How Biologically Modified Hydroxyapatite Affects Epigenetic Regulation
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Modification and Epigenetic Goals
The field of biologically modified hydroxyapatite and its impact on epigenetic regulation represents a cutting-edge intersection of materials science and molecular biology. This research area aims to explore how engineered hydroxyapatite, a calcium phosphate mineral naturally found in bone and teeth, can be tailored to influence epigenetic processes within cells. The primary goal is to develop novel biomaterials that can modulate gene expression without altering the underlying DNA sequence, potentially offering new therapeutic approaches for various diseases and tissue engineering applications.
Hydroxyapatite modification techniques have evolved significantly in recent years, incorporating bioactive molecules, ions, and nanostructures to enhance its biological properties. These modifications can include the incorporation of trace elements like strontium or magnesium, surface functionalization with growth factors or peptides, and alterations in crystal structure or porosity. The epigenetic goals associated with these modifications are multifaceted, focusing on influencing key epigenetic mechanisms such as DNA methylation, histone modifications, and chromatin remodeling.
One of the primary objectives is to create hydroxyapatite-based materials that can selectively target and modulate the activity of epigenetic enzymes, such as DNA methyltransferases (DNMTs) or histone deacetylases (HDACs). By doing so, researchers aim to control gene expression patterns in specific cell types, potentially guiding cell fate decisions or reversing aberrant epigenetic states associated with disease.
Another important goal is to develop hydroxyapatite scaffolds that can create a microenvironment conducive to epigenetic reprogramming. This could involve the controlled release of epigenetic modifiers or the presentation of specific surface topographies that influence cell-material interactions and subsequent epigenetic states. Such scaffolds could have significant implications for tissue regeneration and stem cell therapies.
Researchers are also exploring the potential of biologically modified hydroxyapatite to act as a delivery system for epigenetic drugs or small interfering RNAs (siRNAs). By leveraging the biocompatibility and osteoconductivity of hydroxyapatite, these modified materials could provide targeted and sustained delivery of epigenetic modulators to specific tissues or cell populations.
The long-term vision for this field includes the development of "smart" biomaterials that can dynamically respond to cellular cues and adjust their epigenetic influence accordingly. This could lead to adaptive tissue engineering constructs capable of guiding complex regenerative processes or personalized therapeutic implants that can fine-tune gene expression based on individual patient needs.
Hydroxyapatite modification techniques have evolved significantly in recent years, incorporating bioactive molecules, ions, and nanostructures to enhance its biological properties. These modifications can include the incorporation of trace elements like strontium or magnesium, surface functionalization with growth factors or peptides, and alterations in crystal structure or porosity. The epigenetic goals associated with these modifications are multifaceted, focusing on influencing key epigenetic mechanisms such as DNA methylation, histone modifications, and chromatin remodeling.
One of the primary objectives is to create hydroxyapatite-based materials that can selectively target and modulate the activity of epigenetic enzymes, such as DNA methyltransferases (DNMTs) or histone deacetylases (HDACs). By doing so, researchers aim to control gene expression patterns in specific cell types, potentially guiding cell fate decisions or reversing aberrant epigenetic states associated with disease.
Another important goal is to develop hydroxyapatite scaffolds that can create a microenvironment conducive to epigenetic reprogramming. This could involve the controlled release of epigenetic modifiers or the presentation of specific surface topographies that influence cell-material interactions and subsequent epigenetic states. Such scaffolds could have significant implications for tissue regeneration and stem cell therapies.
Researchers are also exploring the potential of biologically modified hydroxyapatite to act as a delivery system for epigenetic drugs or small interfering RNAs (siRNAs). By leveraging the biocompatibility and osteoconductivity of hydroxyapatite, these modified materials could provide targeted and sustained delivery of epigenetic modulators to specific tissues or cell populations.
The long-term vision for this field includes the development of "smart" biomaterials that can dynamically respond to cellular cues and adjust their epigenetic influence accordingly. This could lead to adaptive tissue engineering constructs capable of guiding complex regenerative processes or personalized therapeutic implants that can fine-tune gene expression based on individual patient needs.
Market Demand for Epigenetic Therapies
The market demand for epigenetic therapies has been steadily growing in recent years, driven by the increasing understanding of epigenetic mechanisms and their role in various diseases. This emerging field offers promising opportunities for developing novel therapeutic approaches, particularly in oncology, neurodegenerative disorders, and rare genetic diseases.
