Emerging Applications of PEMF Therapy in Pediatric Care
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF in Pediatrics: Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in various medical fields. In recent years, its potential applications in pediatric care have garnered significant attention from researchers and clinicians alike. The evolution of PEMF technology can be traced back to the mid-20th century, with initial focus on bone healing and pain management in adults. However, the unique physiological characteristics of children and the growing need for gentle, non-pharmacological interventions have led to an increased interest in exploring PEMF's potential in pediatric medicine.
The primary objective of investigating PEMF therapy in pediatric care is to harness its potential benefits while ensuring safety and efficacy for young patients. This exploration aims to address various pediatric conditions, including but not limited to musculoskeletal disorders, neurological issues, and chronic pain syndromes. By leveraging the non-invasive nature of PEMF, researchers and clinicians hope to develop treatment protocols that minimize side effects and reduce the reliance on pharmaceutical interventions in children.
The technological advancements in PEMF devices have played a crucial role in expanding its applicability to pediatric populations. Modern PEMF systems offer precise control over field strength, frequency, and waveform, allowing for tailored treatments that account for the unique physiological needs of children at different developmental stages. This customization potential has opened up new avenues for research and clinical applications, ranging from accelerating fracture healing in young athletes to managing symptoms of neurodevelopmental disorders.
As the field progresses, several key trends are shaping the future of PEMF therapy in pediatrics. There is a growing emphasis on conducting rigorous clinical trials specifically designed for pediatric populations, addressing the historical lack of child-specific data in many medical technologies. Additionally, researchers are exploring the integration of PEMF therapy with other treatment modalities to create comprehensive care plans for complex pediatric conditions.
The potential impact of PEMF therapy in pediatric care extends beyond immediate medical outcomes. By offering a non-invasive and potentially drug-free treatment option, PEMF could significantly improve the quality of life for young patients and their families. It may reduce the need for invasive procedures, minimize medication-related side effects, and potentially shorten recovery times for various conditions. These benefits align with the broader trend in pediatric medicine towards more patient-friendly and holistic treatment approaches.
The primary objective of investigating PEMF therapy in pediatric care is to harness its potential benefits while ensuring safety and efficacy for young patients. This exploration aims to address various pediatric conditions, including but not limited to musculoskeletal disorders, neurological issues, and chronic pain syndromes. By leveraging the non-invasive nature of PEMF, researchers and clinicians hope to develop treatment protocols that minimize side effects and reduce the reliance on pharmaceutical interventions in children.
The technological advancements in PEMF devices have played a crucial role in expanding its applicability to pediatric populations. Modern PEMF systems offer precise control over field strength, frequency, and waveform, allowing for tailored treatments that account for the unique physiological needs of children at different developmental stages. This customization potential has opened up new avenues for research and clinical applications, ranging from accelerating fracture healing in young athletes to managing symptoms of neurodevelopmental disorders.
As the field progresses, several key trends are shaping the future of PEMF therapy in pediatrics. There is a growing emphasis on conducting rigorous clinical trials specifically designed for pediatric populations, addressing the historical lack of child-specific data in many medical technologies. Additionally, researchers are exploring the integration of PEMF therapy with other treatment modalities to create comprehensive care plans for complex pediatric conditions.
The potential impact of PEMF therapy in pediatric care extends beyond immediate medical outcomes. By offering a non-invasive and potentially drug-free treatment option, PEMF could significantly improve the quality of life for young patients and their families. It may reduce the need for invasive procedures, minimize medication-related side effects, and potentially shorten recovery times for various conditions. These benefits align with the broader trend in pediatric medicine towards more patient-friendly and holistic treatment approaches.
Market Analysis for Pediatric PEMF Devices
The market for pediatric PEMF (Pulsed Electromagnetic Field) devices is experiencing significant growth, driven by increasing awareness of non-invasive treatment options for various pediatric conditions. This emerging sector within the broader medical device industry is attracting attention from healthcare providers, parents, and investors alike.
The global pediatric PEMF device market is currently in its nascent stage but shows promising potential. The market size, while still relatively small compared to other pediatric medical device segments, is expected to expand rapidly over the next five to ten years. This growth is primarily fueled by the rising prevalence of chronic conditions in children, such as musculoskeletal disorders, neurological issues, and pain management needs.
Key factors driving market demand include the non-invasive nature of PEMF therapy, its potential for reducing medication dependence, and the growing body of clinical evidence supporting its efficacy in pediatric applications. Parents are increasingly seeking alternative or complementary treatments for their children, particularly those with fewer side effects than traditional pharmacological interventions.
The market is segmented based on application areas, including bone healing, pain management, neurological disorders, and post-surgical recovery. Among these, bone healing and pain management applications are currently leading in terms of market share, with neurological applications showing the highest growth potential.
