Implementing Hydroxyapatite Fibers in Biofilm Adhesion Prevention
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Fibers: Background and Objectives
Hydroxyapatite (HA) fibers have emerged as a promising material in the field of biomedical engineering, particularly in the context of biofilm adhesion prevention. The development of these fibers represents a significant advancement in addressing the persistent challenge of bacterial colonization on medical devices and implants.
The journey of hydroxyapatite in biomedical applications began with its discovery as the primary inorganic component of bone and teeth. Its biocompatibility and osteoconductive properties quickly garnered attention in the scientific community. However, it was not until recent years that researchers began exploring the potential of HA in fiber form for combating biofilm formation.
The evolution of HA fiber technology has been driven by the increasing prevalence of healthcare-associated infections and the growing resistance of bacteria to traditional antibiotics. This has created an urgent need for innovative approaches to prevent bacterial adhesion and subsequent biofilm formation on medical surfaces.
The primary objective of implementing hydroxyapatite fibers in biofilm adhesion prevention is to create a hostile environment for bacterial attachment while maintaining biocompatibility with host tissues. This dual functionality sets HA fibers apart from conventional antimicrobial coatings, which often rely on the release of toxic substances to inhibit bacterial growth.
Recent technological advancements have enabled the production of HA fibers with tailored properties, including controlled porosity, surface area, and aspect ratio. These characteristics play a crucial role in determining the fiber's effectiveness in preventing bacterial adhesion. Researchers are now focusing on optimizing these parameters to enhance the anti-biofilm properties of HA fibers without compromising their biocompatibility.
The potential applications of HA fibers in biofilm prevention span a wide range of medical devices, including orthopedic implants, dental materials, and wound dressings. The versatility of HA fibers allows for their integration into various substrate materials, opening up new possibilities for creating infection-resistant surfaces in diverse healthcare settings.
As the field progresses, the development of HA fibers is expected to intersect with other emerging technologies, such as nanotechnology and smart materials. This convergence may lead to the creation of advanced HA-based composites with enhanced anti-biofilm properties and the ability to respond dynamically to the presence of bacteria.
In conclusion, the implementation of hydroxyapatite fibers in biofilm adhesion prevention represents a significant technological leap in the ongoing battle against healthcare-associated infections. As research in this area continues to evolve, HA fibers hold the promise of revolutionizing the design of medical devices and implants, ultimately improving patient outcomes and reducing the economic burden of biofilm-related complications in healthcare systems worldwide.
The journey of hydroxyapatite in biomedical applications began with its discovery as the primary inorganic component of bone and teeth. Its biocompatibility and osteoconductive properties quickly garnered attention in the scientific community. However, it was not until recent years that researchers began exploring the potential of HA in fiber form for combating biofilm formation.
The evolution of HA fiber technology has been driven by the increasing prevalence of healthcare-associated infections and the growing resistance of bacteria to traditional antibiotics. This has created an urgent need for innovative approaches to prevent bacterial adhesion and subsequent biofilm formation on medical surfaces.
The primary objective of implementing hydroxyapatite fibers in biofilm adhesion prevention is to create a hostile environment for bacterial attachment while maintaining biocompatibility with host tissues. This dual functionality sets HA fibers apart from conventional antimicrobial coatings, which often rely on the release of toxic substances to inhibit bacterial growth.
Recent technological advancements have enabled the production of HA fibers with tailored properties, including controlled porosity, surface area, and aspect ratio. These characteristics play a crucial role in determining the fiber's effectiveness in preventing bacterial adhesion. Researchers are now focusing on optimizing these parameters to enhance the anti-biofilm properties of HA fibers without compromising their biocompatibility.
The potential applications of HA fibers in biofilm prevention span a wide range of medical devices, including orthopedic implants, dental materials, and wound dressings. The versatility of HA fibers allows for their integration into various substrate materials, opening up new possibilities for creating infection-resistant surfaces in diverse healthcare settings.
