Enhancing Osteoconductive Properties Using Layered Hydroxyapatite Nanocomposites
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Nanocomposite Background and Objectives
Hydroxyapatite (HA) has been a cornerstone in bone tissue engineering for decades due to its remarkable similarity to the mineral component of natural bone. As research in this field has progressed, the focus has shifted towards enhancing the osteoconductive properties of HA through the development of nanocomposites. This technological evolution aims to address the limitations of conventional HA, such as brittleness and slow resorption rates, while amplifying its beneficial properties for bone regeneration.
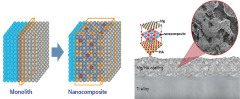
The concept of layered hydroxyapatite nanocomposites represents a significant leap forward in biomaterials science. By incorporating nanoscale structures and layered architectures, researchers aim to mimic the hierarchical organization of natural bone more closely. This biomimetic approach not only enhances the mechanical properties of the material but also provides a more favorable environment for cell attachment, proliferation, and differentiation.
The development of these advanced nanocomposites is driven by the increasing demand for more effective bone graft substitutes in orthopedic and dental applications. With an aging global population and a rise in bone-related disorders, there is a pressing need for materials that can accelerate bone healing and integration. Layered hydroxyapatite nanocomposites offer the potential to meet these clinical needs by providing superior osteoconductive properties compared to traditional HA-based materials.
The primary objectives in enhancing the osteoconductive properties of HA through nanocomposite technology are multifaceted. Researchers aim to improve the material's surface area and roughness, which are crucial factors in cell adhesion and bone formation. Additionally, there is a focus on optimizing the porosity and interconnectivity of the nanocomposite structure to facilitate vascularization and nutrient transport, essential elements for successful bone regeneration.
Another key goal is to enhance the mechanical strength and toughness of the material without compromising its biocompatibility. This is particularly important for load-bearing applications where traditional HA often falls short. By incorporating various nanomaterials and creating layered structures, scientists seek to develop composites that can withstand physiological stresses while maintaining their bioactive properties.
Furthermore, the research aims to fine-tune the degradation rate of these nanocomposites to match the pace of new bone formation. This controlled resorption is crucial for ensuring a gradual transfer of load-bearing responsibilities from the implant to the newly formed bone tissue. The layered structure of these nanocomposites offers the potential for tailoring degradation profiles, allowing for more precise control over the bone regeneration process.
In summary, the development of layered hydroxyapatite nanocomposites represents a convergence of materials science, nanotechnology, and tissue engineering. The overarching goal is to create a new generation of biomaterials that can more effectively stimulate and support bone regeneration, ultimately leading to improved patient outcomes in orthopedic and dental treatments.
The concept of layered hydroxyapatite nanocomposites represents a significant leap forward in biomaterials science. By incorporating nanoscale structures and layered architectures, researchers aim to mimic the hierarchical organization of natural bone more closely. This biomimetic approach not only enhances the mechanical properties of the material but also provides a more favorable environment for cell attachment, proliferation, and differentiation.
The development of these advanced nanocomposites is driven by the increasing demand for more effective bone graft substitutes in orthopedic and dental applications. With an aging global population and a rise in bone-related disorders, there is a pressing need for materials that can accelerate bone healing and integration. Layered hydroxyapatite nanocomposites offer the potential to meet these clinical needs by providing superior osteoconductive properties compared to traditional HA-based materials.
The primary objectives in enhancing the osteoconductive properties of HA through nanocomposite technology are multifaceted. Researchers aim to improve the material's surface area and roughness, which are crucial factors in cell adhesion and bone formation. Additionally, there is a focus on optimizing the porosity and interconnectivity of the nanocomposite structure to facilitate vascularization and nutrient transport, essential elements for successful bone regeneration.
Another key goal is to enhance the mechanical strength and toughness of the material without compromising its biocompatibility. This is particularly important for load-bearing applications where traditional HA often falls short. By incorporating various nanomaterials and creating layered structures, scientists seek to develop composites that can withstand physiological stresses while maintaining their bioactive properties.
Furthermore, the research aims to fine-tune the degradation rate of these nanocomposites to match the pace of new bone formation. This controlled resorption is crucial for ensuring a gradual transfer of load-bearing responsibilities from the implant to the newly formed bone tissue. The layered structure of these nanocomposites offers the potential for tailoring degradation profiles, allowing for more precise control over the bone regeneration process.
