The Role of Hydroxyapatite Nanodots in Nanomedicine for Molecular Imaging
JUL 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Nanodots: Background and Objectives
Hydroxyapatite nanodots have emerged as a promising tool in the field of nanomedicine, particularly for molecular imaging applications. These nanoparticles, composed of calcium phosphate minerals, have garnered significant attention due to their unique properties and potential to revolutionize diagnostic and therapeutic approaches in healthcare.
The development of hydroxyapatite nanodots can be traced back to the early 2000s when researchers began exploring the potential of nanostructured materials for biomedical applications. The natural occurrence of hydroxyapatite in human bones and teeth made it an attractive candidate for biocompatible nanomaterials. Over the past two decades, advancements in synthesis techniques and characterization methods have led to the creation of highly controlled and functionalized hydroxyapatite nanodots.
The primary objective of utilizing hydroxyapatite nanodots in molecular imaging is to enhance the visualization and tracking of biological processes at the cellular and molecular levels. These nanoparticles offer several advantages over conventional imaging agents, including improved biocompatibility, high stability, and the ability to be easily functionalized with various targeting moieties and imaging probes.
One of the key goals in the development of hydroxyapatite nanodots for molecular imaging is to achieve high sensitivity and specificity in detecting and monitoring disease progression. Researchers aim to create nanodots that can selectively accumulate in target tissues or cells, providing clear and accurate imaging results. This targeted approach has the potential to significantly improve early disease detection and treatment monitoring.
Another important objective is to develop multimodal imaging capabilities using hydroxyapatite nanodots. By incorporating different imaging agents or combining various imaging modalities, researchers seek to obtain complementary information about biological processes, enhancing the overall diagnostic accuracy and providing a more comprehensive understanding of disease mechanisms.
The integration of therapeutic agents with hydroxyapatite nanodots is also a key focus area. This approach, known as theranostics, aims to combine diagnostic imaging with targeted drug delivery, offering a powerful tool for personalized medicine. The ultimate goal is to create multifunctional nanoplatforms that can simultaneously diagnose, monitor, and treat diseases at the molecular level.
As research in this field progresses, there is a growing emphasis on optimizing the physicochemical properties of hydroxyapatite nanodots to enhance their performance in vivo. This includes improving their colloidal stability, controlling their size and shape distribution, and modifying their surface chemistry to increase circulation time and reduce non-specific interactions.
In conclusion, the background and objectives of hydroxyapatite nanodots in molecular imaging reflect a rapidly evolving field with immense potential to transform healthcare. By leveraging the unique properties of these nanoparticles, researchers aim to develop advanced imaging tools that can provide unprecedented insights into biological processes and pave the way for more effective diagnostic and therapeutic strategies.
The development of hydroxyapatite nanodots can be traced back to the early 2000s when researchers began exploring the potential of nanostructured materials for biomedical applications. The natural occurrence of hydroxyapatite in human bones and teeth made it an attractive candidate for biocompatible nanomaterials. Over the past two decades, advancements in synthesis techniques and characterization methods have led to the creation of highly controlled and functionalized hydroxyapatite nanodots.
The primary objective of utilizing hydroxyapatite nanodots in molecular imaging is to enhance the visualization and tracking of biological processes at the cellular and molecular levels. These nanoparticles offer several advantages over conventional imaging agents, including improved biocompatibility, high stability, and the ability to be easily functionalized with various targeting moieties and imaging probes.
One of the key goals in the development of hydroxyapatite nanodots for molecular imaging is to achieve high sensitivity and specificity in detecting and monitoring disease progression. Researchers aim to create nanodots that can selectively accumulate in target tissues or cells, providing clear and accurate imaging results. This targeted approach has the potential to significantly improve early disease detection and treatment monitoring.
Another important objective is to develop multimodal imaging capabilities using hydroxyapatite nanodots. By incorporating different imaging agents or combining various imaging modalities, researchers seek to obtain complementary information about biological processes, enhancing the overall diagnostic accuracy and providing a more comprehensive understanding of disease mechanisms.