In the oncology sector, epigenetic therapies have gained significant traction due to their potential to target cancer cells more specifically than traditional chemotherapy. The global market for epigenetic cancer drugs is expected to expand substantially, with several pharmaceutical companies investing heavily in research and development. This growth is fueled by the rising incidence of cancer worldwide and the need for more effective, less toxic treatment options.
Neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, represent another key area driving demand for epigenetic therapies. As the global population ages, the prevalence of these conditions is increasing, creating a pressing need for innovative treatment approaches. Epigenetic interventions show promise in addressing the complex nature of these diseases by potentially reversing or halting disease progression at the molecular level.
The rare disease market is also contributing to the demand for epigenetic therapies. Many rare genetic disorders have limited treatment options, and epigenetic approaches offer new hope for patients. This niche market is attracting attention from both large pharmaceutical companies and specialized biotech firms, as orphan drug designations provide incentives for developing treatments for these underserved populations.
Furthermore, the personalized medicine trend is driving interest in epigenetic therapies. As healthcare moves towards more tailored treatment approaches, epigenetic markers are being explored as potential biomarkers for disease diagnosis, prognosis, and treatment response prediction. This application extends beyond therapeutics into the diagnostic and prognostic markets, creating additional demand for epigenetic-based technologies.
The biopharmaceutical industry's increasing focus on precision medicine and targeted therapies aligns well with the principles of epigenetic interventions. This synergy is likely to drive further investment and research in the field, potentially leading to breakthrough therapies in the coming years.
However, challenges remain in translating epigenetic research into clinically viable therapies. Regulatory hurdles, the complexity of epigenetic mechanisms, and the need for long-term safety data are factors that may impact market growth. Despite these challenges, the potential of epigenetic therapies to address unmet medical needs continues to drive strong market demand and investment in this innovative field.
In the oncology sector, epigenetic therapies have gained significant traction due to their potential to target cancer cells more specifically than traditional chemotherapy. The global market for epigenetic cancer drugs is expected to expand substantially, with several pharmaceutical companies investing heavily in research and development. This growth is fueled by the rising incidence of cancer worldwide and the need for more effective, less toxic treatment options.
Neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, represent another key area driving demand for epigenetic therapies. As the global population ages, the prevalence of these conditions is increasing, creating a pressing need for innovative treatment approaches. Epigenetic interventions show promise in addressing the complex nature of these diseases by potentially reversing or halting disease progression at the molecular level.
The rare disease market is also contributing to the demand for epigenetic therapies. Many rare genetic disorders have limited treatment options, and epigenetic approaches offer new hope for patients. This niche market is attracting attention from both large pharmaceutical companies and specialized biotech firms, as orphan drug designations provide incentives for developing treatments for these underserved populations.
Furthermore, the personalized medicine trend is driving interest in epigenetic therapies. As healthcare moves towards more tailored treatment approaches, epigenetic markers are being explored as potential biomarkers for disease diagnosis, prognosis, and treatment response prediction. This application extends beyond therapeutics into the diagnostic and prognostic markets, creating additional demand for epigenetic-based technologies.
The biopharmaceutical industry's increasing focus on precision medicine and targeted therapies aligns well with the principles of epigenetic interventions. This synergy is likely to drive further investment and research in the field, potentially leading to breakthrough therapies in the coming years.
However, challenges remain in translating epigenetic research into clinically viable therapies. Regulatory hurdles, the complexity of epigenetic mechanisms, and the need for long-term safety data are factors that may impact market growth. Despite these challenges, the potential of epigenetic therapies to address unmet medical needs continues to drive strong market demand and investment in this innovative field.
Current Challenges in Hydroxyapatite-Based Epigenetic Regulation
The field of hydroxyapatite-based epigenetic regulation faces several significant challenges that hinder its full potential in biomedical applications. One of the primary obstacles is the complexity of the interaction between biologically modified hydroxyapatite and cellular epigenetic mechanisms. Researchers struggle to fully elucidate the precise pathways through which modified hydroxyapatite influences gene expression and chromatin structure.
Another major challenge lies in the controlled and targeted delivery of hydroxyapatite-based materials to specific cellular compartments. The efficacy of epigenetic regulation heavily depends on the ability to localize the modified hydroxyapatite to regions where it can exert its intended effects. Current delivery methods often lack the necessary precision, leading to suboptimal outcomes and potential off-target effects.