As the field progresses, the development of HA fibers is expected to intersect with other emerging technologies, such as nanotechnology and smart materials. This convergence may lead to the creation of advanced HA-based composites with enhanced anti-biofilm properties and the ability to respond dynamically to the presence of bacteria.
In conclusion, the implementation of hydroxyapatite fibers in biofilm adhesion prevention represents a significant technological leap in the ongoing battle against healthcare-associated infections. As research in this area continues to evolve, HA fibers hold the promise of revolutionizing the design of medical devices and implants, ultimately improving patient outcomes and reducing the economic burden of biofilm-related complications in healthcare systems worldwide.
Market Analysis for Biofilm Prevention Solutions
The global market for biofilm prevention solutions has been experiencing significant growth, driven by increasing awareness of the detrimental effects of biofilms in various industries. The healthcare sector, in particular, has been a major contributor to this growth, with hospitals and medical device manufacturers seeking innovative solutions to combat biofilm-related infections. The implementation of hydroxyapatite fibers in biofilm adhesion prevention represents a promising avenue within this market.
The biofilm prevention market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by the rising incidence of healthcare-associated infections (HAIs) and the increasing adoption of advanced materials in medical devices and implants. The dental industry has also emerged as a key market segment, with a growing demand for biofilm prevention solutions in oral care products and dental implants.
Geographically, North America and Europe currently dominate the biofilm prevention market, owing to their advanced healthcare infrastructure and stringent regulations regarding infection control. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing healthcare expenditure, and a growing awareness of biofilm-related issues in emerging economies such as China and India.
The market for hydroxyapatite-based biofilm prevention solutions is still in its nascent stage but shows considerable potential. Hydroxyapatite, being biocompatible and possessing unique properties that can inhibit bacterial adhesion, is gaining traction in various applications. The dental sector has been quick to adopt hydroxyapatite-based products, particularly in toothpaste and mouthwash formulations. The orthopedic implant market is another area where hydroxyapatite fibers are expected to make significant inroads, given their ability to promote osseointegration while preventing biofilm formation.
Key market players in the biofilm prevention sector include multinational corporations with diverse product portfolios, as well as specialized biotechnology firms focusing on novel antimicrobial technologies. These companies are investing heavily in research and development to create innovative solutions, with a growing interest in nanotechnology and biomimetic approaches. The integration of hydroxyapatite fibers into existing product lines represents a significant opportunity for these companies to differentiate themselves in a competitive market.
Challenges in the market include the high cost of advanced biofilm prevention technologies, regulatory hurdles in bringing new products to market, and the need for extensive clinical trials to demonstrate efficacy and safety. However, the potential benefits of effective biofilm prevention, including reduced healthcare costs and improved patient outcomes, continue to drive investment and innovation in this field.
The biofilm prevention market is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. This growth is fueled by the rising incidence of healthcare-associated infections (HAIs) and the increasing adoption of advanced materials in medical devices and implants. The dental industry has also emerged as a key market segment, with a growing demand for biofilm prevention solutions in oral care products and dental implants.
Geographically, North America and Europe currently dominate the biofilm prevention market, owing to their advanced healthcare infrastructure and stringent regulations regarding infection control. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by improving healthcare facilities, increasing healthcare expenditure, and a growing awareness of biofilm-related issues in emerging economies such as China and India.
The market for hydroxyapatite-based biofilm prevention solutions is still in its nascent stage but shows considerable potential. Hydroxyapatite, being biocompatible and possessing unique properties that can inhibit bacterial adhesion, is gaining traction in various applications. The dental sector has been quick to adopt hydroxyapatite-based products, particularly in toothpaste and mouthwash formulations. The orthopedic implant market is another area where hydroxyapatite fibers are expected to make significant inroads, given their ability to promote osseointegration while preventing biofilm formation.
Key market players in the biofilm prevention sector include multinational corporations with diverse product portfolios, as well as specialized biotechnology firms focusing on novel antimicrobial technologies. These companies are investing heavily in research and development to create innovative solutions, with a growing interest in nanotechnology and biomimetic approaches. The integration of hydroxyapatite fibers into existing product lines represents a significant opportunity for these companies to differentiate themselves in a competitive market.