The integration of therapeutic agents with hydroxyapatite nanodots is also a key focus area. This approach, known as theranostics, aims to combine diagnostic imaging with targeted drug delivery, offering a powerful tool for personalized medicine. The ultimate goal is to create multifunctional nanoplatforms that can simultaneously diagnose, monitor, and treat diseases at the molecular level.
As research in this field progresses, there is a growing emphasis on optimizing the physicochemical properties of hydroxyapatite nanodots to enhance their performance in vivo. This includes improving their colloidal stability, controlling their size and shape distribution, and modifying their surface chemistry to increase circulation time and reduce non-specific interactions.
In conclusion, the background and objectives of hydroxyapatite nanodots in molecular imaging reflect a rapidly evolving field with immense potential to transform healthcare. By leveraging the unique properties of these nanoparticles, researchers aim to develop advanced imaging tools that can provide unprecedented insights into biological processes and pave the way for more effective diagnostic and therapeutic strategies.
Market Analysis for Molecular Imaging Nanomaterials
The molecular imaging nanomaterials market is experiencing significant growth, driven by the increasing demand for early and accurate disease diagnosis, particularly in oncology and neurology. Hydroxyapatite nanodots, as a promising nanomaterial for molecular imaging, are poised to play a crucial role in this expanding market.
The global molecular imaging market was valued at approximately $4.2 billion in 2020 and is projected to reach $6.8 billion by 2025, growing at a CAGR of 10.2%. Within this market, nanomaterials for molecular imaging are expected to witness even higher growth rates due to their superior imaging capabilities and potential for theranostic applications.
Hydroxyapatite nanodots, with their unique properties such as biocompatibility, biodegradability, and high affinity for various biomolecules, are attracting significant attention in the nanomedicine field. These nanomaterials offer advantages in terms of enhanced contrast, improved targeting efficiency, and reduced toxicity compared to conventional imaging agents.
The market for hydroxyapatite nanodots in molecular imaging is primarily driven by their applications in cancer diagnosis and treatment monitoring. Oncology remains the largest application segment for molecular imaging, accounting for over 40% of the market share. The rising incidence of cancer worldwide and the need for precise imaging techniques are expected to fuel the demand for advanced nanomaterials like hydroxyapatite nanodots.
Geographically, North America dominates the molecular imaging nanomaterials market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced diagnostic techniques.
Key market players in the molecular imaging nanomaterials sector include Nanoprobes, Nanospectra Biosciences, and Nanosys. These companies are actively investing in research and development to enhance the performance of nanomaterials for molecular imaging applications. Collaborations between academic institutions and industry players are also accelerating the development and commercialization of novel nanomaterials like hydroxyapatite nanodots.
Despite the promising outlook, challenges such as regulatory hurdles, high development costs, and concerns regarding long-term safety of nanomaterials need to be addressed to fully realize the market potential of hydroxyapatite nanodots in molecular imaging. Nevertheless, the growing focus on personalized medicine and the continuous advancements in nanotechnology are expected to drive the adoption of these innovative nanomaterials in clinical practice, thereby expanding the market opportunities in the coming years.
The global molecular imaging market was valued at approximately $4.2 billion in 2020 and is projected to reach $6.8 billion by 2025, growing at a CAGR of 10.2%. Within this market, nanomaterials for molecular imaging are expected to witness even higher growth rates due to their superior imaging capabilities and potential for theranostic applications.
Hydroxyapatite nanodots, with their unique properties such as biocompatibility, biodegradability, and high affinity for various biomolecules, are attracting significant attention in the nanomedicine field. These nanomaterials offer advantages in terms of enhanced contrast, improved targeting efficiency, and reduced toxicity compared to conventional imaging agents.
The market for hydroxyapatite nanodots in molecular imaging is primarily driven by their applications in cancer diagnosis and treatment monitoring. Oncology remains the largest application segment for molecular imaging, accounting for over 40% of the market share. The rising incidence of cancer worldwide and the need for precise imaging techniques are expected to fuel the demand for advanced nanomaterials like hydroxyapatite nanodots.
Geographically, North America dominates the molecular imaging nanomaterials market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced diagnostic techniques.