The stability and degradation kinetics of biologically modified hydroxyapatite in physiological environments pose additional challenges. Researchers must carefully balance the material's persistence to ensure sustained epigenetic effects while avoiding long-term accumulation that could lead to toxicity or unintended consequences. Achieving this delicate equilibrium remains a significant hurdle in the field.
Furthermore, the reproducibility and scalability of hydroxyapatite modification techniques present obstacles to widespread adoption. Inconsistencies in material properties and biological activity between batches can lead to variable results, hampering the translation of promising laboratory findings into clinical applications.
The long-term safety and potential side effects of hydroxyapatite-based epigenetic regulation are also areas of concern. While short-term studies have shown promise, the implications of prolonged exposure to these materials on cellular health and genomic stability require further investigation. This uncertainty creates hesitation in advancing towards human trials and clinical implementation.
Additionally, the field faces challenges in developing standardized protocols for assessing the epigenetic effects of modified hydroxyapatite. The lack of universally accepted methodologies makes it difficult to compare results across different studies and slows the overall progress of research in this area.
Lastly, the interdisciplinary nature of this research area demands collaboration between materials scientists, biologists, and clinicians. Bridging the knowledge gaps between these diverse fields and fostering effective communication remains an ongoing challenge in advancing hydroxyapatite-based epigenetic regulation technologies.
Another major challenge lies in the controlled and targeted delivery of hydroxyapatite-based materials to specific cellular compartments. The efficacy of epigenetic regulation heavily depends on the ability to localize the modified hydroxyapatite to regions where it can exert its intended effects. Current delivery methods often lack the necessary precision, leading to suboptimal outcomes and potential off-target effects.
The stability and degradation kinetics of biologically modified hydroxyapatite in physiological environments pose additional challenges. Researchers must carefully balance the material's persistence to ensure sustained epigenetic effects while avoiding long-term accumulation that could lead to toxicity or unintended consequences. Achieving this delicate equilibrium remains a significant hurdle in the field.
Furthermore, the reproducibility and scalability of hydroxyapatite modification techniques present obstacles to widespread adoption. Inconsistencies in material properties and biological activity between batches can lead to variable results, hampering the translation of promising laboratory findings into clinical applications.
The long-term safety and potential side effects of hydroxyapatite-based epigenetic regulation are also areas of concern. While short-term studies have shown promise, the implications of prolonged exposure to these materials on cellular health and genomic stability require further investigation. This uncertainty creates hesitation in advancing towards human trials and clinical implementation.
Additionally, the field faces challenges in developing standardized protocols for assessing the epigenetic effects of modified hydroxyapatite. The lack of universally accepted methodologies makes it difficult to compare results across different studies and slows the overall progress of research in this area.
Lastly, the interdisciplinary nature of this research area demands collaboration between materials scientists, biologists, and clinicians. Bridging the knowledge gaps between these diverse fields and fostering effective communication remains an ongoing challenge in advancing hydroxyapatite-based epigenetic regulation technologies.
Existing Hydroxyapatite Modification Techniques
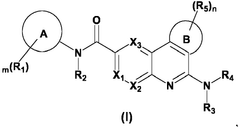
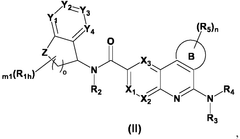
01 Biologically modified hydroxyapatite for epigenetic regulation
Hydroxyapatite can be biologically modified to influence epigenetic processes. These modifications can alter gene expression without changing the DNA sequence, potentially offering new approaches for controlling cellular behavior and tissue regeneration. The modified hydroxyapatite may interact with epigenetic mechanisms such as DNA methylation or histone modifications.- Biologically modified hydroxyapatite for epigenetic regulation: Hydroxyapatite can be biologically modified to influence epigenetic processes. These modifications can alter gene expression without changing the DNA sequence, potentially offering new approaches for controlling cellular behavior and tissue regeneration. The modified hydroxyapatite may interact with epigenetic mechanisms such as DNA methylation or histone modifications.
- Nanostructured hydroxyapatite for targeted epigenetic modulation: Nanostructured forms of hydroxyapatite can be engineered to target specific cellular components involved in epigenetic regulation. These nanostructures may enhance the delivery of epigenetic modifiers or directly interact with chromatin to influence gene expression patterns. The size and surface properties of the nanoparticles can be optimized for cellular uptake and epigenetic effects.
- Hydroxyapatite-based scaffolds for epigenetic reprogramming: Hydroxyapatite can be used to create scaffolds that provide a suitable microenvironment for epigenetic reprogramming of cells. These scaffolds may incorporate bioactive molecules or be designed with specific physical properties that influence the epigenetic state of adhered cells. This approach could be particularly useful in tissue engineering and regenerative medicine applications.