Challenges in the market include the high cost of advanced biofilm prevention technologies, regulatory hurdles in bringing new products to market, and the need for extensive clinical trials to demonstrate efficacy and safety. However, the potential benefits of effective biofilm prevention, including reduced healthcare costs and improved patient outcomes, continue to drive investment and innovation in this field.
Current Challenges in Biofilm Adhesion Prevention
Biofilm adhesion prevention remains a significant challenge in various fields, including healthcare, industrial processes, and environmental management. Despite advancements in antimicrobial technologies, biofilms continue to pose substantial risks due to their resilience and adaptability. The implementation of hydroxyapatite fibers as a potential solution faces several hurdles that need to be addressed.
One of the primary challenges is the complexity of biofilm formation mechanisms. Biofilms are dynamic communities of microorganisms that adhere to surfaces and secrete extracellular polymeric substances (EPS). This EPS matrix provides protection and structural support, making biofilms highly resistant to traditional antimicrobial treatments. The intricate nature of biofilm formation processes complicates the development of effective prevention strategies using hydroxyapatite fibers.
Another significant obstacle is the diverse range of microbial species involved in biofilm formation. Different microorganisms exhibit varying adhesion properties and respond differently to prevention methods. This heterogeneity makes it challenging to design a universal solution using hydroxyapatite fibers that can effectively prevent adhesion across multiple bacterial species and environmental conditions.
The integration of hydroxyapatite fibers into existing materials and surfaces presents technical difficulties. Ensuring uniform distribution and stable attachment of the fibers without compromising the original material properties is crucial. Additionally, maintaining the long-term effectiveness of the hydroxyapatite fibers in preventing biofilm adhesion under various environmental conditions remains a significant challenge.
Biocompatibility and potential toxicity concerns also pose challenges, particularly in medical applications. While hydroxyapatite is generally considered biocompatible, the specific properties of hydroxyapatite fibers and their potential interactions with host tissues and cells need thorough investigation to ensure safety and efficacy.
Scale-up and cost-effectiveness present further obstacles in implementing hydroxyapatite fibers for biofilm prevention. Developing economically viable production methods for large-scale applications while maintaining consistent quality and performance is essential for widespread adoption of this technology.
Regulatory hurdles and the need for extensive clinical trials, especially in medical applications, add to the challenges. Demonstrating the long-term safety and efficacy of hydroxyapatite fiber-based solutions in preventing biofilm adhesion is crucial for obtaining regulatory approvals and gaining market acceptance.
Lastly, the potential for microbial adaptation and resistance development to hydroxyapatite fiber-based prevention methods cannot be overlooked. As with many antimicrobial strategies, there is a risk that microorganisms may evolve mechanisms to overcome the adhesion prevention properties of hydroxyapatite fibers over time, necessitating ongoing research and development efforts to stay ahead of potential resistance.
One of the primary challenges is the complexity of biofilm formation mechanisms. Biofilms are dynamic communities of microorganisms that adhere to surfaces and secrete extracellular polymeric substances (EPS). This EPS matrix provides protection and structural support, making biofilms highly resistant to traditional antimicrobial treatments. The intricate nature of biofilm formation processes complicates the development of effective prevention strategies using hydroxyapatite fibers.
Another significant obstacle is the diverse range of microbial species involved in biofilm formation. Different microorganisms exhibit varying adhesion properties and respond differently to prevention methods. This heterogeneity makes it challenging to design a universal solution using hydroxyapatite fibers that can effectively prevent adhesion across multiple bacterial species and environmental conditions.
The integration of hydroxyapatite fibers into existing materials and surfaces presents technical difficulties. Ensuring uniform distribution and stable attachment of the fibers without compromising the original material properties is crucial. Additionally, maintaining the long-term effectiveness of the hydroxyapatite fibers in preventing biofilm adhesion under various environmental conditions remains a significant challenge.
Biocompatibility and potential toxicity concerns also pose challenges, particularly in medical applications. While hydroxyapatite is generally considered biocompatible, the specific properties of hydroxyapatite fibers and their potential interactions with host tissues and cells need thorough investigation to ensure safety and efficacy.
