development cycles. Specifically, CV techniques that can interpret peaks across different SOC levels and cycling conditions are identified as high-priority tools by 67% of respondents, highlighting the critical market need for sophisticated electrochemical analysis capabilities that can provide deeper insights into battery material behavior under real-world operating conditions.
Market research indicates that the global battery testing equipment market is projected to reach $2.5 billion by 2027, growing at a CAGR of approximately 6.8% from 2022. Within this segment, electrochemical characterization tools like CV systems represent a significant and rapidly expanding portion, with estimated annual growth rates exceeding 8%.
The EV sector stands as the primary driver for advanced battery characterization demand. With major automotive manufacturers committing to electrification targets, the need for precise battery material evaluation has become critical for improving energy density, cycle life, and safety profiles. Tesla, Volkswagen, and BYD have all established dedicated battery research facilities with substantial investments in electrochemical characterization equipment.
Energy storage system (ESS) applications represent another substantial market segment. Grid-scale storage projects have increased by 62% year-over-year, creating demand for battery technologies that can be thoroughly characterized across various operational conditions. Utility companies and renewable energy developers require detailed electrochemical analysis to ensure battery performance under fluctuating charge-discharge cycles.
Consumer electronics manufacturers constitute the third major market segment, with companies like Apple, Samsung, and Xiaomi investing heavily in battery research to extend device runtime while reducing form factors. These companies rely on advanced CV techniques to evaluate new battery materials and optimize existing formulations.
Geographically, Asia-Pacific dominates the market demand, accounting for approximately 45% of global battery characterization equipment sales. This concentration aligns with the region's dominance in battery manufacturing, with China, Japan, and South Korea leading production capacity. North America and Europe follow with growing demand driven by domestic battery production initiatives and research activities.
Industry surveys reveal that 78% of battery researchers consider advanced characterization techniques essential for accelerating material development cycles. Specifically, CV techniques that can interpret peaks across different SOC levels and cycling conditions are identified as high-priority tools by 67% of respondents, highlighting the critical market need for sophisticated electrochemical analysis capabilities that can provide deeper insights into battery material behavior under real-world operating conditions.
Current Challenges in CV Peak Interpretation
Despite significant advancements in cyclic voltammetry (CV) techniques for battery material characterization, several persistent challenges impede accurate peak interpretation across state of charge (SOC) and cycling conditions. The complexity of electrochemical reactions occurring simultaneously during battery operation creates overlapping peaks that are difficult to deconvolute. This overlap often obscures critical information about specific redox processes, particularly in composite electrode materials where multiple active components contribute to the overall electrochemical signature.
Signal-to-noise ratio remains problematic, especially at high scan rates or when examining materials with low electrochemical activity. Background currents from capacitive effects, solution resistance, and electrode double-layer formation frequently mask smaller faradaic peaks associated with minor but potentially significant degradation mechanisms or phase transitions.
Peak shifts across different SOC levels present another significant challenge. As lithium concentration changes within electrode materials, the thermodynamic and kinetic properties of redox reactions evolve, causing peaks to shift in potential. These shifts complicate direct comparison between different operational states and make it difficult to establish consistent reference points for degradation analysis.
The interpretation of peak evolution during extended cycling faces additional hurdles. Gradual changes in peak intensity, position, and shape must be distinguished from experimental artifacts and irreversible capacity loss. Current analytical frameworks struggle to separate reversible capacity fading from permanent structural changes in active materials, particularly when both processes occur simultaneously.
Temperature dependence of CV responses introduces another layer of complexity. Peaks can shift significantly with temperature variations, affecting reaction kinetics and diffusion processes. This temperature sensitivity complicates the comparison of data collected under different environmental conditions, limiting the standardization of CV interpretation protocols.
Electrode surface phenomena, including SEI formation and evolution, present interpretation challenges that evolve throughout battery lifetime. The dynamic nature of these interfaces creates time-dependent CV responses that conventional static analysis methods fail to capture adequately. Current models often oversimplify these complex interfacial processes, leading to incomplete or inaccurate interpretations.
Finally, the lack of standardized methodologies for peak deconvolution and quantitative analysis remains a significant barrier. Different research groups employ varied approaches to baseline correction, peak fitting, and data normalization, making cross-study comparisons challenging and limiting the development of universal interpretation frameworks for CV data in battery materials research.
Signal-to-noise ratio remains problematic, especially at high scan rates or when examining materials with low electrochemical activity. Background currents from capacitive effects, solution resistance, and electrode double-layer formation frequently mask smaller faradaic peaks associated with minor but potentially significant degradation mechanisms or phase transitions.