Geographically, North America dominates the pediatric PEMF device market, followed by Europe. This is largely due to higher healthcare expenditure, advanced medical infrastructure, and greater acceptance of innovative therapies in these regions. However, Asia-Pacific is emerging as a lucrative market, driven by improving healthcare access and rising disposable incomes in countries like China and India.
The competitive landscape of the pediatric PEMF device market is characterized by a mix of established medical device companies and innovative startups. Major players are investing in research and development to enhance device efficacy and expand application areas. Collaborations with pediatric hospitals and research institutions are becoming increasingly common as companies seek to build clinical evidence and gain market credibility.
Regulatory environment plays a crucial role in market dynamics. In the United States, the FDA's clearance process for pediatric medical devices, including PEMF devices, is rigorous, which can impact market entry timelines but also ensures product safety and efficacy. Similar regulatory frameworks in other regions are shaping the global market landscape.
Looking ahead, the pediatric PEMF device market is poised for substantial growth. Technological advancements, such as the development of more compact and user-friendly devices, are expected to drive adoption. Additionally, the increasing integration of PEMF therapy into pediatric care protocols is likely to expand the market further, creating opportunities for both existing players and new entrants in this promising healthcare segment.
The global pediatric PEMF device market is currently in its nascent stage but shows promising potential. The market size, while still relatively small compared to other pediatric medical device segments, is expected to expand rapidly over the next five to ten years. This growth is primarily fueled by the rising prevalence of chronic conditions in children, such as musculoskeletal disorders, neurological issues, and pain management needs.
Key factors driving market demand include the non-invasive nature of PEMF therapy, its potential for reducing medication dependence, and the growing body of clinical evidence supporting its efficacy in pediatric applications. Parents are increasingly seeking alternative or complementary treatments for their children, particularly those with fewer side effects than traditional pharmacological interventions.
The market is segmented based on application areas, including bone healing, pain management, neurological disorders, and post-surgical recovery. Among these, bone healing and pain management applications are currently leading in terms of market share, with neurological applications showing the highest growth potential.
Geographically, North America dominates the pediatric PEMF device market, followed by Europe. This is largely due to higher healthcare expenditure, advanced medical infrastructure, and greater acceptance of innovative therapies in these regions. However, Asia-Pacific is emerging as a lucrative market, driven by improving healthcare access and rising disposable incomes in countries like China and India.
The competitive landscape of the pediatric PEMF device market is characterized by a mix of established medical device companies and innovative startups. Major players are investing in research and development to enhance device efficacy and expand application areas. Collaborations with pediatric hospitals and research institutions are becoming increasingly common as companies seek to build clinical evidence and gain market credibility.
Regulatory environment plays a crucial role in market dynamics. In the United States, the FDA's clearance process for pediatric medical devices, including PEMF devices, is rigorous, which can impact market entry timelines but also ensures product safety and efficacy. Similar regulatory frameworks in other regions are shaping the global market landscape.
Looking ahead, the pediatric PEMF device market is poised for substantial growth. Technological advancements, such as the development of more compact and user-friendly devices, are expected to drive adoption. Additionally, the increasing integration of PEMF therapy into pediatric care protocols is likely to expand the market further, creating opportunities for both existing players and new entrants in this promising healthcare segment.
Current PEMF Technology in Child Healthcare
Pulsed Electromagnetic Field (PEMF) therapy has gained significant traction in pediatric care, offering a non-invasive and drug-free approach to various health conditions. In child healthcare, PEMF technology is currently being applied to address a range of issues, from pain management to accelerated healing of fractures and soft tissue injuries.
One of the primary applications of PEMF in pediatric care is in the treatment of bone-related conditions. The technology has shown promising results in stimulating bone growth and accelerating fracture healing in children. PEMF devices designed for this purpose typically emit low-frequency electromagnetic fields that penetrate the skin and stimulate cellular activity within the bone tissue. This stimulation enhances the production of growth factors and promotes the proliferation of osteoblasts, the cells responsible for bone formation.
In addition to bone health, PEMF therapy is being utilized for pain management in pediatric patients. Children suffering from chronic pain conditions, such as fibromyalgia or complex regional pain syndrome, have shown improvements with PEMF treatment. The technology works by modulating pain signals and reducing inflammation, offering a non-pharmacological alternative to traditional pain management approaches.
PEMF devices used in pediatric care come in various forms, including mats, pads, and localized applicators. These devices are designed to be child-friendly, with adjustable intensity levels and treatment durations to ensure safety and efficacy. Some PEMF systems are portable, allowing for home-based treatments under parental supervision, while others are used in clinical settings under professional guidance.
