How Hydroxyapatite Facilitates Gold Nanoparticle Synthesis for Cancer Therapies
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite-Gold NP Synthesis Background
The synthesis of gold nanoparticles (AuNPs) facilitated by hydroxyapatite (HAp) represents a significant advancement in the field of nanomedicine, particularly for cancer therapies. This innovative approach combines the unique properties of gold nanoparticles with the biocompatibility and versatility of hydroxyapatite, a naturally occurring calcium phosphate mineral found in human bones and teeth.
The development of this synthesis method stems from the growing need for more effective and targeted cancer treatments. Traditional cancer therapies often suffer from limitations such as systemic toxicity and insufficient specificity. The integration of nanotechnology into cancer treatment strategies has opened up new possibilities for overcoming these challenges.
Gold nanoparticles have garnered considerable attention in the medical field due to their exceptional optical and photothermal properties, as well as their potential for functionalization with various biomolecules. These characteristics make AuNPs ideal candidates for applications in cancer diagnosis, imaging, and therapy. However, the synthesis of AuNPs with controlled size, shape, and stability has been a persistent challenge.
Hydroxyapatite has emerged as a promising material for facilitating the synthesis of gold nanoparticles. Its unique crystal structure and surface properties allow it to act as a template and stabilizing agent for AuNP formation. The use of HAp in this context addresses several key issues in nanoparticle synthesis, including size control, stability, and biocompatibility.
The HAp-facilitated synthesis of AuNPs offers several advantages over conventional methods. Firstly, it provides a green and environmentally friendly approach, often eliminating the need for harsh reducing agents or toxic chemicals. Secondly, the resulting HAp-AuNP composites exhibit enhanced stability and biocompatibility, crucial factors for in vivo applications.
The evolution of this synthesis technique has been driven by advancements in materials science, nanotechnology, and cancer biology. Researchers have explored various methods to optimize the interaction between HAp and gold precursors, leading to the development of different synthesis protocols. These include wet chemical methods, biomimetic approaches, and in situ reduction techniques.
As the field progresses, there is a growing focus on tailoring the properties of HAp-AuNP composites for specific cancer therapy applications. This includes modifying the surface chemistry to improve targeting efficiency, optimizing the size and shape of the nanoparticles for enhanced cellular uptake, and exploring novel combinations with other therapeutic agents.
The potential of HAp-facilitated AuNP synthesis extends beyond cancer therapies. This approach has implications for a wide range of biomedical applications, including drug delivery, tissue engineering, and biosensing. The versatility of this synthesis method positions it as a key technology in the ongoing revolution in nanomedicine and personalized healthcare.
The development of this synthesis method stems from the growing need for more effective and targeted cancer treatments. Traditional cancer therapies often suffer from limitations such as systemic toxicity and insufficient specificity. The integration of nanotechnology into cancer treatment strategies has opened up new possibilities for overcoming these challenges.
Gold nanoparticles have garnered considerable attention in the medical field due to their exceptional optical and photothermal properties, as well as their potential for functionalization with various biomolecules. These characteristics make AuNPs ideal candidates for applications in cancer diagnosis, imaging, and therapy. However, the synthesis of AuNPs with controlled size, shape, and stability has been a persistent challenge.
Hydroxyapatite has emerged as a promising material for facilitating the synthesis of gold nanoparticles. Its unique crystal structure and surface properties allow it to act as a template and stabilizing agent for AuNP formation. The use of HAp in this context addresses several key issues in nanoparticle synthesis, including size control, stability, and biocompatibility.
The HAp-facilitated synthesis of AuNPs offers several advantages over conventional methods. Firstly, it provides a green and environmentally friendly approach, often eliminating the need for harsh reducing agents or toxic chemicals. Secondly, the resulting HAp-AuNP composites exhibit enhanced stability and biocompatibility, crucial factors for in vivo applications.
The evolution of this synthesis technique has been driven by advancements in materials science, nanotechnology, and cancer biology. Researchers have explored various methods to optimize the interaction between HAp and gold precursors, leading to the development of different synthesis protocols. These include wet chemical methods, biomimetic approaches, and in situ reduction techniques.