In summary, the development of layered hydroxyapatite nanocomposites represents a convergence of materials science, nanotechnology, and tissue engineering. The overarching goal is to create a new generation of biomaterials that can more effectively stimulate and support bone regeneration, ultimately leading to improved patient outcomes in orthopedic and dental treatments.
Market Analysis for Bone Graft Substitutes
The global bone graft substitutes market has been experiencing significant growth, driven by the increasing prevalence of bone and joint disorders, rising geriatric population, and advancements in medical technology. The market for bone graft substitutes is expected to continue its upward trajectory, with a particular focus on innovative materials such as layered hydroxyapatite nanocomposites.
Demographic trends play a crucial role in market expansion, as the aging population is more susceptible to osteoporosis, fractures, and joint diseases. This demographic shift has led to a surge in demand for orthopedic procedures, consequently boosting the need for bone graft substitutes. Additionally, the rising incidence of sports-related injuries and road accidents contributes to market growth.
The market is segmented based on product type, including allografts, synthetic bone grafts, xenografts, and others. Synthetic bone grafts, particularly those incorporating advanced materials like layered hydroxyapatite nanocomposites, are gaining traction due to their enhanced osteoconductive properties and reduced risk of disease transmission compared to traditional options.
Geographically, North America holds the largest market share, attributed to its well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced medical technologies. Europe follows closely, while the Asia-Pacific region is emerging as the fastest-growing market, driven by improving healthcare access and rising disposable incomes in countries like China and India.
Key market players are investing heavily in research and development to introduce innovative products with superior osteoconductive properties. The development of layered hydroxyapatite nanocomposites represents a significant advancement in this field, offering improved bone regeneration capabilities and potentially faster healing times.
Factors such as the increasing number of orthopedic surgeries, dental procedures, and spinal fusion surgeries are expected to fuel market growth. However, the high cost of bone graft procedures and stringent regulatory approval processes may pose challenges to market expansion.
The COVID-19 pandemic initially caused a temporary slowdown in the market due to the postponement of elective surgeries. However, the market has shown resilience and is expected to rebound strongly as healthcare systems adapt to the new normal and address the backlog of postponed procedures.
Demographic trends play a crucial role in market expansion, as the aging population is more susceptible to osteoporosis, fractures, and joint diseases. This demographic shift has led to a surge in demand for orthopedic procedures, consequently boosting the need for bone graft substitutes. Additionally, the rising incidence of sports-related injuries and road accidents contributes to market growth.
The market is segmented based on product type, including allografts, synthetic bone grafts, xenografts, and others. Synthetic bone grafts, particularly those incorporating advanced materials like layered hydroxyapatite nanocomposites, are gaining traction due to their enhanced osteoconductive properties and reduced risk of disease transmission compared to traditional options.
Geographically, North America holds the largest market share, attributed to its well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced medical technologies. Europe follows closely, while the Asia-Pacific region is emerging as the fastest-growing market, driven by improving healthcare access and rising disposable incomes in countries like China and India.
Key market players are investing heavily in research and development to introduce innovative products with superior osteoconductive properties. The development of layered hydroxyapatite nanocomposites represents a significant advancement in this field, offering improved bone regeneration capabilities and potentially faster healing times.
Factors such as the increasing number of orthopedic surgeries, dental procedures, and spinal fusion surgeries are expected to fuel market growth. However, the high cost of bone graft procedures and stringent regulatory approval processes may pose challenges to market expansion.
The COVID-19 pandemic initially caused a temporary slowdown in the market due to the postponement of elective surgeries. However, the market has shown resilience and is expected to rebound strongly as healthcare systems adapt to the new normal and address the backlog of postponed procedures.
Current Challenges in Osteoconductive Materials
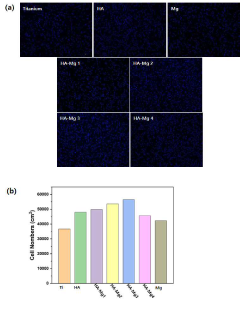
The field of osteoconductive materials has witnessed significant advancements in recent years, yet several challenges persist in achieving optimal bone regeneration and integration. One of the primary obstacles is the limited ability of current materials to mimic the complex hierarchical structure of natural bone. This structural mismatch often leads to suboptimal cell adhesion, proliferation, and differentiation, ultimately affecting the overall osteoconductive properties.