Another emerging application of PEMF in child healthcare is in the treatment of neurological disorders. Preliminary studies have shown potential benefits in conditions such as autism spectrum disorders and attention deficit hyperactivity disorder (ADHD). The therapy is thought to influence neurotransmitter activity and improve brain function, although more research is needed to fully understand its mechanisms and long-term effects in these areas.
PEMF technology is also being explored for its potential in enhancing wound healing and reducing post-operative recovery times in pediatric patients. By promoting cellular regeneration and improving blood circulation, PEMF therapy may accelerate the healing process and reduce the risk of complications following surgical procedures.
As research in this field continues to evolve, the integration of PEMF technology with other treatment modalities is becoming more common. For instance, combining PEMF therapy with physical therapy or occupational therapy has shown synergistic effects in improving outcomes for children with musculoskeletal or neurological conditions.
One of the primary applications of PEMF in pediatric care is in the treatment of bone-related conditions. The technology has shown promising results in stimulating bone growth and accelerating fracture healing in children. PEMF devices designed for this purpose typically emit low-frequency electromagnetic fields that penetrate the skin and stimulate cellular activity within the bone tissue. This stimulation enhances the production of growth factors and promotes the proliferation of osteoblasts, the cells responsible for bone formation.
In addition to bone health, PEMF therapy is being utilized for pain management in pediatric patients. Children suffering from chronic pain conditions, such as fibromyalgia or complex regional pain syndrome, have shown improvements with PEMF treatment. The technology works by modulating pain signals and reducing inflammation, offering a non-pharmacological alternative to traditional pain management approaches.
PEMF devices used in pediatric care come in various forms, including mats, pads, and localized applicators. These devices are designed to be child-friendly, with adjustable intensity levels and treatment durations to ensure safety and efficacy. Some PEMF systems are portable, allowing for home-based treatments under parental supervision, while others are used in clinical settings under professional guidance.
Another emerging application of PEMF in child healthcare is in the treatment of neurological disorders. Preliminary studies have shown potential benefits in conditions such as autism spectrum disorders and attention deficit hyperactivity disorder (ADHD). The therapy is thought to influence neurotransmitter activity and improve brain function, although more research is needed to fully understand its mechanisms and long-term effects in these areas.
PEMF technology is also being explored for its potential in enhancing wound healing and reducing post-operative recovery times in pediatric patients. By promoting cellular regeneration and improving blood circulation, PEMF therapy may accelerate the healing process and reduce the risk of complications following surgical procedures.
As research in this field continues to evolve, the integration of PEMF technology with other treatment modalities is becoming more common. For instance, combining PEMF therapy with physical therapy or occupational therapy has shown synergistic effects in improving outcomes for children with musculoskeletal or neurological conditions.
Existing PEMF Solutions for Children
01 PEMF devices for therapeutic applications
Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular repair and regeneration, potentially treating a wide range of conditions including pain, inflammation, and bone healing.- PEMF devices for therapeutic applications: Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular activity and promote healing. They can be used for pain management, tissue repair, and improving overall well-being.
- PEMF therapy for specific medical conditions: PEMF therapy is applied to treat specific medical conditions such as musculoskeletal disorders, neurological issues, and cardiovascular problems. The therapy can be tailored to target particular areas of the body or specific health concerns, offering a non-invasive treatment option for various ailments.
- Portable and wearable PEMF devices: Advancements in PEMF technology have led to the development of portable and wearable devices. These compact units allow for convenient at-home use or on-the-go treatments, making PEMF therapy more accessible for regular use in daily life.
- PEMF therapy combined with other treatments: PEMF therapy is often combined with other treatment modalities to enhance overall therapeutic effects. This may include integration with traditional medicine, physical therapy, or other alternative therapies to create comprehensive treatment plans for various health conditions.
- Advanced PEMF control systems and protocols: Modern PEMF devices incorporate advanced control systems and treatment protocols. These features allow for precise adjustment of field strength, frequency, and duration of treatment, enabling customized therapy sessions tailored to individual patient needs and specific health conditions.