As the field progresses, there is a growing focus on tailoring the properties of HAp-AuNP composites for specific cancer therapy applications. This includes modifying the surface chemistry to improve targeting efficiency, optimizing the size and shape of the nanoparticles for enhanced cellular uptake, and exploring novel combinations with other therapeutic agents.
The potential of HAp-facilitated AuNP synthesis extends beyond cancer therapies. This approach has implications for a wide range of biomedical applications, including drug delivery, tissue engineering, and biosensing. The versatility of this synthesis method positions it as a key technology in the ongoing revolution in nanomedicine and personalized healthcare.
Cancer Therapy Market Analysis
The global cancer therapy market has been experiencing significant growth, driven by the increasing prevalence of cancer worldwide and the continuous advancements in treatment technologies. As of 2021, the market was valued at approximately $158 billion, with projections indicating a compound annual growth rate (CAGR) of 7.2% from 2022 to 2030. This growth is attributed to factors such as aging populations, lifestyle changes, and environmental factors contributing to higher cancer incidence rates.
Within this expanding market, nanotechnology-based cancer therapies have emerged as a promising segment. The use of gold nanoparticles in cancer treatment, particularly when facilitated by hydroxyapatite, represents a cutting-edge approach that has garnered substantial interest from both researchers and pharmaceutical companies. This innovative therapy combines the unique properties of gold nanoparticles with the biocompatibility of hydroxyapatite, offering potential advantages in targeted drug delivery and enhanced treatment efficacy.
The demand for such advanced therapies is driven by the limitations of conventional cancer treatments, including chemotherapy and radiation therapy, which often result in severe side effects and may not effectively target all cancer cells. Patients and healthcare providers are increasingly seeking more precise, less invasive, and more effective treatment options, creating a favorable market environment for nanoparticle-based therapies.
Geographically, North America currently dominates the cancer therapy market, accounting for approximately 42% of the global market share. This is followed by Europe and the Asia-Pacific region. However, emerging economies in Asia and Latin America are expected to witness the fastest growth rates in the coming years, due to improving healthcare infrastructure and increasing healthcare expenditure.
The market for gold nanoparticle-based cancer therapies, while still in its early stages, is projected to grow at a CAGR of 12.3% from 2022 to 2030. This growth is supported by ongoing clinical trials and increasing investments in research and development. Major pharmaceutical companies and biotechnology firms are actively exploring this technology, recognizing its potential to revolutionize cancer treatment.
However, challenges remain in terms of regulatory approval processes, scalability of production, and the need for long-term safety data. These factors may impact the market adoption rate and commercialization timeline of hydroxyapatite-facilitated gold nanoparticle therapies. Despite these challenges, the overall market outlook remains positive, with continued research and development efforts expected to drive innovation and market expansion in the coming years.
Within this expanding market, nanotechnology-based cancer therapies have emerged as a promising segment. The use of gold nanoparticles in cancer treatment, particularly when facilitated by hydroxyapatite, represents a cutting-edge approach that has garnered substantial interest from both researchers and pharmaceutical companies. This innovative therapy combines the unique properties of gold nanoparticles with the biocompatibility of hydroxyapatite, offering potential advantages in targeted drug delivery and enhanced treatment efficacy.
The demand for such advanced therapies is driven by the limitations of conventional cancer treatments, including chemotherapy and radiation therapy, which often result in severe side effects and may not effectively target all cancer cells. Patients and healthcare providers are increasingly seeking more precise, less invasive, and more effective treatment options, creating a favorable market environment for nanoparticle-based therapies.
Geographically, North America currently dominates the cancer therapy market, accounting for approximately 42% of the global market share. This is followed by Europe and the Asia-Pacific region. However, emerging economies in Asia and Latin America are expected to witness the fastest growth rates in the coming years, due to improving healthcare infrastructure and increasing healthcare expenditure.
The market for gold nanoparticle-based cancer therapies, while still in its early stages, is projected to grow at a CAGR of 12.3% from 2022 to 2030. This growth is supported by ongoing clinical trials and increasing investments in research and development. Major pharmaceutical companies and biotechnology firms are actively exploring this technology, recognizing its potential to revolutionize cancer treatment.