Another critical challenge lies in controlling the degradation rate of osteoconductive materials. Many existing materials either degrade too quickly, compromising mechanical stability, or too slowly, impeding the natural bone remodeling process. Striking the right balance between material degradation and new bone formation remains a significant hurdle in the field.
The biocompatibility of osteoconductive materials also presents ongoing challenges. While many materials show promising results in vitro, their performance in vivo can be compromised by inflammatory responses or foreign body reactions. This discrepancy highlights the need for more sophisticated testing models that better predict in vivo outcomes.
Furthermore, the mechanical properties of osteoconductive materials often fall short of natural bone. Many materials struggle to match the strength and elasticity of native bone tissue, leading to potential stress shielding or implant failure. This mismatch in mechanical properties can hinder long-term integration and functionality of bone implants.
The scalability and reproducibility of advanced osteoconductive materials pose additional challenges. Many promising materials developed in laboratory settings face difficulties in scaling up for clinical applications while maintaining consistent quality and performance. This gap between bench-top success and clinical translation remains a significant bottleneck in the field.
Another persistent challenge is the limited ability of current materials to promote vascularization. Inadequate blood vessel formation within newly formed bone tissue can lead to poor nutrient supply and waste removal, ultimately affecting the long-term viability of the regenerated bone.
Lastly, the integration of growth factors and other bioactive molecules into osteoconductive materials in a controlled and sustained manner remains challenging. While these molecules can significantly enhance bone regeneration, their delivery and release kinetics are often suboptimal, leading to reduced efficacy or potential side effects.
Another critical challenge lies in controlling the degradation rate of osteoconductive materials. Many existing materials either degrade too quickly, compromising mechanical stability, or too slowly, impeding the natural bone remodeling process. Striking the right balance between material degradation and new bone formation remains a significant hurdle in the field.
The biocompatibility of osteoconductive materials also presents ongoing challenges. While many materials show promising results in vitro, their performance in vivo can be compromised by inflammatory responses or foreign body reactions. This discrepancy highlights the need for more sophisticated testing models that better predict in vivo outcomes.
Furthermore, the mechanical properties of osteoconductive materials often fall short of natural bone. Many materials struggle to match the strength and elasticity of native bone tissue, leading to potential stress shielding or implant failure. This mismatch in mechanical properties can hinder long-term integration and functionality of bone implants.
The scalability and reproducibility of advanced osteoconductive materials pose additional challenges. Many promising materials developed in laboratory settings face difficulties in scaling up for clinical applications while maintaining consistent quality and performance. This gap between bench-top success and clinical translation remains a significant bottleneck in the field.
Another persistent challenge is the limited ability of current materials to promote vascularization. Inadequate blood vessel formation within newly formed bone tissue can lead to poor nutrient supply and waste removal, ultimately affecting the long-term viability of the regenerated bone.
Lastly, the integration of growth factors and other bioactive molecules into osteoconductive materials in a controlled and sustained manner remains challenging. While these molecules can significantly enhance bone regeneration, their delivery and release kinetics are often suboptimal, leading to reduced efficacy or potential side effects.
Layered Nanocomposite Fabrication Techniques
01 Layered hydroxyapatite nanocomposite synthesis
Methods for synthesizing layered hydroxyapatite nanocomposites with enhanced osteoconductive properties. These techniques involve the controlled assembly of hydroxyapatite nanoparticles into layered structures, often incorporating other biocompatible materials to improve mechanical strength and bioactivity.- Layered hydroxyapatite nanocomposite synthesis: Methods for synthesizing layered hydroxyapatite nanocomposites with enhanced osteoconductive properties. These techniques involve combining hydroxyapatite with various materials to create nanostructured composites that mimic the natural bone structure, improving biocompatibility and bone regeneration potential.
- Incorporation of bioactive agents: Integration of bioactive agents into layered hydroxyapatite nanocomposites to enhance their osteoconductive properties. These agents may include growth factors, antibiotics, or other therapeutic compounds that promote bone growth, prevent infection, or accelerate healing processes.
- Surface modification techniques: Methods for modifying the surface of layered hydroxyapatite nanocomposites to improve their osteoconductive properties. These techniques may involve chemical treatments, plasma processing, or coating with bioactive materials to enhance cell adhesion, proliferation, and differentiation on the nanocomposite surface.