Scale-up and cost-effectiveness present further obstacles in implementing hydroxyapatite fibers for biofilm prevention. Developing economically viable production methods for large-scale applications while maintaining consistent quality and performance is essential for widespread adoption of this technology.
Regulatory hurdles and the need for extensive clinical trials, especially in medical applications, add to the challenges. Demonstrating the long-term safety and efficacy of hydroxyapatite fiber-based solutions in preventing biofilm adhesion is crucial for obtaining regulatory approvals and gaining market acceptance.
Lastly, the potential for microbial adaptation and resistance development to hydroxyapatite fiber-based prevention methods cannot be overlooked. As with many antimicrobial strategies, there is a risk that microorganisms may evolve mechanisms to overcome the adhesion prevention properties of hydroxyapatite fibers over time, necessitating ongoing research and development efforts to stay ahead of potential resistance.
Existing Hydroxyapatite Fiber Implementation Methods
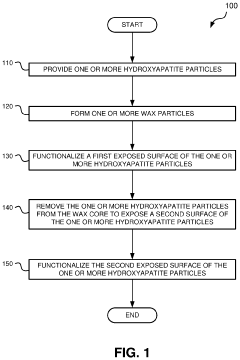
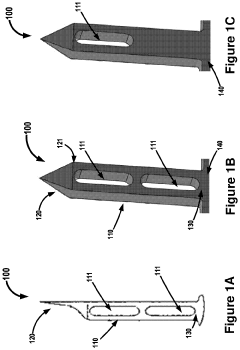
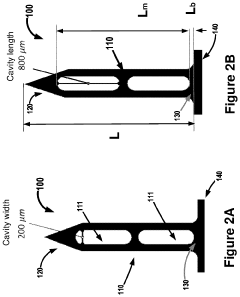
01 Hydroxyapatite fiber synthesis and properties
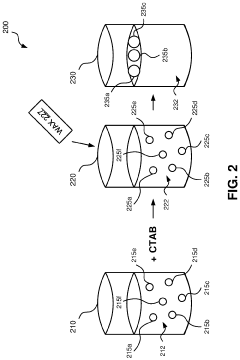
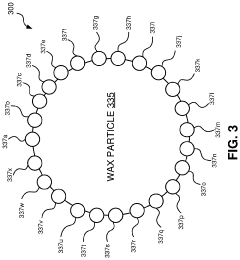
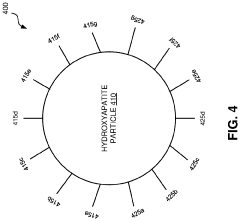
Methods for synthesizing hydroxyapatite fibers with specific properties, such as porosity and surface area, that can influence biofilm adhesion. These fibers can be engineered to have varying dimensions and structures, which affect their interaction with microorganisms and potential for biofilm formation.- Hydroxyapatite fiber synthesis and properties: Methods for synthesizing hydroxyapatite fibers with specific properties that enhance biofilm adhesion. These fibers can be engineered to have controlled porosity, surface area, and morphology, which influence their interaction with microorganisms and promote biofilm formation.
- Surface modification of hydroxyapatite fibers: Techniques for modifying the surface of hydroxyapatite fibers to improve biofilm adhesion. This may include chemical treatments, coating with bioactive molecules, or creating specific surface topographies that enhance bacterial attachment and colonization.
- Biofilm formation mechanisms on hydroxyapatite fibers: Studies on the mechanisms of biofilm formation and adhesion on hydroxyapatite fiber surfaces. This includes understanding the initial bacterial attachment, extracellular matrix production, and the development of mature biofilms on these materials.
- Applications of hydroxyapatite fiber-biofilm composites: Exploration of potential applications for hydroxyapatite fiber-biofilm composites, such as in water treatment, bioremediation, or tissue engineering. These composites leverage the synergistic properties of the hydroxyapatite substrate and the adhered biofilm.