However, challenges remain in terms of regulatory approval processes, scalability of production, and the need for long-term safety data. These factors may impact the market adoption rate and commercialization timeline of hydroxyapatite-facilitated gold nanoparticle therapies. Despite these challenges, the overall market outlook remains positive, with continued research and development efforts expected to drive innovation and market expansion in the coming years.
Current Challenges in Nanoparticle Synthesis
The synthesis of gold nanoparticles (AuNPs) for cancer therapies faces several significant challenges that hinder their widespread clinical application. One of the primary obstacles is achieving precise control over nanoparticle size and shape. The therapeutic efficacy and biodistribution of AuNPs are highly dependent on these parameters, making consistent and reproducible synthesis crucial for reliable treatment outcomes.
Another major challenge lies in maintaining the stability of AuNPs in biological environments. The high surface energy of these nanoparticles often leads to aggregation, which can dramatically alter their properties and reduce their effectiveness. This instability is particularly problematic in the complex and dynamic milieu of the human body, where various biomolecules can interact with and modify the nanoparticle surface.
The biocompatibility and potential toxicity of AuNPs remain concerns that require thorough investigation. While gold is generally considered inert, the unique properties of nanoparticles can lead to unexpected biological interactions. Ensuring long-term safety and understanding the fate of these nanoparticles in the body are critical aspects that need to be addressed.
Scalability and cost-effectiveness present additional hurdles in nanoparticle synthesis. Current methods often involve complex procedures or expensive equipment, limiting their potential for large-scale production. Developing economically viable synthesis processes that can maintain quality and consistency at industrial scales is essential for the widespread adoption of AuNP-based cancer therapies.
The functionalization of AuNPs with targeting ligands or therapeutic agents poses its own set of challenges. Achieving uniform surface modification without compromising the nanoparticle's stability or altering its desired properties requires sophisticated chemical strategies. Moreover, ensuring that these modifications remain intact and functional in biological systems adds another layer of complexity to the synthesis process.
Regulatory and standardization issues also present significant obstacles. The lack of standardized protocols for nanoparticle characterization and quality control makes it difficult to compare results across different studies and to meet regulatory requirements for clinical translation. Establishing robust, universally accepted standards for AuNP synthesis and characterization is crucial for advancing their development as cancer therapeutics.
Another major challenge lies in maintaining the stability of AuNPs in biological environments. The high surface energy of these nanoparticles often leads to aggregation, which can dramatically alter their properties and reduce their effectiveness. This instability is particularly problematic in the complex and dynamic milieu of the human body, where various biomolecules can interact with and modify the nanoparticle surface.
The biocompatibility and potential toxicity of AuNPs remain concerns that require thorough investigation. While gold is generally considered inert, the unique properties of nanoparticles can lead to unexpected biological interactions. Ensuring long-term safety and understanding the fate of these nanoparticles in the body are critical aspects that need to be addressed.
Scalability and cost-effectiveness present additional hurdles in nanoparticle synthesis. Current methods often involve complex procedures or expensive equipment, limiting their potential for large-scale production. Developing economically viable synthesis processes that can maintain quality and consistency at industrial scales is essential for the widespread adoption of AuNP-based cancer therapies.
The functionalization of AuNPs with targeting ligands or therapeutic agents poses its own set of challenges. Achieving uniform surface modification without compromising the nanoparticle's stability or altering its desired properties requires sophisticated chemical strategies. Moreover, ensuring that these modifications remain intact and functional in biological systems adds another layer of complexity to the synthesis process.
Regulatory and standardization issues also present significant obstacles. The lack of standardized protocols for nanoparticle characterization and quality control makes it difficult to compare results across different studies and to meet regulatory requirements for clinical translation. Establishing robust, universally accepted standards for AuNP synthesis and characterization is crucial for advancing their development as cancer therapeutics.