- Porosity control and optimization: Techniques for controlling and optimizing the porosity of layered hydroxyapatite nanocomposites to enhance their osteoconductive properties. This includes methods for creating interconnected pore structures that facilitate cell infiltration, nutrient transport, and vascularization, leading to improved bone ingrowth and integration.
- Mechanical property enhancement: Strategies for improving the mechanical properties of layered hydroxyapatite nanocomposites while maintaining their osteoconductive characteristics. These may include reinforcement with other materials, optimization of nanoparticle size and distribution, or novel processing techniques to enhance strength, toughness, and durability for load-bearing applications.
02 Incorporation of bioactive agents
Integration of bioactive agents, such as growth factors or antibiotics, into layered hydroxyapatite nanocomposites. This approach enhances the osteoconductive properties by promoting bone growth and preventing infections, making the nanocomposites more effective for bone tissue engineering applications.Expand Specific Solutions03 Surface modification techniques
Various surface modification techniques applied to layered hydroxyapatite nanocomposites to improve their osteoconductive properties. These methods include plasma treatment, chemical functionalization, and coating with bioactive molecules, enhancing cell adhesion and proliferation on the nanocomposite surface.Expand Specific Solutions04 Polymer-hydroxyapatite nanocomposites
Development of polymer-hydroxyapatite nanocomposites with improved mechanical properties and osteoconductivity. These composites combine the flexibility of polymers with the bioactivity of hydroxyapatite, creating materials that better mimic the natural bone structure and promote osseointegration.Expand Specific Solutions05 3D printing of layered hydroxyapatite nanocomposites
Utilization of 3D printing technologies to fabricate layered hydroxyapatite nanocomposites with precise control over structure and porosity. This approach allows for the creation of customized bone scaffolds with optimized osteoconductive properties tailored to specific patient needs.Expand Specific Solutions
Key Players in Bone Tissue Engineering
The field of enhancing osteoconductive properties using layered hydroxyapatite nanocomposites is in a growth phase, with increasing market potential due to rising demand for advanced bone regeneration materials. The global market for bone graft substitutes, including hydroxyapatite-based materials, is projected to reach several billion dollars by 2025. Technologically, the field is advancing rapidly, with key players like Promimic AB, DePuy Synthes, and LG Chem Ltd. leading innovation. Academic institutions such as MIT, Zhejiang University, and IIT Bombay are contributing significantly to research and development, indicating a maturing technology landscape with potential for further breakthroughs in biocompatibility and osseointegration.
Promimic AB
Technical Solution: Promimic AB has developed a unique HAnano Surface technology based on layered hydroxyapatite nanocomposites. Their approach involves creating an ultra-thin, nanometer-scale coating of hydroxyapatite nanoparticles on implant surfaces using a wet-chemical deposition process[10]. The layered nanostructure closely resembles the natural apatite found in bone, promoting rapid osseointegration and improving implant stability. Promimic's technology allows for the incorporation of other bioactive ions, such as strontium or fluoride, within the nanocomposite layers to further enhance bone formation[11]. Clinical studies have demonstrated that implants with the HAnano Surface show faster and stronger bone integration compared to traditional implant surfaces, particularly in challenging patient cases with low bone quality[12].
Strengths: Ultra-thin coating preserves implant micro-geometry, versatile application to various implant materials, and clinically proven effectiveness. Weaknesses: Limited to surface modification and potential long-term wear concerns in high-stress applications.
DePuy Synthes Products, Inc.
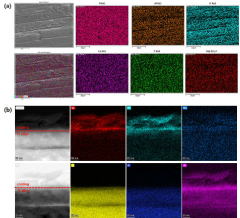
Technical Solution: DePuy Synthes has developed a proprietary layered hydroxyapatite nanocomposite coating for their orthopedic implants. Their technology utilizes a plasma spray process to deposit alternating layers of hydroxyapatite and bioactive glass nanoparticles onto metal implant surfaces[2]. This layered approach creates a graded structure that enhances osseointegration and promotes rapid bone formation. The nanocomposite coating also incorporates trace elements such as strontium and magnesium to further stimulate osteoblast activity and improve bone mineralization[4]. Clinical studies have demonstrated that implants with this layered nanocomposite coating show significantly faster and more robust bone integration compared to traditional hydroxyapatite coatings[6].