Key market players in the molecular imaging nanomaterials sector include Nanoprobes, Nanospectra Biosciences, and Nanosys. These companies are actively investing in research and development to enhance the performance of nanomaterials for molecular imaging applications. Collaborations between academic institutions and industry players are also accelerating the development and commercialization of novel nanomaterials like hydroxyapatite nanodots.
Despite the promising outlook, challenges such as regulatory hurdles, high development costs, and concerns regarding long-term safety of nanomaterials need to be addressed to fully realize the market potential of hydroxyapatite nanodots in molecular imaging. Nevertheless, the growing focus on personalized medicine and the continuous advancements in nanotechnology are expected to drive the adoption of these innovative nanomaterials in clinical practice, thereby expanding the market opportunities in the coming years.
Current Challenges in Nanomedicine Imaging
Nanomedicine imaging faces several significant challenges that hinder its widespread adoption and effectiveness in clinical applications. One of the primary obstacles is the limited sensitivity and specificity of current imaging modalities when applied to nanoscale structures. Traditional imaging techniques often struggle to detect and accurately visualize nanoparticles or molecular interactions at the nanoscale level, leading to potential misinterpretations or missed diagnostic opportunities.
Another critical challenge is the development of biocompatible and stable contrast agents that can effectively enhance imaging quality without causing adverse effects in the body. Many existing contrast agents suffer from rapid clearance, poor targeting efficiency, or potential toxicity, limiting their utility in long-term or repeated imaging studies. The design of nanoparticles that can maintain their integrity and functionality in complex biological environments while providing strong and persistent imaging signals remains a significant hurdle.
The issue of targeted delivery and accumulation of imaging agents at specific sites of interest presents another major challenge. Despite advances in nanoparticle engineering, achieving precise and selective localization of imaging agents to diseased tissues or organs while minimizing off-target accumulation continues to be a complex task. This challenge is particularly pronounced in the context of heterogeneous diseases like cancer, where the dynamic nature of the tumor microenvironment can impede effective targeting.
Furthermore, the translation of promising nanomedicine imaging techniques from laboratory settings to clinical practice faces numerous regulatory and scalability challenges. Ensuring consistent quality, reproducibility, and safety of nanoparticle-based imaging agents at industrial scales is a complex process that requires significant investment and rigorous testing. The regulatory landscape for nanomedicine products is still evolving, creating uncertainty and potential delays in the approval process for new imaging technologies.
Lastly, the integration of multimodal imaging capabilities into a single nanoplatform represents a frontier challenge in the field. While combining different imaging modalities (e.g., optical, magnetic resonance, and nuclear imaging) can provide complementary information and enhance diagnostic accuracy, it also introduces complexities in nanoparticle design, data acquisition, and image processing. Developing nanoparticles that can effectively serve as contrast agents for multiple imaging modalities while maintaining their individual performance characteristics remains an active area of research and development.
Another critical challenge is the development of biocompatible and stable contrast agents that can effectively enhance imaging quality without causing adverse effects in the body. Many existing contrast agents suffer from rapid clearance, poor targeting efficiency, or potential toxicity, limiting their utility in long-term or repeated imaging studies. The design of nanoparticles that can maintain their integrity and functionality in complex biological environments while providing strong and persistent imaging signals remains a significant hurdle.
The issue of targeted delivery and accumulation of imaging agents at specific sites of interest presents another major challenge. Despite advances in nanoparticle engineering, achieving precise and selective localization of imaging agents to diseased tissues or organs while minimizing off-target accumulation continues to be a complex task. This challenge is particularly pronounced in the context of heterogeneous diseases like cancer, where the dynamic nature of the tumor microenvironment can impede effective targeting.
Furthermore, the translation of promising nanomedicine imaging techniques from laboratory settings to clinical practice faces numerous regulatory and scalability challenges. Ensuring consistent quality, reproducibility, and safety of nanoparticle-based imaging agents at industrial scales is a complex process that requires significant investment and rigorous testing. The regulatory landscape for nanomedicine products is still evolving, creating uncertainty and potential delays in the approval process for new imaging technologies.