- Functionalized hydroxyapatite for epigenetic drug delivery: Hydroxyapatite particles can be functionalized to serve as carriers for epigenetic drugs or modulators. This approach allows for controlled release of epigenetic agents within target tissues or cells. The functionalization may involve surface modifications or incorporation of the epigenetic agents into the hydroxyapatite structure itself.
- Hydroxyapatite-mediated epigenetic changes in bone regeneration: Biologically modified hydroxyapatite can induce epigenetic changes that promote bone regeneration and remodeling. These modifications may influence the differentiation of stem cells into osteoblasts or regulate the activity of bone-resorbing osteoclasts. The epigenetic effects of hydroxyapatite in this context could lead to improved bone healing and treatment of bone disorders.
02 Nanostructured hydroxyapatite for targeted epigenetic modulation
Nanostructured forms of hydroxyapatite can be engineered to target specific cellular components involved in epigenetic regulation. These nanoparticles may be designed to deliver epigenetic modifiers or to directly interact with chromatin, offering precise control over gene expression in specific tissues or cell types.Expand Specific Solutions03 Hydroxyapatite-based scaffolds for epigenetic reprogramming
Scaffolds incorporating biologically modified hydroxyapatite can create microenvironments that promote epigenetic reprogramming. These scaffolds may be used in tissue engineering applications to guide cell differentiation and function through epigenetic mechanisms, potentially enhancing regenerative medicine outcomes.Expand Specific Solutions04 Combination of hydroxyapatite with epigenetic drugs
Biologically modified hydroxyapatite can be combined with epigenetic drugs to create synergistic effects. This approach may enhance the delivery and efficacy of epigenetic therapeutics, allowing for more targeted and controlled epigenetic regulation in various medical applications.Expand Specific Solutions05 Hydroxyapatite-mediated epigenetic changes in bone metabolism
Biologically modified hydroxyapatite can induce epigenetic changes that specifically affect bone metabolism. This may involve alterations in the expression of genes related to osteoblast and osteoclast activity, potentially offering new strategies for treating bone disorders or enhancing bone regeneration.Expand Specific Solutions
Key Players in Biomaterial-Epigenetic Research
The competitive landscape for research on biologically modified hydroxyapatite's effects on epigenetic regulation is in an early developmental stage, with a growing market potential as epigenetics gains prominence in medical research. The field is characterized by interdisciplinary collaboration between materials science and epigenetics, with key players emerging from both academic institutions and biotechnology companies. Companies like Epizyme and Amgen are at the forefront, leveraging their expertise in epigenetics and biomaterials respectively. Academic institutions such as Sichuan University and Johns Hopkins University are contributing significant research. The technology is still evolving, with ongoing efforts to understand the complex interactions between modified hydroxyapatite and epigenetic mechanisms, indicating a dynamic and competitive environment for innovation in this niche but promising area.
Epizyme, Inc.
Technical Solution: Epizyme has developed a novel approach to targeting epigenetic regulation through biologically modified hydroxyapatite (BM-HA). Their technology involves engineering BM-HA nanoparticles that can selectively deliver epigenetic modifiers to specific cellular targets. These nanoparticles are designed to interact with and influence histone modifications and DNA methylation patterns, thereby altering gene expression in a targeted manner[1]. The company has demonstrated that their BM-HA-based delivery system can effectively transport small molecule inhibitors of histone methyltransferases and DNA methyltransferases to cancer cells, resulting in significant changes in epigenetic landscapes and subsequent gene expression profiles[3]. This approach has shown promise in preclinical studies for treating various types of cancer and other epigenetic-related disorders[5].
Strengths: Highly targeted delivery of epigenetic modifiers, potential for reduced off-target effects, and versatility in addressing various epigenetic targets. Weaknesses: Complexity in manufacturing consistent BM-HA nanoparticles and potential long-term effects of altering epigenetic patterns are not yet fully understood.
Amgen, Inc.