Strengths: Improved osseointegration, incorporation of bioactive elements, and clinically proven effectiveness. Weaknesses: Limited to coating applications and potential long-term stability concerns in highly loaded implants.
Innovations in Hydroxyapatite Nanostructure Design
Coating layer containing a nanocomposite with improved osteosynthetic properties, manufacturing method thereof and the uses thereof
PatentPendingKR1020240081050A
Innovation
- A nanocomposite coating layer comprising hydroxyapatite and metals like magnesium, titanium, gold, platinum, silver, cobalt, titanium nitride, or chromium nitride is formed using RF sputtering, enhancing adhesion to substrates and improving blood wettability, thereby accelerating osseointegration.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing layered hydroxyapatite nanocomposites for enhancing osteoconductive properties. These materials, designed to interact with living tissues, must undergo rigorous testing to ensure they do not elicit adverse reactions or pose long-term health risks.
The biocompatibility of layered hydroxyapatite nanocomposites is primarily influenced by their chemical composition, surface properties, and degradation behavior. The chemical similarity of hydroxyapatite to natural bone mineral contributes to its inherent biocompatibility. However, the introduction of additional layers and nanostructures may alter the material's interaction with biological systems.
Surface characteristics, including topography, charge, and wettability, play a crucial role in cell adhesion, proliferation, and differentiation. Nanostructured surfaces can enhance these properties, but they may also influence protein adsorption and bacterial adhesion. Careful optimization of surface features is essential to promote desirable cellular responses while minimizing the risk of infection.
Degradation kinetics and by-products are critical factors in assessing the long-term safety of these nanocomposites. Ideally, the material should degrade at a rate that matches new bone formation, releasing non-toxic components that can be metabolized or excreted by the body. Uncontrolled degradation or the release of harmful substances could lead to inflammation, impaired healing, or systemic toxicity.
Potential immunogenicity must be thoroughly evaluated, as nanoparticles can sometimes trigger unexpected immune responses. This includes assessing the risk of complement activation, cytokine production, and the formation of antibodies against the material. In vitro and in vivo studies are essential to characterize the immune response to these nanocomposites.
The possibility of nanoparticle translocation and accumulation in distant organs should be investigated. While hydroxyapatite is generally considered safe, the unique properties of nanostructured materials may alter their biodistribution and clearance pathways. Long-term studies are necessary to rule out any chronic toxicity or carcinogenic potential.
Standardized testing protocols, such as those outlined by ISO 10993, provide a framework for evaluating the biocompatibility of medical devices. These include tests for cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity. Additional specialized tests may be required to address specific concerns related to nanocomposites.
Regulatory considerations must be addressed early in the development process. Different regulatory bodies may have varying requirements for demonstrating the safety of nanostructured materials. Engaging with regulatory agencies and following good laboratory practices (GLP) and good manufacturing practices (GMP) are essential for successful translation to clinical use.
The biocompatibility of layered hydroxyapatite nanocomposites is primarily influenced by their chemical composition, surface properties, and degradation behavior. The chemical similarity of hydroxyapatite to natural bone mineral contributes to its inherent biocompatibility. However, the introduction of additional layers and nanostructures may alter the material's interaction with biological systems.
Surface characteristics, including topography, charge, and wettability, play a crucial role in cell adhesion, proliferation, and differentiation. Nanostructured surfaces can enhance these properties, but they may also influence protein adsorption and bacterial adhesion. Careful optimization of surface features is essential to promote desirable cellular responses while minimizing the risk of infection.
Degradation kinetics and by-products are critical factors in assessing the long-term safety of these nanocomposites. Ideally, the material should degrade at a rate that matches new bone formation, releasing non-toxic components that can be metabolized or excreted by the body. Uncontrolled degradation or the release of harmful substances could lead to inflammation, impaired healing, or systemic toxicity.
Potential immunogenicity must be thoroughly evaluated, as nanoparticles can sometimes trigger unexpected immune responses. This includes assessing the risk of complement activation, cytokine production, and the formation of antibodies against the material. In vitro and in vivo studies are essential to characterize the immune response to these nanocomposites.
The possibility of nanoparticle translocation and accumulation in distant organs should be investigated. While hydroxyapatite is generally considered safe, the unique properties of nanostructured materials may alter their biodistribution and clearance pathways. Long-term studies are necessary to rule out any chronic toxicity or carcinogenic potential.