Peak shifts across different SOC levels present another significant challenge. As lithium concentration changes within electrode materials, the thermodynamic and kinetic properties of redox reactions evolve, causing peaks to shift in potential. These shifts complicate direct comparison between different operational states and make it difficult to establish consistent reference points for degradation analysis.
The interpretation of peak evolution during extended cycling faces additional hurdles. Gradual changes in peak intensity, position, and shape must be distinguished from experimental artifacts and irreversible capacity loss. Current analytical frameworks struggle to separate reversible capacity fading from permanent structural changes in active materials, particularly when both processes occur simultaneously.
Temperature dependence of CV responses introduces another layer of complexity. Peaks can shift significantly with temperature variations, affecting reaction kinetics and diffusion processes. This temperature sensitivity complicates the comparison of data collected under different environmental conditions, limiting the standardization of CV interpretation protocols.
Electrode surface phenomena, including SEI formation and evolution, present interpretation challenges that evolve throughout battery lifetime. The dynamic nature of these interfaces creates time-dependent CV responses that conventional static analysis methods fail to capture adequately. Current models often oversimplify these complex interfacial processes, leading to incomplete or inaccurate interpretations.
Finally, the lack of standardized methodologies for peak deconvolution and quantitative analysis remains a significant barrier. Different research groups employ varied approaches to baseline correction, peak fitting, and data normalization, making cross-study comparisons challenging and limiting the development of universal interpretation frameworks for CV data in battery materials research.
Current CV Methodologies for Battery Materials
01 Redox reaction peak analysis in cyclic voltammetry
Cyclic voltammetry peaks can be interpreted to understand redox reactions occurring at electrode surfaces. The position, height, and shape of peaks provide information about electron transfer processes, reaction kinetics, and thermodynamics. Analysis of anodic (oxidation) and cathodic (reduction) peaks helps determine reversibility of reactions, diffusion coefficients, and reaction mechanisms. Peak separation and current ratios are particularly important parameters for characterizing electrochemical systems.- Interpretation of redox peaks in cyclic voltammetry: Cyclic voltammetry peaks provide information about redox reactions occurring at the electrode surface. The position, height, and shape of these peaks can be interpreted to determine the redox potential, reaction kinetics, and reversibility of electrochemical processes. Anodic peaks indicate oxidation reactions while cathodic peaks represent reduction reactions. The separation between these peaks and their relative heights can be used to assess the reversibility of the electron transfer process.
- Peak analysis for material characterization: Cyclic voltammetry peak analysis is used to characterize various materials including catalysts, batteries, and sensors. The peak positions and intensities can reveal information about the electronic structure, composition, and performance of these materials. Multiple peaks may indicate different redox-active species or multiple electron transfer steps. By analyzing these peaks, researchers can determine the electrochemical properties and potential applications of novel materials.
- Quantitative analysis using peak parameters: Peak parameters in cyclic voltammetry can be used for quantitative analysis of analytes. The peak current is proportional to the concentration of the electroactive species, allowing for determination of unknown concentrations. Mathematical relationships such as the Randles-Sevcik equation relate peak current to scan rate, diffusion coefficient, and concentration. Peak area integration provides information about the total charge transferred during the redox process, which can be used to calculate reaction yields and efficiencies.
- Influence of experimental conditions on peak characteristics: Experimental conditions significantly affect cyclic voltammetry peak characteristics. Scan rate influences peak height and separation, with faster scan rates typically resulting in larger peak currents and increased peak separation. Electrode material, surface condition, and electrolyte composition can alter peak positions and shapes. Temperature affects reaction kinetics and thus peak parameters. Understanding these influences is crucial for correct interpretation of cyclic voltammetry data and for optimizing experimental conditions for specific applications.
- Advanced data processing techniques for peak analysis: Advanced data processing techniques enhance the interpretation of cyclic voltammetry peaks. Digital filtering can improve signal-to-noise ratio, making subtle peaks more discernible. Deconvolution methods separate overlapping peaks for complex systems with multiple redox processes. Machine learning algorithms can identify patterns in voltammetric data and correlate peak characteristics with material properties. Simulation and modeling approaches compare experimental data with theoretical predictions to validate proposed reaction mechanisms and extract kinetic parameters.