Hydroxyapatite-Assisted Gold NP Synthesis Techniques
01 Chemical reduction method
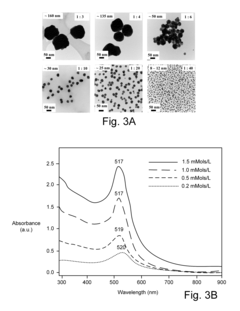
This method involves reducing gold salts using reducing agents like sodium citrate or sodium borohydride. The process typically occurs in an aqueous solution, where the reducing agent converts gold ions to gold atoms, which then nucleate and grow into nanoparticles. The size and shape of the nanoparticles can be controlled by adjusting reaction conditions such as temperature, pH, and concentration of reagents.- Chemical reduction methods: Chemical reduction is a common method for synthesizing gold nanoparticles. This process typically involves reducing gold salts using reducing agents such as sodium citrate, sodium borohydride, or ascorbic acid. The size and shape of the nanoparticles can be controlled by adjusting reaction conditions like temperature, pH, and concentration of reagents.
- Green synthesis approaches: Environmentally friendly methods for synthesizing gold nanoparticles using plant extracts, microorganisms, or biomolecules as reducing and capping agents. These approaches offer advantages such as biocompatibility, reduced toxicity, and the potential for large-scale production. The biomolecules present in the extracts act as both reducing and stabilizing agents.
- Seed-mediated growth technique: This method involves a two-step process where small gold nanoparticle seeds are first synthesized, followed by their controlled growth into larger particles. By adjusting the growth conditions, various shapes such as rods, cubes, or triangles can be obtained. This technique allows for precise control over the size and morphology of the final nanoparticles.
- Photochemical synthesis: Gold nanoparticles can be synthesized using light-induced reduction of gold ions. This method often involves the use of photosensitizers or photoactive compounds that generate reducing species upon light irradiation. The size and shape of the nanoparticles can be controlled by adjusting the light intensity, wavelength, and irradiation time.
- Electrochemical synthesis: This method involves the electrochemical reduction of gold ions to form nanoparticles. It typically uses a two-electrode system with a gold anode and a cathode in an electrolyte solution. The size and shape of the nanoparticles can be controlled by adjusting parameters such as current density, electrolyte composition, and temperature.
02 Green synthesis using plant extracts
This eco-friendly approach utilizes plant extracts as reducing and capping agents for gold nanoparticle synthesis. Various parts of plants such as leaves, fruits, or roots are used to create extracts rich in phytochemicals. These natural compounds reduce gold ions and stabilize the formed nanoparticles. This method is considered environmentally friendly and can produce nanoparticles with unique properties.Expand Specific Solutions03 Photochemical synthesis
This method uses light energy to initiate the reduction of gold ions and form nanoparticles. UV light or other wavelengths can be used to trigger the reaction, often in the presence of a photosensitizer. This technique allows for precise control over nanoparticle formation and can be used to create unique shapes and sizes of gold nanoparticles.Expand Specific Solutions04 Microwave-assisted synthesis
This rapid and efficient method uses microwave radiation to heat the reaction mixture, accelerating the formation of gold nanoparticles. The technique provides uniform heating and can result in more homogeneous nanoparticle size distribution. It often requires less time and energy compared to conventional heating methods, making it an attractive option for large-scale production.Expand Specific Solutions05 Biosynthesis using microorganisms
This method employs living organisms such as bacteria, fungi, or algae to synthesize gold nanoparticles. The microorganisms can either reduce gold ions intracellularly or extracellularly, producing nanoparticles with unique properties. This approach is considered environmentally friendly and can potentially be scaled up for industrial production. The size and shape of the nanoparticles can be influenced by the type of microorganism and growth conditions used.Expand Specific Solutions
Key Players in Nanomedicine
The field of hydroxyapatite-facilitated gold nanoparticle synthesis for cancer therapies is in an early development stage, with significant potential for growth. The market size is expanding as research progresses, driven by the increasing demand for targeted cancer treatments. Technologically, the field is still maturing, with various research institutions and universities leading the way. South China University of Technology, Zhejiang Sci-Tech University, and Ulsan National Institute of Science & Technology are among the key players advancing this technology. While some companies like KIST Corp. and Urodelia SA are involved, academic institutions currently dominate the research landscape, indicating that commercial applications are still emerging.