Lastly, the integration of multimodal imaging capabilities into a single nanoplatform represents a frontier challenge in the field. While combining different imaging modalities (e.g., optical, magnetic resonance, and nuclear imaging) can provide complementary information and enhance diagnostic accuracy, it also introduces complexities in nanoparticle design, data acquisition, and image processing. Developing nanoparticles that can effectively serve as contrast agents for multiple imaging modalities while maintaining their individual performance characteristics remains an active area of research and development.
Existing Hydroxyapatite Nanodot Applications
01 Synthesis and preparation of hydroxyapatite nanodots
Various methods are employed to synthesize and prepare hydroxyapatite nanodots, including chemical precipitation, hydrothermal processes, and sol-gel techniques. These methods allow for precise control over the size, shape, and composition of the nanodots, enabling tailored properties for specific applications.- Synthesis and preparation of hydroxyapatite nanodots: Various methods are employed to synthesize and prepare hydroxyapatite nanodots, including chemical precipitation, hydrothermal processes, and sol-gel techniques. These methods allow for control over particle size, morphology, and composition of the nanodots, which are crucial for their specific applications in biomedicine and materials science.
- Applications in biomedical engineering: Hydroxyapatite nanodots find extensive use in biomedical engineering, particularly in bone tissue engineering, drug delivery systems, and dental applications. Their biocompatibility, osteoconductivity, and ability to mimic natural bone mineral make them ideal for bone regeneration and implant coatings.
- Integration in nanoelectronic devices: Hydroxyapatite nanodots are being explored for use in nanoelectronic devices, particularly in memory applications. Their unique properties allow for potential improvements in data storage capacity and efficiency in electronic components.
- Surface modification and functionalization: Techniques for surface modification and functionalization of hydroxyapatite nanodots are developed to enhance their properties and expand their applications. These modifications can improve dispersion, bioactivity, and allow for targeted drug delivery or specific interactions with biological systems.
- Environmental and industrial applications: Hydroxyapatite nanodots are being investigated for environmental remediation and industrial processes. Their high surface area and adsorption properties make them potential candidates for water purification, heavy metal removal, and catalytic applications in various industrial settings.
02 Applications in biomedical engineering
Hydroxyapatite nanodots find extensive use in biomedical engineering, particularly in bone tissue engineering, drug delivery systems, and dental applications. Their biocompatibility and similarity to natural bone mineral make them ideal for enhancing bone regeneration and integration of implants.Expand Specific Solutions03 Use in electronic and optoelectronic devices
Hydroxyapatite nanodots are utilized in the fabrication of electronic and optoelectronic devices, such as memory devices, sensors, and light-emitting diodes. Their unique properties, including high surface area and quantum confinement effects, contribute to enhanced device performance.Expand Specific Solutions04 Surface modification and functionalization
Various techniques are employed to modify and functionalize the surface of hydroxyapatite nanodots, enhancing their properties and expanding their applications. These modifications can improve dispersion, bioactivity, and compatibility with different matrices or biological systems.Expand Specific Solutions05 Environmental and industrial applications
Hydroxyapatite nanodots are explored for environmental remediation and industrial processes, such as water treatment, catalysis, and adsorption of pollutants. Their high surface area and reactive properties make them effective in removing contaminants and catalyzing chemical reactions.Expand Specific Solutions
Key Players in Nanomedicine Imaging Industry
The field of hydroxyapatite nanodots in nanomedicine for molecular imaging is in its early developmental stage, with significant potential for growth. The market size is expanding as research interest increases, driven by the unique properties of these nanomaterials for biomedical applications. While the technology is still maturing, several key players are advancing its development. Academic institutions like Massachusetts Institute of Technology, Cornell University, and Zhejiang University are conducting fundamental research, while companies such as 3M Innovative Properties Co. and research organizations like Mayo Foundation for Medical Education & Research are exploring practical applications. The involvement of diverse entities indicates growing recognition of the technology's promise in molecular imaging and nanomedicine.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced hydroxyapatite nanodots for molecular imaging in nanomedicine. Their approach involves synthesizing ultra-small hydroxyapatite nanoparticles (less than 5 nm in diameter) with precise control over size and composition[1]. These nanodots are functionalized with targeting ligands and imaging agents, allowing for multimodal imaging capabilities. MIT's technology enables simultaneous fluorescence and magnetic resonance imaging, providing high-resolution visualization of biological processes at the molecular level[2]. The nanodots are designed to have excellent biocompatibility and biodegradability, addressing safety concerns in clinical applications. MIT researchers have also demonstrated the potential of these nanodots for drug delivery, combining imaging and therapeutic functions in a single nanoplatform[3].