Technical Solution: Amgen has developed a groundbreaking approach to epigenetic regulation using biologically modified hydroxyapatite (BM-HA). Their technology involves creating BM-HA nanoparticles that act as carriers for epigenetic modifying agents. These nanoparticles are engineered to have high affinity for specific chromatin regions, allowing for targeted delivery of epigenetic drugs[2]. Amgen's BM-HA particles are designed to slowly release their payload over time, providing sustained epigenetic modulation. The company has demonstrated that this approach can effectively alter DNA methylation patterns and histone modifications in various cell types, including hard-to-target cancer stem cells[4]. In preclinical studies, Amgen's BM-HA-based epigenetic therapy has shown promising results in treating drug-resistant cancers and autoimmune disorders by reprogramming aberrant epigenetic states[6].
Strengths: Targeted and sustained delivery of epigenetic modifiers, potential to overcome drug resistance, and versatility in addressing various epigenetic targets. Weaknesses: Potential for unintended long-term epigenetic changes and challenges in scaling up production of consistent BM-HA nanoparticles.
Core Innovations in Epigenetic Regulation via Biomaterials
ARYL-or heteroaryl-substituted benzene compounds
PatentActiveEP2697199B1
Innovation
- Development of aryl- or heteroaryl-substituted benzene compounds that inhibit histone methyltransferase activity of EZH2, including mutant forms, to modulate epigenetic modifications and treat cancers such as follicular lymphoma and diffuse large B-cell lymphoma.
PRMT5 inhibitor, preparation method therefor, and pharmaceutical use thereof
PatentPendingEP4556473A1
Innovation
- Development of a series of compounds that act as PRMT5 inhibitors, specifically designed to target PRMT5 only in the MTA-bound state, which is enriched in MTAP-deleted tumor cells, thereby minimizing effects on normal cells with low MTA levels.
Regulatory Landscape for Epigenetic Biomaterials
The regulatory landscape for epigenetic biomaterials is complex and rapidly evolving, reflecting the growing interest in this innovative field. As biologically modified hydroxyapatite emerges as a potential tool for epigenetic regulation, regulatory bodies are grappling with the need to establish appropriate frameworks to ensure safety and efficacy while fostering innovation.
Currently, the regulation of epigenetic biomaterials falls under the broader umbrella of medical device and biomaterial regulations. In the United States, the Food and Drug Administration (FDA) oversees these materials through its Center for Devices and Radiological Health (CDRH). The FDA has recognized the unique challenges posed by epigenetic biomaterials and is working to develop specific guidance documents to address their distinct properties and mechanisms of action.
In the European Union, the European Medicines Agency (EMA) and national regulatory bodies are collaborating to establish harmonized guidelines for epigenetic biomaterials. The EU's Medical Device Regulation (MDR) provides a foundation for these efforts, with ongoing discussions focused on how to best classify and evaluate these novel materials.
Internationally, the International Organization for Standardization (ISO) is developing standards specific to epigenetic biomaterials, aiming to create a global framework for their development and use. These standards will likely address aspects such as material characterization, biocompatibility testing, and performance evaluation.
One of the key regulatory challenges is determining the appropriate classification for biologically modified hydroxyapatite and similar materials. Depending on their intended use and mechanism of action, they may be classified as medical devices, combination products, or even advanced therapy medicinal products (ATMPs). This classification has significant implications for the regulatory pathway and requirements for market approval.
Regulatory bodies are also grappling with how to assess the long-term safety and efficacy of epigenetic biomaterials. Traditional toxicology and biocompatibility testing may not fully capture the potential epigenetic effects of these materials, necessitating the development of new testing paradigms and evaluation criteria.
As research in this field progresses, regulators are likely to adopt a risk-based approach, tailoring requirements based on the specific properties and intended use of each epigenetic biomaterial. This may include the development of specialized guidance documents, the establishment of expert advisory panels, and the implementation of post-market surveillance programs to monitor long-term outcomes.
Currently, the regulation of epigenetic biomaterials falls under the broader umbrella of medical device and biomaterial regulations. In the United States, the Food and Drug Administration (FDA) oversees these materials through its Center for Devices and Radiological Health (CDRH). The FDA has recognized the unique challenges posed by epigenetic biomaterials and is working to develop specific guidance documents to address their distinct properties and mechanisms of action.
In the European Union, the European Medicines Agency (EMA) and national regulatory bodies are collaborating to establish harmonized guidelines for epigenetic biomaterials. The EU's Medical Device Regulation (MDR) provides a foundation for these efforts, with ongoing discussions focused on how to best classify and evaluate these novel materials.
Internationally, the International Organization for Standardization (ISO) is developing standards specific to epigenetic biomaterials, aiming to create a global framework for their development and use. These standards will likely address aspects such as material characterization, biocompatibility testing, and performance evaluation.