- Characterization and analysis of hydroxyapatite fiber-biofilm interactions: Methods and techniques for characterizing and analyzing the interactions between hydroxyapatite fibers and biofilms. This includes advanced imaging techniques, spectroscopic analysis, and mechanical testing to assess the strength and nature of the adhesion.
02 Surface modification of hydroxyapatite fibers
Techniques for modifying the surface of hydroxyapatite fibers to enhance or inhibit biofilm adhesion. This can include coating the fibers with antimicrobial agents, altering surface charge, or creating specific topographies that influence bacterial attachment and biofilm formation.Expand Specific Solutions03 Biofilm formation mechanisms on hydroxyapatite fibers
Studies on the mechanisms of biofilm formation and adhesion on hydroxyapatite fiber surfaces. This includes investigating the initial attachment of bacteria, the production of extracellular polymeric substances, and the development of mature biofilms on these materials.Expand Specific Solutions04 Applications of hydroxyapatite fibers in biofilm-related fields
Utilization of hydroxyapatite fibers in various applications where biofilm adhesion is a concern or a desired feature. This can include medical implants, water treatment systems, and industrial processes where controlled biofilm growth is beneficial or detrimental.Expand Specific Solutions05 Characterization and testing of biofilm adhesion on hydroxyapatite fibers
Methods and techniques for characterizing and testing biofilm adhesion on hydroxyapatite fibers. This includes microscopy techniques, quantitative assays, and in vitro models to assess the extent and strength of biofilm formation on these materials.Expand Specific Solutions
Key Players in Hydroxyapatite and Biofilm Research
The implementation of hydroxyapatite fibers in biofilm adhesion prevention is in an emerging stage, with growing market potential due to increasing focus on healthcare-associated infections. The global market for antimicrobial coatings is expanding, driven by rising awareness of infection control. Technologically, the field is advancing rapidly, with companies like Promimic AB and Tomita Pharmaceutical Co. Ltd. leading in nano-sized hydroxyapatite development. Academic institutions such as Rutgers State University and Shandong University are contributing significant research. The technology's maturity varies, with some commercial applications already available, while more advanced formulations are still in development stages, indicating a dynamic and competitive landscape.
Promimic AB
Technical Solution: Promimic AB has developed a proprietary HAnano Surface technology for applying hydroxyapatite nanocrystals to medical implants. Their approach involves creating a nanometer-thin layer of synthetic hydroxyapatite on implant surfaces, mimicking the natural bone mineral. This technology enhances osseointegration and reduces bacterial adhesion[1]. The company has successfully applied this method to various implant materials, including titanium, stainless steel, and polymers. Their process allows for precise control of the nanocrystal size and distribution, resulting in a uniform and highly bioactive surface[2].
Strengths: Highly biocompatible, promotes rapid osseointegration, and reduces bacterial adhesion. Versatile application across different implant materials. Weaknesses: May require specialized equipment for application, potentially increasing production costs.
Rutgers State University of New Jersey
Technical Solution: Researchers at Rutgers have developed a novel approach using electrospun hydroxyapatite nanofibers for biofilm prevention. Their method involves creating a nanofiber scaffold that combines hydroxyapatite with biodegradable polymers. This composite material is designed to release antimicrobial agents in a controlled manner while promoting tissue integration. The electrospinning process allows for the creation of fibers with high surface area-to-volume ratios, enhancing their effectiveness in preventing bacterial adhesion[3]. Additionally, the team has explored incorporating silver nanoparticles into the hydroxyapatite fibers to further boost antimicrobial properties[4].
Strengths: Combines biofilm prevention with tissue integration promotion, controlled release of antimicrobial agents. Weaknesses: Complexity of manufacturing process may limit large-scale production, potential concerns about long-term effects of silver nanoparticles.
Innovative Approaches in Hydroxyapatite Fiber Design
Hydroxyapatite janus particles
PatentActiveUS20200062788A1
Innovation
- Development of hydroxyapatite Janus particles with multiple functionalities on their surface, where one side is functionalized to bind to biodegradable polymer constructs using moieties like azide or alkyne groups, and the other side is functionalized with bioactive molecules to promote bone growth and integration.