02 PEMF therapy for pain management and tissue healing
PEMF therapy is utilized for pain management and tissue healing. The electromagnetic fields generated by PEMF devices can penetrate deep into tissues, promoting cellular repair, reducing inflammation, and accelerating the healing process in various musculoskeletal conditions.Expand Specific Solutions03 Portable and wearable PEMF devices
Advancements in PEMF technology have led to the development of portable and wearable devices. These compact units allow for convenient, on-the-go treatment, enabling patients to receive therapy while performing daily activities or during sleep, potentially improving compliance and treatment outcomes.Expand Specific Solutions04 PEMF therapy in veterinary medicine
PEMF therapy has found applications in veterinary medicine. The technology is being used to treat various conditions in animals, including pain management, wound healing, and musculoskeletal disorders, offering a non-invasive treatment option for pets and livestock.Expand Specific Solutions05 Combination of PEMF with other therapies
Research is exploring the combination of PEMF therapy with other treatment modalities. This approach aims to enhance therapeutic outcomes by synergizing the benefits of PEMF with other therapies such as light therapy, heat therapy, or traditional medical treatments, potentially offering more comprehensive and effective treatment options.Expand Specific Solutions
Key Players in Pediatric PEMF Industry
The emerging field of PEMF therapy in pediatric care is in its early developmental stages, with a growing market potential as research progresses. The technology's maturity is still evolving, with companies like Venus Concept Ltd. and Regenesis Biomedical, Inc. leading innovation in medical aesthetics and regenerative medicine, respectively. While the market size is currently modest, it shows promise for expansion as clinical evidence accumulates. Established players such as Medtronic and Novartis AG bring significant resources to the field, potentially accelerating technological advancements and market adoption. Academic institutions like the National University of Singapore and the University of Basel are contributing valuable research, further driving the technology's development and potential applications in pediatric care.
Venus Concept Ltd.
Technical Solution: Venus Concept has developed a proprietary PEMF technology called Venus Pulse™, which is being explored for pediatric applications. Their approach focuses on non-invasive, low-intensity PEMF therapy that can be safely applied to children. The technology utilizes precise electromagnetic pulses to stimulate cellular repair and regeneration, potentially addressing various pediatric conditions such as musculoskeletal disorders and wound healing[1]. Venus Concept's PEMF devices are designed with adjustable parameters to cater to the specific needs of pediatric patients, ensuring gentle yet effective treatment[2].
Strengths: Customizable for pediatric use, non-invasive nature suitable for children. Weaknesses: Limited long-term studies on pediatric applications, potential concerns about electromagnetic exposure in developing bodies.