02 Electrochemical sensor development using cyclic voltammetry
Cyclic voltammetry peak interpretation is essential for developing and optimizing electrochemical sensors. By analyzing peak characteristics, researchers can determine sensor sensitivity, selectivity, and detection limits. Modified electrodes with various materials show distinctive voltammetric responses that can be used to detect specific analytes. Peak shifts and current changes in the presence of target molecules provide the basis for quantitative and qualitative analysis in sensing applications.Expand Specific Solutions03 Battery and energy storage material characterization
Cyclic voltammetry is widely used to characterize battery materials and energy storage systems. Peak interpretation reveals information about charge-discharge mechanisms, intercalation processes, and electrode stability. Multiple peaks often indicate multi-step electron transfer processes or phase transitions in battery materials. The evolution of peaks during cycling provides insights into capacity fading mechanisms, electrode degradation, and the formation of solid-electrolyte interphase layers.Expand Specific Solutions04 Electrodeposition and surface modification analysis
Interpretation of cyclic voltammetry peaks is crucial for understanding electrodeposition processes and surface modifications. Nucleation and growth mechanisms can be determined by analyzing the shape and position of deposition and stripping peaks. The appearance of new peaks after surface modification indicates successful functionalization or coating. Peak characteristics also reveal information about surface coverage, layer thickness, and the presence of defects in modified electrodes.Expand Specific Solutions05 Advanced data processing for complex voltammograms
Complex cyclic voltammograms with overlapping peaks require advanced data processing techniques for accurate interpretation. Mathematical methods such as deconvolution, baseline correction, and peak fitting algorithms help separate and quantify individual redox processes. Machine learning and artificial intelligence approaches are increasingly used to identify patterns in voltammetric data and extract meaningful parameters from peaks. These computational methods enhance the information obtained from cyclic voltammetry experiments, particularly for systems with multiple electroactive species.Expand Specific Solutions
Key Players in Battery Characterization Industry
The cyclic voltammetry market for battery materials analysis is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The market size is projected to reach significant value as battery technology research intensifies globally. Leading players demonstrate varying levels of technical maturity, with established companies like LG Energy Solution, Samsung SDI, and Toyota Motor Corp focusing on advanced peak interpretation methodologies across state-of-charge ranges. Research institutions including Caltech and CNRS contribute fundamental innovations, while specialized battery manufacturers such as A123 Systems and Saft Groupe are developing proprietary CV analysis techniques. Automotive companies like GM, Ford, and Daimler are increasingly investing in this technology to optimize battery performance and longevity through improved cycling analysis capabilities.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced cyclic voltammetry (CV) techniques for battery material characterization across different states of charge (SOC) and cycling conditions. Their approach integrates multi-scale CV analysis with differential capacity analysis to identify specific electrochemical reactions occurring at various potentials. The company employs temperature-controlled CV measurements (10-60°C) to separate diffusion-controlled processes from surface-confined reactions, enabling precise identification of degradation mechanisms during cycling. Their proprietary algorithms correlate peak shifts and intensity changes with specific material transformations, allowing real-time monitoring of SEI formation, lithium plating, and transition metal dissolution. LG's system can detect subtle changes in peak characteristics as early indicators of capacity fade, particularly in their NCM and NCMA cathode materials, where they've mapped specific CV signatures to structural changes during extended cycling.
Strengths: Superior integration with production processes allowing for high-throughput screening of new materials; advanced data analytics capabilities that connect CV results with practical battery performance metrics. Weaknesses: Their approach requires sophisticated equipment and expertise, making it less accessible for general research applications; some proprietary algorithms limit transparency in interpretation methodology.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The CEA has developed a sophisticated multi-dimensional CV analysis framework for battery materials that combines traditional potential sweep techniques with advanced operando characterization methods. Their approach utilizes synchrotron-based X-ray diffraction synchronized with CV measurements to directly correlate structural changes with electrochemical signatures across different SOC ranges. The CEA's methodology incorporates temperature-dependent CV (from -20°C to 60°C) to distinguish between kinetically limited and diffusion-controlled processes in various battery chemistries. They've pioneered the use of microelectrode arrays to map spatial heterogeneities in electrode materials during cycling, revealing how local variations in current density affect degradation patterns. Their analytical framework includes machine learning algorithms that identify subtle changes in CV peak characteristics as early indicators of capacity fade mechanisms. The CEA has successfully applied this approach to next-generation materials including high-voltage spinel cathodes and conversion-type anodes, establishing specific CV signatures for various degradation modes including electrolyte decomposition, transition metal dissolution, and structural transformations.