High-load microneedles and compositions for skin augmentation
PatentPendingUS20210060321A1
Innovation
- A microneedle device with an array of microneedles and a biocompatible skin augmentation composition that includes a dispersant, allowing for easy application and precise delivery of the composition into the dermis or hypodermis layer, eliminating the need for painful injections and reducing material waste.
Regulatory Framework for Biomedical Materials
The regulatory framework for biomedical materials plays a crucial role in ensuring the safety and efficacy of medical devices and implants, including those incorporating hydroxyapatite fibers for biofilm adhesion prevention. In the United States, the Food and Drug Administration (FDA) oversees the approval process for such materials through its Center for Devices and Radiological Health (CDRH). The FDA classifies medical devices into three categories based on their risk level, with Class III devices requiring the most stringent regulatory oversight.
For hydroxyapatite fiber-based materials, the regulatory pathway typically involves premarket approval (PMA) or the 510(k) clearance process, depending on the specific application and intended use. The manufacturer must demonstrate substantial equivalence to a predicate device or provide comprehensive clinical data to support the safety and effectiveness of the new material.
In the European Union, the regulatory landscape is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR introduces more stringent requirements for clinical evidence, post-market surveillance, and traceability of medical devices. Manufacturers seeking to market hydroxyapatite fiber-based materials in the EU must obtain CE marking, which involves a conformity assessment procedure conducted by a notified body.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates biomedical materials through a system similar to that of the FDA. The PMDA requires manufacturers to submit extensive documentation on the material's composition, manufacturing process, and clinical performance before granting approval.
Internationally, the International Organization for Standardization (ISO) provides guidelines for the biological evaluation of medical devices (ISO 10993 series) and specific standards for ceramic materials used in surgical implants (ISO 13175). These standards are often referenced by regulatory bodies worldwide and serve as a foundation for assessing the biocompatibility and performance of hydroxyapatite fiber-based materials.
Regulatory frameworks also address the long-term safety and performance of biomedical materials. Manufacturers are required to implement robust post-market surveillance systems to monitor the real-world performance of their products and report any adverse events or complications. This ongoing monitoring helps identify potential risks and informs future regulatory decisions.
As the field of biofilm adhesion prevention using hydroxyapatite fibers continues to evolve, regulatory bodies are likely to adapt their frameworks to address emerging challenges and technologies. This may include the development of specific guidance documents for novel biomaterials and the incorporation of advanced testing methodologies to assess their long-term behavior in the human body.
For hydroxyapatite fiber-based materials, the regulatory pathway typically involves premarket approval (PMA) or the 510(k) clearance process, depending on the specific application and intended use. The manufacturer must demonstrate substantial equivalence to a predicate device or provide comprehensive clinical data to support the safety and effectiveness of the new material.
In the European Union, the regulatory landscape is governed by the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR introduces more stringent requirements for clinical evidence, post-market surveillance, and traceability of medical devices. Manufacturers seeking to market hydroxyapatite fiber-based materials in the EU must obtain CE marking, which involves a conformity assessment procedure conducted by a notified body.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates biomedical materials through a system similar to that of the FDA. The PMDA requires manufacturers to submit extensive documentation on the material's composition, manufacturing process, and clinical performance before granting approval.
Internationally, the International Organization for Standardization (ISO) provides guidelines for the biological evaluation of medical devices (ISO 10993 series) and specific standards for ceramic materials used in surgical implants (ISO 13175). These standards are often referenced by regulatory bodies worldwide and serve as a foundation for assessing the biocompatibility and performance of hydroxyapatite fiber-based materials.
Regulatory frameworks also address the long-term safety and performance of biomedical materials. Manufacturers are required to implement robust post-market surveillance systems to monitor the real-world performance of their products and report any adverse events or complications. This ongoing monitoring helps identify potential risks and informs future regulatory decisions.
As the field of biofilm adhesion prevention using hydroxyapatite fibers continues to evolve, regulatory bodies are likely to adapt their frameworks to address emerging challenges and technologies. This may include the development of specific guidance documents for novel biomaterials and the incorporation of advanced testing methodologies to assess their long-term behavior in the human body.