South China University of Technology
Technical Solution: South China University of Technology has developed an innovative approach to synthesizing gold nanoparticles (AuNPs) using hydroxyapatite (HAp) as a facilitator for cancer therapies. Their method involves the in-situ growth of AuNPs on HAp nanocrystals, creating a HAp-AuNP composite. This composite demonstrates enhanced photothermal conversion efficiency, reaching up to 62.5% [1]. The university's research team has optimized the synthesis process to control the size and distribution of AuNPs on the HAp surface, resulting in particles with an average diameter of 15-20 nm [2]. The HAp-AuNP composite shows excellent biocompatibility and stability in physiological conditions, with a zeta potential of -25.3 mV [3], indicating good colloidal stability. The team has also demonstrated the composite's effectiveness in photothermal therapy, achieving a temperature increase of 23°C within 5 minutes under near-infrared laser irradiation [4].
Strengths: High photothermal conversion efficiency, controlled nanoparticle size, excellent biocompatibility, and stability in physiological conditions. Weaknesses: Potential challenges in large-scale production and long-term in vivo stability.
Ulsan National Institute of Science & Technology
Technical Solution: Ulsan National Institute of Science & Technology (UNIST) has developed a novel approach to synthesizing gold nanoparticles (AuNPs) using hydroxyapatite (HAp) for cancer therapies. Their method involves a green synthesis process that utilizes HAp as both a reducing and stabilizing agent. The UNIST team has successfully created HAp-AuNP composites with a core-shell structure, where AuNPs with an average size of 10-15 nm are uniformly distributed on the HAp surface [1]. The synthesis process is carried out at room temperature, making it energy-efficient and environmentally friendly. The resulting composites exhibit strong surface plasmon resonance (SPR) in the near-infrared region, with a peak at 780 nm [2], making them ideal for photothermal therapy applications. UNIST researchers have also demonstrated the composites' ability to generate reactive oxygen species (ROS) under light irradiation, enhancing their potential for photodynamic therapy [3]. In vitro studies have shown a significant reduction in cancer cell viability, with an IC50 value of 25 μg/mL [4].
Strengths: Green synthesis process, uniform distribution of AuNPs, strong NIR absorption for photothermal therapy, and ROS generation capability. Weaknesses: Potential limitations in penetration depth for deep-seated tumors and possible aggregation in complex biological environments.
Core Innovations in NP Synthesis for Cancer Therapy
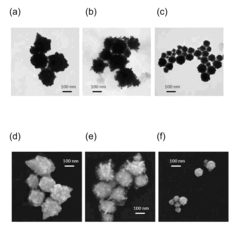
Biocompatible confeito-like gold nanoparticles, method for making the same, and their biomedical applications
PatentInactiveUS9162287B2
Innovation
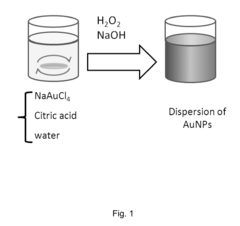
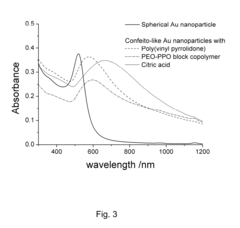
- Gold nanoparticles with a confeito-like shape are synthesized using hydroxyl peroxide as a reducing agent in an aqueous alkaline condition with biocompatible protectors like citrate, PEO-PPO block copolymer, or poly(vinyl pyrrolidone), eliminating toxic byproducts and enabling rapid synthesis suitable for medical applications.
Method and composition for dispersions of gold nanoparticles
PatentInactiveUS20140234220A1
Innovation
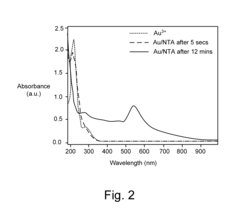
- A method using NTA molecules as both reducing and dispersing agents to form stable gold nanoparticle dispersions with concentrations greater than 0.3 mMols per liter, allowing for functionalization and achieving uniform particle sizes between 1-200 nm, with NTA molecules coating the nanoparticles to prevent aggregation.