Strengths: Exceptional fundamental scientific approach combining multiple characterization techniques; strong capabilities in correlating atomic/molecular level changes with macroscopic electrochemical behavior. Weaknesses: Their highly sophisticated approach requires specialized equipment and expertise not readily available in industrial settings; longer analysis timeframes may limit applicability for rapid material screening.
Critical CV Peak Interpretation Mechanisms
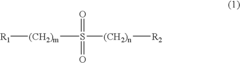
Electrolyte for a lithium battery and a lithium battery comprising the same
PatentInactiveUS20040214091A1
Innovation
- A non-aqueous electrolyte for lithium batteries incorporating specific additive compounds that initiate polymerization at high voltages, forming a coating layer on electrodes to reduce internal resistance and consume overcharge current, thereby enhancing safety and electrochemical characteristics.
Device and method of estimating an amount of charge of a battery
PatentWO2020188284A1
Innovation
- A method that calculates a current offset error between predicted and observed charge, using this error to iteratively estimate and adapt the battery's charge, incorporating self-recalibration routines to improve accuracy and compensate for internal impedance variations and aging.
Battery Degradation Mechanisms and CV Signatures
Battery degradation in lithium-ion systems manifests through multiple interconnected mechanisms that can be effectively monitored using cyclic voltammetry (CV) techniques. These degradation pathways leave distinctive electrochemical signatures that evolve across the battery's state of charge (SOC) and throughout its cycling lifetime.
The solid electrolyte interphase (SEI) formation and growth represents a primary degradation mechanism, characterized in CV profiles by diminishing peak intensities and increasing separation between anodic and cathodic peaks. This signature typically appears most prominently during early cycling stages, with the SEI layer consuming lithium ions and electrolyte components, resulting in capacity fade that can be quantified through integrated CV peak areas.
Active material dissolution, particularly for transition metal-based cathodes, produces characteristic shifts in redox peak positions. Manganese dissolution from LiMn₂O₄ cathodes, for example, manifests as a gradual negative shift in oxidation peaks coupled with broadening effects. These signatures become more pronounced at elevated temperatures and extreme SOC conditions, making CV an invaluable diagnostic tool for accelerated aging studies.
Lithium plating, occurring predominantly during fast charging or low-temperature operations, generates distinctive stripping peaks in the anodic scan around 0V versus Li/Li⁺. The intensity of these peaks correlates directly with the severity of lithium deposition, providing quantitative insights into this particularly dangerous degradation mode that can lead to dendrite formation and safety concerns.
Structural transformations within electrode materials create complex CV signatures, including the emergence of new redox couples or the disappearance of existing peaks. Phase transitions in layered cathode materials often manifest as peak splitting or merging phenomena that evolve systematically with cycling. These transformations frequently correlate with capacity fade mechanisms and can be monitored through peak position analysis across multiple CV cycles.
Electrolyte decomposition signatures appear as irreversible current contributions outside the normal operating voltage window. The progressive expansion of these regions in successive CV scans indicates accelerating electrolyte degradation, often accompanied by increasing cell impedance that manifests as greater peak separation in the voltammograms.
By systematically analyzing these CV signatures across different SOC ranges and throughout cycling, researchers can develop diagnostic protocols that enable early detection of specific degradation mechanisms. This approach facilitates more targeted intervention strategies and supports the development of advanced battery management systems capable of adaptive operation based on real-time degradation assessment.
The solid electrolyte interphase (SEI) formation and growth represents a primary degradation mechanism, characterized in CV profiles by diminishing peak intensities and increasing separation between anodic and cathodic peaks. This signature typically appears most prominently during early cycling stages, with the SEI layer consuming lithium ions and electrolyte components, resulting in capacity fade that can be quantified through integrated CV peak areas.
Active material dissolution, particularly for transition metal-based cathodes, produces characteristic shifts in redox peak positions. Manganese dissolution from LiMn₂O₄ cathodes, for example, manifests as a gradual negative shift in oxidation peaks coupled with broadening effects. These signatures become more pronounced at elevated temperatures and extreme SOC conditions, making CV an invaluable diagnostic tool for accelerated aging studies.
Lithium plating, occurring predominantly during fast charging or low-temperature operations, generates distinctive stripping peaks in the anodic scan around 0V versus Li/Li⁺. The intensity of these peaks correlates directly with the severity of lithium deposition, providing quantitative insights into this particularly dangerous degradation mode that can lead to dendrite formation and safety concerns.