Environmental Impact of Hydroxyapatite Production
The production of hydroxyapatite (HAp) for biofilm adhesion prevention applications has significant environmental implications that warrant careful consideration. The synthesis of HAp fibers typically involves chemical processes that may generate waste products and consume energy resources. Traditional methods, such as wet chemical precipitation and hydrothermal synthesis, often require high temperatures and pressures, leading to increased energy consumption and greenhouse gas emissions.
One of the primary environmental concerns is the use of chemical precursors in HAp production. These may include calcium and phosphate sources, as well as additives to control crystal growth and morphology. The disposal of unreacted chemicals and byproducts can potentially impact water quality if not properly managed. Additionally, the use of organic solvents in some synthesis routes may contribute to air pollution and pose health risks if not adequately contained and treated.
Water consumption is another critical factor in the environmental impact of HAp production. Many synthesis methods require substantial amounts of water for reactions, washing, and purification steps. This can strain local water resources, particularly in water-scarce regions. Furthermore, the wastewater generated during production may contain dissolved ions and particulates that require treatment before discharge to prevent contamination of natural water bodies.
On the positive side, recent advancements in green chemistry approaches have led to more environmentally friendly HAp synthesis methods. These include the use of bio-based precursors, such as eggshells or seashells, as calcium sources, which can reduce the reliance on mined minerals. Sol-gel techniques and microwave-assisted synthesis have also shown promise in reducing energy consumption and reaction times, thereby minimizing the overall environmental footprint of HAp production.
The lifecycle assessment of HAp fibers used in biofilm adhesion prevention should also consider the end-of-life disposal or potential for recycling. While HAp is generally considered biocompatible and biodegradable, the fate of these materials in the environment after their intended use must be evaluated. There is potential for HAp to be reabsorbed into natural calcium and phosphate cycles, but this process and its ecological impacts require further study.
In conclusion, while the production of HAp fibers for biofilm prevention offers significant medical benefits, it is crucial to balance these advantages against potential environmental costs. Ongoing research into sustainable synthesis methods, waste reduction strategies, and closed-loop production systems will be essential in minimizing the environmental impact of HAp production and ensuring its long-term viability as a biomedical material.
One of the primary environmental concerns is the use of chemical precursors in HAp production. These may include calcium and phosphate sources, as well as additives to control crystal growth and morphology. The disposal of unreacted chemicals and byproducts can potentially impact water quality if not properly managed. Additionally, the use of organic solvents in some synthesis routes may contribute to air pollution and pose health risks if not adequately contained and treated.
Water consumption is another critical factor in the environmental impact of HAp production. Many synthesis methods require substantial amounts of water for reactions, washing, and purification steps. This can strain local water resources, particularly in water-scarce regions. Furthermore, the wastewater generated during production may contain dissolved ions and particulates that require treatment before discharge to prevent contamination of natural water bodies.
On the positive side, recent advancements in green chemistry approaches have led to more environmentally friendly HAp synthesis methods. These include the use of bio-based precursors, such as eggshells or seashells, as calcium sources, which can reduce the reliance on mined minerals. Sol-gel techniques and microwave-assisted synthesis have also shown promise in reducing energy consumption and reaction times, thereby minimizing the overall environmental footprint of HAp production.
The lifecycle assessment of HAp fibers used in biofilm adhesion prevention should also consider the end-of-life disposal or potential for recycling. While HAp is generally considered biocompatible and biodegradable, the fate of these materials in the environment after their intended use must be evaluated. There is potential for HAp to be reabsorbed into natural calcium and phosphate cycles, but this process and its ecological impacts require further study.
In conclusion, while the production of HAp fibers for biofilm prevention offers significant medical benefits, it is crucial to balance these advantages against potential environmental costs. Ongoing research into sustainable synthesis methods, waste reduction strategies, and closed-loop production systems will be essential in minimizing the environmental impact of HAp production and ensuring its long-term viability as a biomedical material.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!