Strengths: Cutting-edge synthesis techniques, multimodal imaging capabilities, and potential for theranostic applications. Weaknesses: Potential challenges in scaling up production and regulatory hurdles for clinical translation.
Memorial Sloan Kettering Cancer Center
Technical Solution: Memorial Sloan Kettering Cancer Center has pioneered the use of hydroxyapatite nanodots for cancer-specific molecular imaging. Their approach focuses on developing targeted nanodots that can selectively accumulate in tumor tissues. The center has engineered hydroxyapatite nanodots with surface modifications to enhance tumor penetration and retention[4]. These nanodots are coupled with radionuclides for positron emission tomography (PET) imaging, allowing for highly sensitive detection of cancer cells. The center has also explored the use of these nanodots for image-guided surgery, where real-time visualization of tumor margins can improve surgical outcomes[5]. Additionally, they have investigated the potential of hydroxyapatite nanodots as carriers for chemotherapeutic agents, combining diagnostic and therapeutic functions.
Strengths: Specialized focus on cancer applications, integration with clinical practice, and potential for improving cancer diagnosis and treatment. Weaknesses: Limited application outside of oncology and potential concerns about long-term effects of nanoparticle accumulation in the body.
Core Innovations in Hydroxyapatite Nanodot Technology
The art, method, manner, process and system of multifunctional nanobiomaterial for molecular imaging and drug- delivery
PatentWO2014141287A1
Innovation
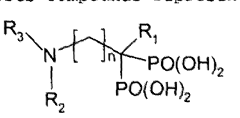
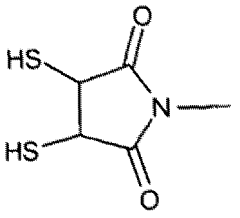
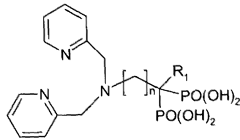
- Development of multifunctional nanobiomaterials based on doped calcium apatite nanoparticles that exhibit near-infrared fluorescence, magnetism, and X-ray absorption, allowing for simultaneous contrast enhancement across multiple imaging modalities and targeted drug delivery to specific disease sites.
Nanoparticles and their uses in molecular imaging
PatentWO2011151631A1
Innovation
- Development of nanoparticle compositions formed from inorganic ionic materials that can intrinsically bind to imaging probes, allowing for targeted delivery and amplification of imaging agents, enabling simpler radiolabelling and reduced toxicity.
Regulatory Framework for Nanomedicine
The regulatory framework for nanomedicine, particularly in the context of hydroxyapatite nanodots for molecular imaging, is a complex and evolving landscape. As nanotechnology continues to advance in medical applications, regulatory bodies worldwide are adapting their approaches to ensure safety and efficacy while fostering innovation.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing guidelines for nanomedicine. The FDA's approach is product-specific, considering the unique properties of nanomaterials and their intended use. For hydroxyapatite nanodots, which fall under the category of nanoparticles used in imaging, the FDA's Center for Devices and Radiological Health (CDRH) would likely be the primary regulatory body involved.
The European Medicines Agency (EMA) has also been proactive in addressing the regulatory challenges of nanomedicine. The EMA has established a Nanomedicines Expert Group to provide specialized guidance on the development and evaluation of nanomedicine products. This group has been instrumental in developing reflection papers and guidelines specific to nanoparticle-based imaging agents.
In Asia, countries like Japan and China have been developing their own regulatory frameworks for nanomedicine. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has issued guidelines for the evaluation of liposomes and other nanoparticle-based drug delivery systems, which may have implications for imaging applications using hydroxyapatite nanodots.