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed the Provant® Therapy System, which utilizes PEMF technology for pain management and tissue repair. While primarily focused on adult applications, the company is exploring the potential of adapting this technology for pediatric care. Their approach involves using pulsed radiofrequency energy to stimulate cellular activity and promote healing. For pediatric applications, Regenesis is investigating lower-intensity settings and modified treatment protocols to ensure safety and efficacy in younger patients[3]. The company is particularly interested in addressing pediatric chronic pain conditions and post-surgical recovery[4].
Strengths: Established technology with potential for pediatric adaptation, focus on non-pharmacological pain management. Weaknesses: Limited pediatric-specific clinical data, may require significant modifications for child-friendly use.
Innovative PEMF Techniques for Pediatric Use
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
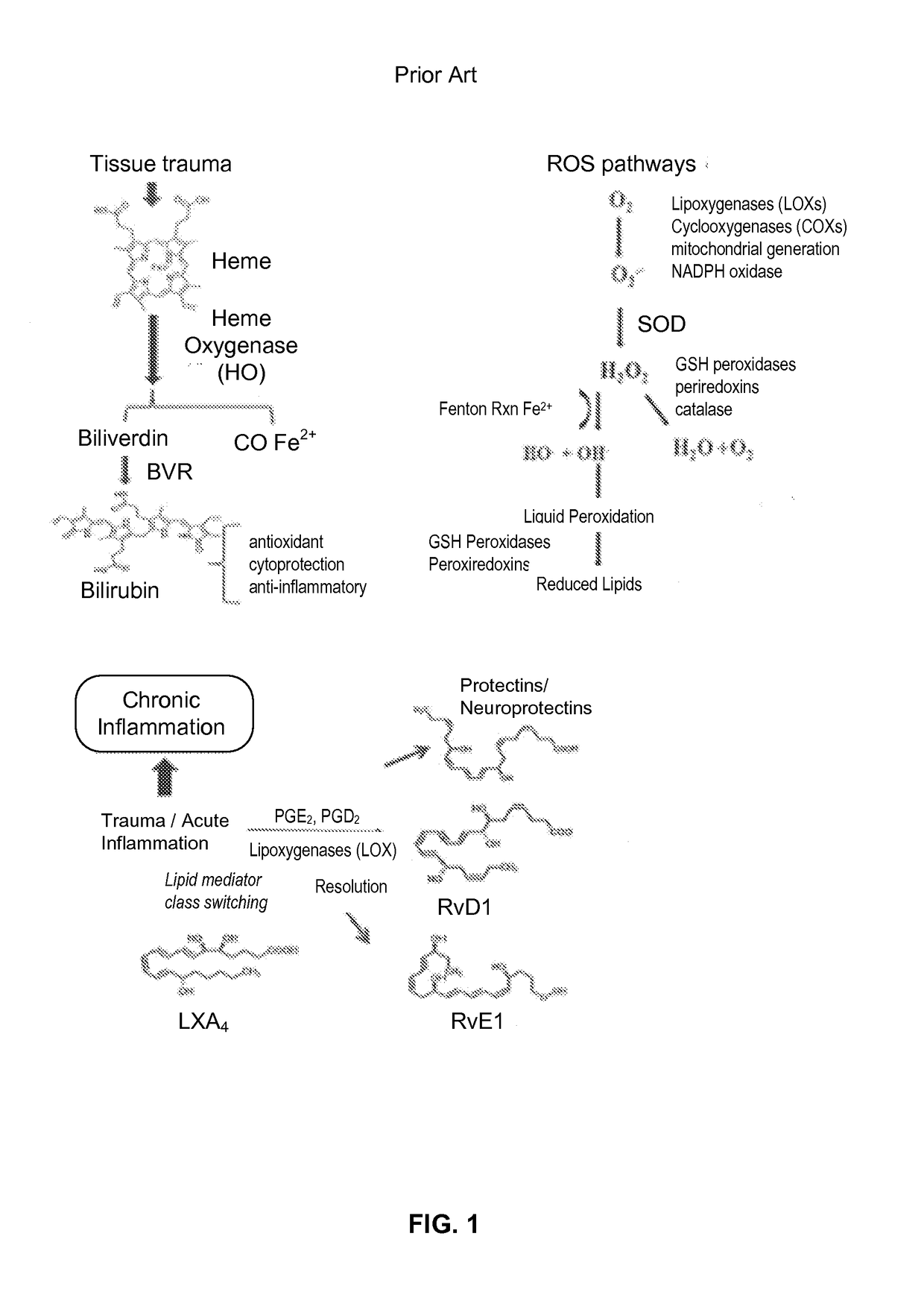
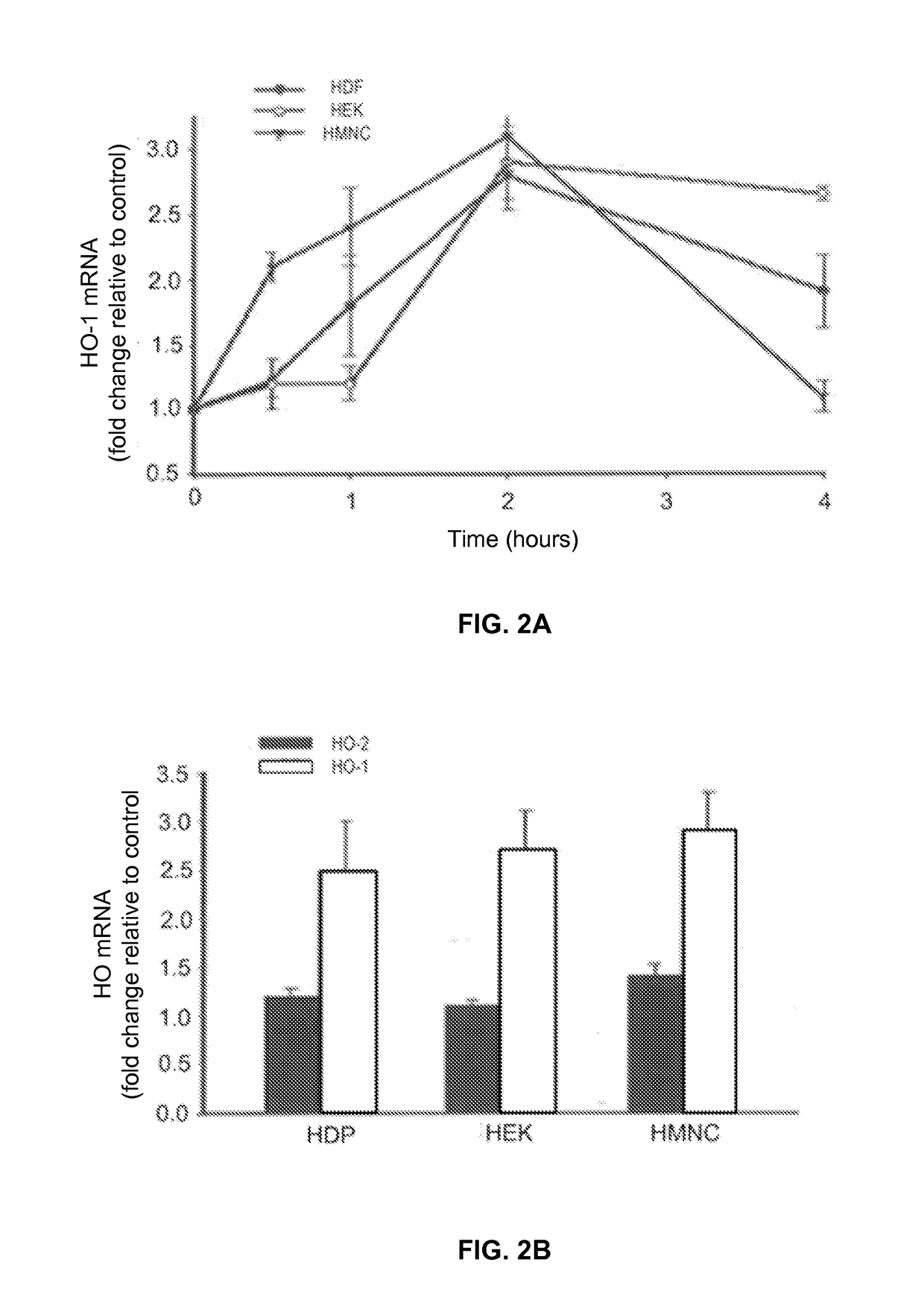
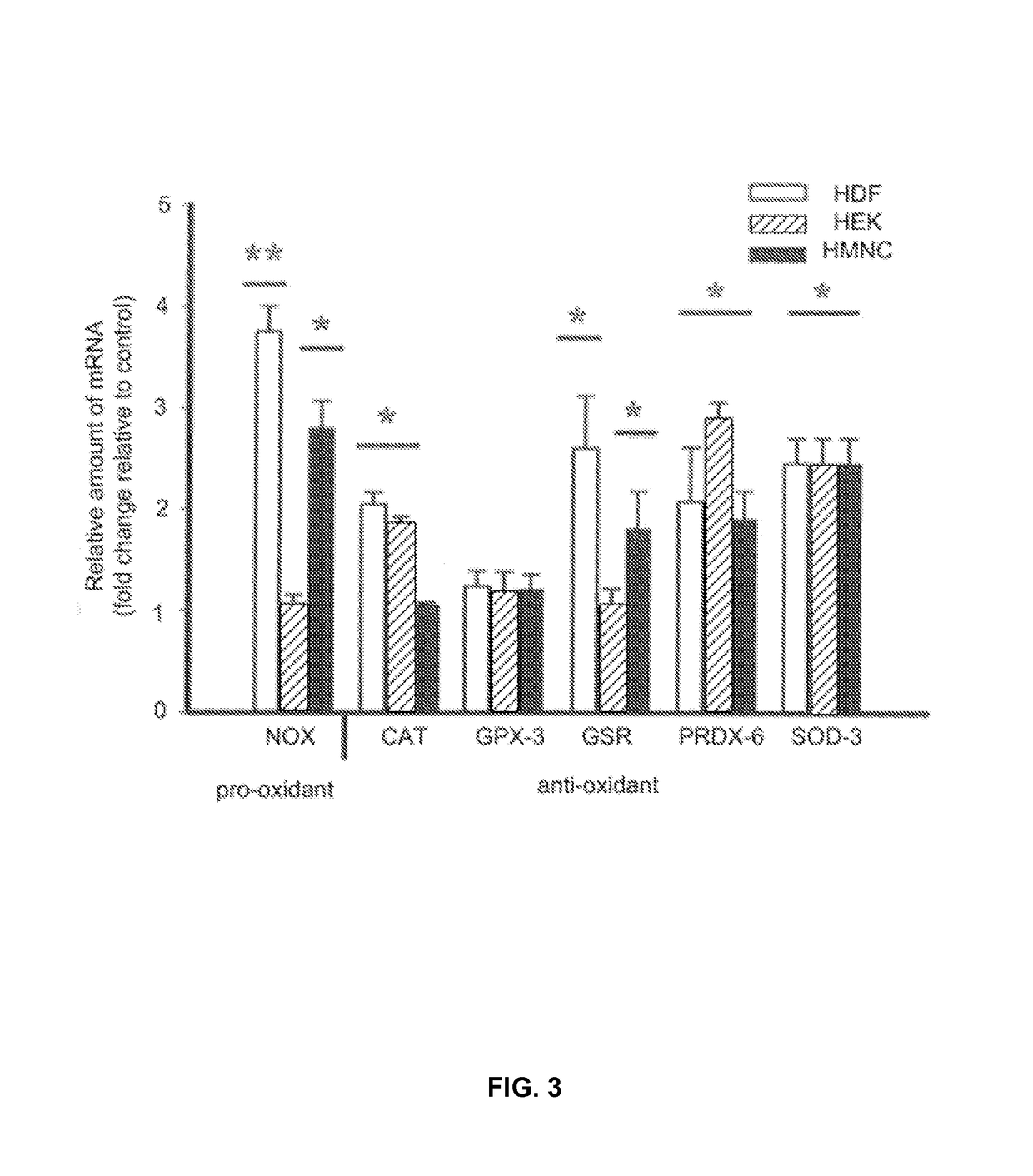
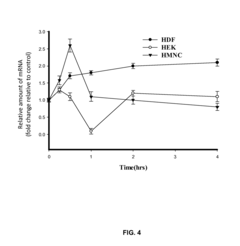
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Pulsed Electromagnetic Field (PEMF) Therapy Whole Body Wellness Device to increase cells energy, strengthen immune system and promote cell regeneration
PatentInactiveUS20190054308A1
Innovation
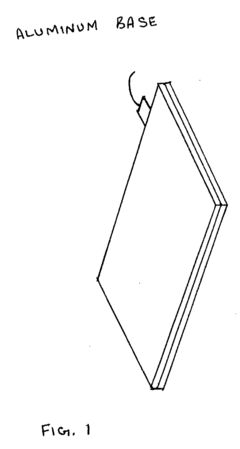
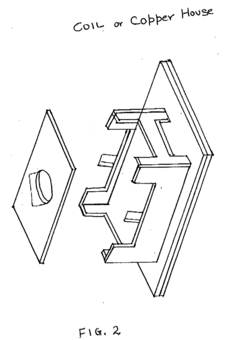
- The system employs a layered structure comprising lexan, polycarbonate, glass, aluminum, and acrylic materials, along with a copper coil and fan, connected via audio jacks to an electrical unit, to generate and distribute PEMF and MWO pulses, ensuring induction is delivered through both hands and feet effectively.