Standardized testing protocols, such as those outlined by ISO 10993, provide a framework for evaluating the biocompatibility of medical devices. These include tests for cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity. Additional specialized tests may be required to address specific concerns related to nanocomposites.
Regulatory considerations must be addressed early in the development process. Different regulatory bodies may have varying requirements for demonstrating the safety of nanostructured materials. Engaging with regulatory agencies and following good laboratory practices (GLP) and good manufacturing practices (GMP) are essential for successful translation to clinical use.
Regulatory Pathway for Novel Bone Graft Materials
The regulatory pathway for novel bone graft materials, such as layered hydroxyapatite nanocomposites, is a complex process that requires careful navigation to ensure compliance with regulatory standards and successful market entry. This pathway typically involves several key stages and considerations.
Initially, developers must determine the appropriate regulatory classification for their bone graft material. In the United States, the Food and Drug Administration (FDA) classifies most bone graft substitutes as Class II medical devices, which require a 510(k) premarket notification. However, some innovative materials may be classified as Class III devices, necessitating a more rigorous premarket approval (PMA) process.
The next crucial step is to design and conduct preclinical studies that demonstrate the safety and efficacy of the layered hydroxyapatite nanocomposite. These studies should evaluate biocompatibility, osteoconductivity, and potential toxicity. In vitro tests may include cell culture assays to assess cytotoxicity and cell proliferation, while in vivo studies often involve animal models to evaluate bone formation and integration.
Following successful preclinical trials, developers must prepare and submit the appropriate regulatory application. For a 510(k) submission, this involves demonstrating substantial equivalence to a predicate device already on the market. The application should include detailed information on the material's composition, manufacturing process, and performance data from preclinical studies.
Once the application is submitted, the regulatory agency will review the data and may request additional information or clarification. This review process can take several months, and developers should be prepared to respond promptly to any queries to avoid delays.
If the regulatory agency determines that clinical trials are necessary, developers must design and conduct these studies in accordance with Good Clinical Practice (GCP) guidelines. Clinical trials for bone graft materials typically assess safety, efficacy, and performance in human subjects, often comparing the novel material to standard-of-care treatments.
Throughout the regulatory process, it is essential to maintain open communication with the regulatory agency. Pre-submission meetings can be valuable for clarifying expectations and addressing potential concerns early in the development process.
Finally, upon receiving regulatory approval, developers must implement post-market surveillance programs to monitor the long-term safety and performance of their bone graft material in real-world clinical settings. This ongoing vigilance is crucial for maintaining regulatory compliance and ensuring patient safety.
Initially, developers must determine the appropriate regulatory classification for their bone graft material. In the United States, the Food and Drug Administration (FDA) classifies most bone graft substitutes as Class II medical devices, which require a 510(k) premarket notification. However, some innovative materials may be classified as Class III devices, necessitating a more rigorous premarket approval (PMA) process.
The next crucial step is to design and conduct preclinical studies that demonstrate the safety and efficacy of the layered hydroxyapatite nanocomposite. These studies should evaluate biocompatibility, osteoconductivity, and potential toxicity. In vitro tests may include cell culture assays to assess cytotoxicity and cell proliferation, while in vivo studies often involve animal models to evaluate bone formation and integration.
Following successful preclinical trials, developers must prepare and submit the appropriate regulatory application. For a 510(k) submission, this involves demonstrating substantial equivalence to a predicate device already on the market. The application should include detailed information on the material's composition, manufacturing process, and performance data from preclinical studies.
Once the application is submitted, the regulatory agency will review the data and may request additional information or clarification. This review process can take several months, and developers should be prepared to respond promptly to any queries to avoid delays.
If the regulatory agency determines that clinical trials are necessary, developers must design and conduct these studies in accordance with Good Clinical Practice (GCP) guidelines. Clinical trials for bone graft materials typically assess safety, efficacy, and performance in human subjects, often comparing the novel material to standard-of-care treatments.
Throughout the regulatory process, it is essential to maintain open communication with the regulatory agency. Pre-submission meetings can be valuable for clarifying expectations and addressing potential concerns early in the development process.
Finally, upon receiving regulatory approval, developers must implement post-market surveillance programs to monitor the long-term safety and performance of their bone graft material in real-world clinical settings. This ongoing vigilance is crucial for maintaining regulatory compliance and ensuring patient safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!