Internationally, the Organization for Economic Co-operation and Development (OECD) has been working on harmonizing regulatory approaches to nanomaterials across different countries. Their efforts include developing standardized test guidelines and risk assessment methodologies that could be applied to nanomedicine products like hydroxyapatite nanodots.
One of the key challenges in regulating nanomedicine is the need for standardized characterization methods. Regulatory bodies are increasingly requiring detailed physicochemical characterization of nanoparticles, including size distribution, surface properties, and stability. For hydroxyapatite nanodots, this may involve specialized imaging techniques and analytical methods to ensure consistent quality and performance.
Safety assessment is another critical aspect of the regulatory framework. Given the unique properties of nanomaterials, traditional toxicology testing methods may not always be sufficient. Regulatory agencies are calling for more comprehensive in vitro and in vivo studies to evaluate the potential long-term effects of nanoparticles in the body, including their biodistribution, clearance, and potential for accumulation.
As the field of nanomedicine continues to evolve, regulatory frameworks are likely to become more refined and specific. There is a growing recognition of the need for a balanced approach that ensures patient safety while also facilitating the development of innovative nanomedicine technologies. For researchers and developers working with hydroxyapatite nanodots in molecular imaging, staying abreast of these regulatory developments and engaging early with regulatory bodies will be crucial for successful translation of their technologies to clinical applications.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing guidelines for nanomedicine. The FDA's approach is product-specific, considering the unique properties of nanomaterials and their intended use. For hydroxyapatite nanodots, which fall under the category of nanoparticles used in imaging, the FDA's Center for Devices and Radiological Health (CDRH) would likely be the primary regulatory body involved.
The European Medicines Agency (EMA) has also been proactive in addressing the regulatory challenges of nanomedicine. The EMA has established a Nanomedicines Expert Group to provide specialized guidance on the development and evaluation of nanomedicine products. This group has been instrumental in developing reflection papers and guidelines specific to nanoparticle-based imaging agents.
In Asia, countries like Japan and China have been developing their own regulatory frameworks for nanomedicine. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has issued guidelines for the evaluation of liposomes and other nanoparticle-based drug delivery systems, which may have implications for imaging applications using hydroxyapatite nanodots.
Internationally, the Organization for Economic Co-operation and Development (OECD) has been working on harmonizing regulatory approaches to nanomaterials across different countries. Their efforts include developing standardized test guidelines and risk assessment methodologies that could be applied to nanomedicine products like hydroxyapatite nanodots.
One of the key challenges in regulating nanomedicine is the need for standardized characterization methods. Regulatory bodies are increasingly requiring detailed physicochemical characterization of nanoparticles, including size distribution, surface properties, and stability. For hydroxyapatite nanodots, this may involve specialized imaging techniques and analytical methods to ensure consistent quality and performance.
Safety assessment is another critical aspect of the regulatory framework. Given the unique properties of nanomaterials, traditional toxicology testing methods may not always be sufficient. Regulatory agencies are calling for more comprehensive in vitro and in vivo studies to evaluate the potential long-term effects of nanoparticles in the body, including their biodistribution, clearance, and potential for accumulation.
As the field of nanomedicine continues to evolve, regulatory frameworks are likely to become more refined and specific. There is a growing recognition of the need for a balanced approach that ensures patient safety while also facilitating the development of innovative nanomedicine technologies. For researchers and developers working with hydroxyapatite nanodots in molecular imaging, staying abreast of these regulatory developments and engaging early with regulatory bodies will be crucial for successful translation of their technologies to clinical applications.
Biocompatibility and Safety Considerations
The biocompatibility and safety of hydroxyapatite nanodots (HAp-NDs) are crucial considerations for their application in nanomedicine and molecular imaging. HAp-NDs have shown promising potential due to their inherent biocompatibility, as hydroxyapatite is a naturally occurring mineral in human bones and teeth. This familiarity with the human body contributes to their reduced likelihood of triggering adverse immune responses.
In vitro studies have demonstrated that HAp-NDs exhibit low cytotoxicity across various cell lines, including both healthy and cancerous cells. The nanoparticles' size, typically ranging from 20 to 100 nm, allows for efficient cellular uptake without causing significant disruption to cellular functions. However, the exact mechanisms of cellular internalization and intracellular trafficking of HAp-NDs require further investigation to fully understand their long-term effects on cellular processes.