Safety and Efficacy in Pediatric PEMF Therapy
The safety and efficacy of Pulsed Electromagnetic Field (PEMF) therapy in pediatric care are paramount considerations as this emerging technology gains traction in treating various childhood conditions. Extensive research has been conducted to evaluate the potential benefits and risks associated with PEMF therapy in young patients, with promising results in several areas.
Safety studies have shown that PEMF therapy, when applied within recommended parameters, poses minimal risk to pediatric patients. The non-invasive nature of the treatment and the absence of ionizing radiation make it an attractive option for children. However, long-term studies are still ongoing to fully assess any potential delayed effects on developing tissues and organs.
Efficacy studies have demonstrated positive outcomes in several pediatric applications. In the treatment of bone fractures, PEMF therapy has shown to accelerate healing and reduce recovery time in children. This is particularly beneficial for active youngsters eager to return to normal activities. Additionally, PEMF therapy has exhibited potential in managing chronic pain conditions in pediatric patients, offering a non-pharmacological alternative to traditional pain management strategies.
Neurological disorders in children have also been a focus of PEMF research. Preliminary studies suggest that PEMF therapy may have neuroprotective effects and could potentially aid in the treatment of conditions such as cerebral palsy and autism spectrum disorders. However, more robust clinical trials are needed to confirm these findings and establish standardized treatment protocols.
One area of particular interest is the application of PEMF therapy in pediatric oncology. Some studies have indicated that PEMF may enhance the efficacy of certain cancer treatments while potentially reducing side effects. This dual benefit could be especially valuable in pediatric patients, where minimizing treatment-related toxicity is crucial.
Despite the promising results, it is essential to note that the optimal parameters for PEMF therapy in children may differ from those used in adults. Factors such as tissue depth, body size, and developmental stage must be carefully considered when designing treatment protocols for pediatric patients. Ongoing research is focused on refining these parameters to ensure maximum efficacy and safety.
As with any medical intervention, close monitoring and follow-up are crucial when applying PEMF therapy to pediatric patients. Healthcare providers must remain vigilant for any unexpected side effects or adverse reactions, particularly in long-term use scenarios. The development of standardized guidelines for pediatric PEMF therapy is an ongoing process, with professional medical organizations working to establish best practices based on the growing body of evidence.
Safety studies have shown that PEMF therapy, when applied within recommended parameters, poses minimal risk to pediatric patients. The non-invasive nature of the treatment and the absence of ionizing radiation make it an attractive option for children. However, long-term studies are still ongoing to fully assess any potential delayed effects on developing tissues and organs.
Efficacy studies have demonstrated positive outcomes in several pediatric applications. In the treatment of bone fractures, PEMF therapy has shown to accelerate healing and reduce recovery time in children. This is particularly beneficial for active youngsters eager to return to normal activities. Additionally, PEMF therapy has exhibited potential in managing chronic pain conditions in pediatric patients, offering a non-pharmacological alternative to traditional pain management strategies.
Neurological disorders in children have also been a focus of PEMF research. Preliminary studies suggest that PEMF therapy may have neuroprotective effects and could potentially aid in the treatment of conditions such as cerebral palsy and autism spectrum disorders. However, more robust clinical trials are needed to confirm these findings and establish standardized treatment protocols.
One area of particular interest is the application of PEMF therapy in pediatric oncology. Some studies have indicated that PEMF may enhance the efficacy of certain cancer treatments while potentially reducing side effects. This dual benefit could be especially valuable in pediatric patients, where minimizing treatment-related toxicity is crucial.