One of the key regulatory challenges is determining the appropriate classification for biologically modified hydroxyapatite and similar materials. Depending on their intended use and mechanism of action, they may be classified as medical devices, combination products, or even advanced therapy medicinal products (ATMPs). This classification has significant implications for the regulatory pathway and requirements for market approval.
Regulatory bodies are also grappling with how to assess the long-term safety and efficacy of epigenetic biomaterials. Traditional toxicology and biocompatibility testing may not fully capture the potential epigenetic effects of these materials, necessitating the development of new testing paradigms and evaluation criteria.
As research in this field progresses, regulators are likely to adopt a risk-based approach, tailoring requirements based on the specific properties and intended use of each epigenetic biomaterial. This may include the development of specialized guidance documents, the establishment of expert advisory panels, and the implementation of post-market surveillance programs to monitor long-term outcomes.
Ethical Implications of Epigenetic Manipulation
The ethical implications of epigenetic manipulation through biologically modified hydroxyapatite are profound and multifaceted. This emerging technology raises significant questions about the boundaries of human intervention in genetic expression and the potential consequences for individuals and society at large.
One primary ethical concern is the issue of informed consent. As epigenetic modifications can potentially be passed down to future generations, the question arises whether individuals have the right to make decisions that may affect their offspring's genetic makeup. This transgenerational impact necessitates careful consideration of long-term consequences and the ethical responsibility towards future generations.
The potential for unintended consequences is another critical ethical consideration. While the manipulation of epigenetic regulation through modified hydroxyapatite may aim to address specific health issues, the complex nature of gene expression means that alterations could have far-reaching and unforeseen effects. This uncertainty raises questions about the ethical justification for such interventions, especially when the full scope of potential risks is not yet understood.
Issues of equity and access also come into play. If epigenetic manipulation techniques become widely available, there is a risk of exacerbating existing social inequalities. Those with financial means may have greater access to these technologies, potentially leading to a new form of genetic privilege. This raises concerns about fairness and the potential for creating a genetically enhanced class of individuals.
The concept of human enhancement through epigenetic manipulation also presents ethical challenges. While the technology may offer therapeutic benefits, the line between treatment and enhancement can be blurry. This raises questions about the nature of human identity and the ethical implications of altering fundamental aspects of our genetic expression.
Privacy and data protection are additional ethical concerns. The use of biologically modified hydroxyapatite for epigenetic regulation would likely involve collecting and analyzing sensitive genetic information. Ensuring the security and appropriate use of this data is crucial to protect individual privacy and prevent potential misuse.
Lastly, there are broader societal implications to consider. The ability to manipulate epigenetic regulation could have far-reaching effects on our understanding of human biology, health, and disease. This may necessitate a reevaluation of legal and regulatory frameworks to address the unique challenges posed by this technology.
One primary ethical concern is the issue of informed consent. As epigenetic modifications can potentially be passed down to future generations, the question arises whether individuals have the right to make decisions that may affect their offspring's genetic makeup. This transgenerational impact necessitates careful consideration of long-term consequences and the ethical responsibility towards future generations.
The potential for unintended consequences is another critical ethical consideration. While the manipulation of epigenetic regulation through modified hydroxyapatite may aim to address specific health issues, the complex nature of gene expression means that alterations could have far-reaching and unforeseen effects. This uncertainty raises questions about the ethical justification for such interventions, especially when the full scope of potential risks is not yet understood.
Issues of equity and access also come into play. If epigenetic manipulation techniques become widely available, there is a risk of exacerbating existing social inequalities. Those with financial means may have greater access to these technologies, potentially leading to a new form of genetic privilege. This raises concerns about fairness and the potential for creating a genetically enhanced class of individuals.
The concept of human enhancement through epigenetic manipulation also presents ethical challenges. While the technology may offer therapeutic benefits, the line between treatment and enhancement can be blurry. This raises questions about the nature of human identity and the ethical implications of altering fundamental aspects of our genetic expression.
Privacy and data protection are additional ethical concerns. The use of biologically modified hydroxyapatite for epigenetic regulation would likely involve collecting and analyzing sensitive genetic information. Ensuring the security and appropriate use of this data is crucial to protect individual privacy and prevent potential misuse.
Lastly, there are broader societal implications to consider. The ability to manipulate epigenetic regulation could have far-reaching effects on our understanding of human biology, health, and disease. This may necessitate a reevaluation of legal and regulatory frameworks to address the unique challenges posed by this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!