In vivo studies have provided encouraging results regarding the systemic toxicity of HAp-NDs. When administered intravenously, these nanoparticles show minimal accumulation in vital organs such as the liver, spleen, and kidneys. The body's ability to metabolize and excrete HAp-NDs through natural pathways contributes to their favorable safety profile. Nevertheless, long-term studies are necessary to evaluate any potential chronic effects of repeated exposure.
The surface chemistry of HAp-NDs plays a significant role in their biocompatibility and safety. Modifications to the nanoparticle surface, such as PEGylation or the addition of targeting ligands, can influence their interaction with biological systems. These modifications may alter the nanoparticles' biodistribution, cellular uptake, and clearance rates, potentially impacting their overall safety profile.
One of the key safety considerations for HAp-NDs in molecular imaging applications is their potential to generate reactive oxygen species (ROS). While HAp-NDs generally exhibit low ROS production compared to other inorganic nanoparticles, this aspect requires careful monitoring, especially in long-term or repeated exposure scenarios. Strategies to mitigate ROS generation, such as incorporating antioxidant coatings, are being explored to enhance the safety of HAp-NDs in clinical applications.
The biocompatibility of HAp-NDs extends to their potential for biodegradation. Unlike some other inorganic nanoparticles, HAp-NDs can be broken down by the body's natural processes, reducing the risk of long-term accumulation. However, the rate and extent of biodegradation may vary depending on the specific formulation and surface modifications of the nanoparticles, necessitating thorough characterization for each application.
In conclusion, while initial studies indicate a favorable safety profile for HAp-NDs in nanomedicine and molecular imaging, continued research is essential to fully elucidate their long-term effects and optimize their biocompatibility. Standardized protocols for safety assessment and regulatory guidelines specific to HAp-NDs will be crucial for their successful translation from bench to bedside.
In vitro studies have demonstrated that HAp-NDs exhibit low cytotoxicity across various cell lines, including both healthy and cancerous cells. The nanoparticles' size, typically ranging from 20 to 100 nm, allows for efficient cellular uptake without causing significant disruption to cellular functions. However, the exact mechanisms of cellular internalization and intracellular trafficking of HAp-NDs require further investigation to fully understand their long-term effects on cellular processes.
In vivo studies have provided encouraging results regarding the systemic toxicity of HAp-NDs. When administered intravenously, these nanoparticles show minimal accumulation in vital organs such as the liver, spleen, and kidneys. The body's ability to metabolize and excrete HAp-NDs through natural pathways contributes to their favorable safety profile. Nevertheless, long-term studies are necessary to evaluate any potential chronic effects of repeated exposure.
The surface chemistry of HAp-NDs plays a significant role in their biocompatibility and safety. Modifications to the nanoparticle surface, such as PEGylation or the addition of targeting ligands, can influence their interaction with biological systems. These modifications may alter the nanoparticles' biodistribution, cellular uptake, and clearance rates, potentially impacting their overall safety profile.
One of the key safety considerations for HAp-NDs in molecular imaging applications is their potential to generate reactive oxygen species (ROS). While HAp-NDs generally exhibit low ROS production compared to other inorganic nanoparticles, this aspect requires careful monitoring, especially in long-term or repeated exposure scenarios. Strategies to mitigate ROS generation, such as incorporating antioxidant coatings, are being explored to enhance the safety of HAp-NDs in clinical applications.
The biocompatibility of HAp-NDs extends to their potential for biodegradation. Unlike some other inorganic nanoparticles, HAp-NDs can be broken down by the body's natural processes, reducing the risk of long-term accumulation. However, the rate and extent of biodegradation may vary depending on the specific formulation and surface modifications of the nanoparticles, necessitating thorough characterization for each application.
In conclusion, while initial studies indicate a favorable safety profile for HAp-NDs in nanomedicine and molecular imaging, continued research is essential to fully elucidate their long-term effects and optimize their biocompatibility. Standardized protocols for safety assessment and regulatory guidelines specific to HAp-NDs will be crucial for their successful translation from bench to bedside.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!