Despite the promising results, it is essential to note that the optimal parameters for PEMF therapy in children may differ from those used in adults. Factors such as tissue depth, body size, and developmental stage must be carefully considered when designing treatment protocols for pediatric patients. Ongoing research is focused on refining these parameters to ensure maximum efficacy and safety.
As with any medical intervention, close monitoring and follow-up are crucial when applying PEMF therapy to pediatric patients. Healthcare providers must remain vigilant for any unexpected side effects or adverse reactions, particularly in long-term use scenarios. The development of standardized guidelines for pediatric PEMF therapy is an ongoing process, with professional medical organizations working to establish best practices based on the growing body of evidence.
Regulatory Framework for Pediatric PEMF Devices
The regulatory framework for pediatric PEMF devices is a critical aspect of ensuring the safety and efficacy of these emerging therapeutic tools in pediatric care. As PEMF therapy gains traction in treating various conditions in children, regulatory bodies worldwide are adapting their guidelines to address the unique considerations of this patient population.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating PEMF devices for pediatric use. These devices are typically classified as Class II medical devices, requiring a 510(k) premarket notification submission. The FDA has established specific guidelines for pediatric medical devices, emphasizing the importance of age-appropriate design, safety features, and clinical evidence tailored to children's physiological differences.
The European Union, through its Medical Device Regulation (MDR), has implemented stringent requirements for pediatric medical devices, including PEMF therapy equipment. The MDR mandates comprehensive clinical investigations and post-market surveillance for devices intended for pediatric use, ensuring ongoing safety monitoring and efficacy assessment.
Regulatory bodies are particularly focused on the potential long-term effects of PEMF therapy on developing tissues and organs in children. As a result, manufacturers are required to conduct extensive pre-clinical and clinical studies to demonstrate both short-term and long-term safety profiles. These studies must address concerns such as the impact on bone growth, neurological development, and potential interactions with other medical treatments commonly used in pediatric care.
The regulatory framework also emphasizes the importance of age-specific dosing and treatment protocols. Manufacturers must provide clear guidelines on the appropriate field strengths, frequencies, and duration of PEMF therapy for different age groups within the pediatric population. This requirement stems from the recognition that children's bodies respond differently to electromagnetic fields compared to adults, necessitating carefully calibrated treatment parameters.
Additionally, regulatory agencies are increasingly focusing on the usability and design of PEMF devices for pediatric applications. Manufacturers are expected to incorporate child-friendly features, such as tamper-resistant controls, clear and simple user interfaces, and safeguards against accidental misuse. These design considerations are crucial for ensuring that PEMF devices can be safely and effectively used in home settings under parental supervision.
As the field of pediatric PEMF therapy continues to evolve, regulatory frameworks are likely to undergo further refinement. Ongoing dialogue between regulatory bodies, medical professionals, and device manufacturers will be essential in shaping future guidelines that balance innovation with patient safety in this promising area of pediatric care.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating PEMF devices for pediatric use. These devices are typically classified as Class II medical devices, requiring a 510(k) premarket notification submission. The FDA has established specific guidelines for pediatric medical devices, emphasizing the importance of age-appropriate design, safety features, and clinical evidence tailored to children's physiological differences.
The European Union, through its Medical Device Regulation (MDR), has implemented stringent requirements for pediatric medical devices, including PEMF therapy equipment. The MDR mandates comprehensive clinical investigations and post-market surveillance for devices intended for pediatric use, ensuring ongoing safety monitoring and efficacy assessment.
Regulatory bodies are particularly focused on the potential long-term effects of PEMF therapy on developing tissues and organs in children. As a result, manufacturers are required to conduct extensive pre-clinical and clinical studies to demonstrate both short-term and long-term safety profiles. These studies must address concerns such as the impact on bone growth, neurological development, and potential interactions with other medical treatments commonly used in pediatric care.
The regulatory framework also emphasizes the importance of age-specific dosing and treatment protocols. Manufacturers must provide clear guidelines on the appropriate field strengths, frequencies, and duration of PEMF therapy for different age groups within the pediatric population. This requirement stems from the recognition that children's bodies respond differently to electromagnetic fields compared to adults, necessitating carefully calibrated treatment parameters.
Additionally, regulatory agencies are increasingly focusing on the usability and design of PEMF devices for pediatric applications. Manufacturers are expected to incorporate child-friendly features, such as tamper-resistant controls, clear and simple user interfaces, and safeguards against accidental misuse. These design considerations are crucial for ensuring that PEMF devices can be safely and effectively used in home settings under parental supervision.
As the field of pediatric PEMF therapy continues to evolve, regulatory frameworks are likely to undergo further refinement. Ongoing dialogue between regulatory bodies, medical professionals, and device manufacturers will be essential in shaping future guidelines that balance innovation with patient safety in this promising area of pediatric care.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!