Structural transformations within electrode materials create complex CV signatures, including the emergence of new redox couples or the disappearance of existing peaks. Phase transitions in layered cathode materials often manifest as peak splitting or merging phenomena that evolve systematically with cycling. These transformations frequently correlate with capacity fade mechanisms and can be monitored through peak position analysis across multiple CV cycles.
Electrolyte decomposition signatures appear as irreversible current contributions outside the normal operating voltage window. The progressive expansion of these regions in successive CV scans indicates accelerating electrolyte degradation, often accompanied by increasing cell impedance that manifests as greater peak separation in the voltammograms.
By systematically analyzing these CV signatures across different SOC ranges and throughout cycling, researchers can develop diagnostic protocols that enable early detection of specific degradation mechanisms. This approach facilitates more targeted intervention strategies and supports the development of advanced battery management systems capable of adaptive operation based on real-time degradation assessment.
Standardization of CV Protocols for Battery Research
The standardization of cyclic voltammetry (CV) protocols represents a critical need in battery research, particularly as this electroanalytical technique continues to gain prominence for characterizing battery materials across different states of charge (SOC) and cycling conditions. Current CV practices in battery research suffer from significant inconsistencies in experimental parameters, data collection methodologies, and reporting standards, which hinder meaningful comparisons between studies and impede scientific progress.
A comprehensive standardization framework must address multiple dimensions of CV experimentation. Scan rate selection requires particular attention, as rates ranging from 0.1 mV/s to 100 mV/s are commonly employed across different studies, yielding vastly different peak profiles and interpretations. The establishment of recommended scan rate ranges for specific battery chemistries would facilitate more consistent data generation and interpretation.
Temperature control during CV measurements represents another critical parameter requiring standardization. Battery redox reactions exhibit strong temperature dependence, yet many publications fail to report precise temperature conditions. A standardized protocol should mandate temperature reporting with ±1°C precision and recommend specific temperature ranges for different battery chemistries.
Reference electrode selection and placement significantly impact CV measurements but lack consistent guidelines. The standardization effort should establish preferred reference electrodes for various electrolyte systems and specify optimal placement configurations to minimize IR drop and measurement artifacts that can distort peak positions and intensities.
Data processing and peak analysis methods vary considerably across research groups. Standardized baseline correction techniques, peak identification algorithms, and quantification methods would ensure that redox peaks are consistently interpreted across different states of charge and cycling histories. This would be particularly valuable for tracking subtle changes in peak characteristics that indicate degradation mechanisms.
Reporting requirements constitute another essential component of standardization. Minimum reporting standards should include detailed experimental conditions, cell configuration specifications, material preparation methods, and raw data availability to enable reproducibility and meaningful cross-study comparisons.
Implementation of these standardized protocols would significantly enhance the utility of CV as a diagnostic tool for battery research, enabling more reliable interpretation of electrochemical signatures across different states of charge and cycling conditions. This would accelerate the development of next-generation battery materials by providing a common analytical framework for the research community.
A comprehensive standardization framework must address multiple dimensions of CV experimentation. Scan rate selection requires particular attention, as rates ranging from 0.1 mV/s to 100 mV/s are commonly employed across different studies, yielding vastly different peak profiles and interpretations. The establishment of recommended scan rate ranges for specific battery chemistries would facilitate more consistent data generation and interpretation.
Temperature control during CV measurements represents another critical parameter requiring standardization. Battery redox reactions exhibit strong temperature dependence, yet many publications fail to report precise temperature conditions. A standardized protocol should mandate temperature reporting with ±1°C precision and recommend specific temperature ranges for different battery chemistries.
Reference electrode selection and placement significantly impact CV measurements but lack consistent guidelines. The standardization effort should establish preferred reference electrodes for various electrolyte systems and specify optimal placement configurations to minimize IR drop and measurement artifacts that can distort peak positions and intensities.
Data processing and peak analysis methods vary considerably across research groups. Standardized baseline correction techniques, peak identification algorithms, and quantification methods would ensure that redox peaks are consistently interpreted across different states of charge and cycling histories. This would be particularly valuable for tracking subtle changes in peak characteristics that indicate degradation mechanisms.
Reporting requirements constitute another essential component of standardization. Minimum reporting standards should include detailed experimental conditions, cell configuration specifications, material preparation methods, and raw data availability to enable reproducibility and meaningful cross-study comparisons.
Implementation of these standardized protocols would significantly enhance the utility of CV as a diagnostic tool for battery research, enabling more reliable interpretation of electrochemical signatures across different states of charge and cycling conditions. This would accelerate the development of next-generation battery materials by providing a common analytical framework for the research community.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!