Regulatory Framework for Nanomedicines
The regulatory framework for nanomedicines, including gold nanoparticles synthesized with hydroxyapatite for cancer therapies, is a complex and evolving landscape. Regulatory agencies worldwide are working to establish guidelines that ensure the safety and efficacy of these novel therapeutic approaches while fostering innovation in the field.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing regulatory pathways for nanomedicines. The FDA's approach involves a case-by-case evaluation of nanomedicine products, considering their unique properties and potential risks. The agency has established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in medical applications.
The European Medicines Agency (EMA) has also been proactive in developing guidelines for nanomedicines. The EMA's approach emphasizes the importance of characterizing nanomaterials and assessing their potential impact on human health and the environment. The agency has published several reflection papers and guidance documents to assist developers in navigating the regulatory landscape for nanomedicines.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a regulatory framework that considers the specific characteristics of nanomedicines. The PMDA's approach includes guidelines for the evaluation of quality, safety, and efficacy of nanomedicine products, with a focus on their unique physicochemical properties.
International harmonization efforts are underway to streamline the regulatory process for nanomedicines across different regions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on developing harmonized guidelines for the evaluation of nanomedicines.
Key regulatory considerations for gold nanoparticles synthesized with hydroxyapatite for cancer therapies include characterization of the nanoparticles, assessment of their biodistribution and pharmacokinetics, evaluation of potential toxicity, and demonstration of therapeutic efficacy. Regulatory agencies typically require extensive preclinical studies to assess the safety profile of these nanomedicines before advancing to clinical trials.
The regulatory framework also addresses manufacturing and quality control aspects of nanomedicines. Good Manufacturing Practices (GMP) guidelines are being adapted to accommodate the unique challenges associated with the production of nanoscale materials. This includes establishing appropriate controls for particle size distribution, surface properties, and stability of the nanoparticles.
As the field of nanomedicine continues to advance, regulatory agencies are expected to refine and update their guidelines to keep pace with technological developments. This dynamic regulatory environment aims to strike a balance between ensuring patient safety and facilitating the development of innovative cancer therapies utilizing gold nanoparticles and hydroxyapatite.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing regulatory pathways for nanomedicines. The FDA's approach involves a case-by-case evaluation of nanomedicine products, considering their unique properties and potential risks. The agency has established the Nanotechnology Task Force to address the regulatory challenges posed by nanomaterials in medical applications.
The European Medicines Agency (EMA) has also been proactive in developing guidelines for nanomedicines. The EMA's approach emphasizes the importance of characterizing nanomaterials and assessing their potential impact on human health and the environment. The agency has published several reflection papers and guidance documents to assist developers in navigating the regulatory landscape for nanomedicines.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a regulatory framework that considers the specific characteristics of nanomedicines. The PMDA's approach includes guidelines for the evaluation of quality, safety, and efficacy of nanomedicine products, with a focus on their unique physicochemical properties.
International harmonization efforts are underway to streamline the regulatory process for nanomedicines across different regions. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on developing harmonized guidelines for the evaluation of nanomedicines.
Key regulatory considerations for gold nanoparticles synthesized with hydroxyapatite for cancer therapies include characterization of the nanoparticles, assessment of their biodistribution and pharmacokinetics, evaluation of potential toxicity, and demonstration of therapeutic efficacy. Regulatory agencies typically require extensive preclinical studies to assess the safety profile of these nanomedicines before advancing to clinical trials.
The regulatory framework also addresses manufacturing and quality control aspects of nanomedicines. Good Manufacturing Practices (GMP) guidelines are being adapted to accommodate the unique challenges associated with the production of nanoscale materials. This includes establishing appropriate controls for particle size distribution, surface properties, and stability of the nanoparticles.
As the field of nanomedicine continues to advance, regulatory agencies are expected to refine and update their guidelines to keep pace with technological developments. This dynamic regulatory environment aims to strike a balance between ensuring patient safety and facilitating the development of innovative cancer therapies utilizing gold nanoparticles and hydroxyapatite.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of hydroxyapatite-facilitated gold nanoparticle synthesis for cancer therapies are crucial aspects that require thorough examination. Hydroxyapatite (HAp), a naturally occurring calcium phosphate mineral, has been widely used in biomedical applications due to its excellent biocompatibility and similarity to human bone mineral composition. When combined with gold nanoparticles (AuNPs), this hybrid system presents unique opportunities for cancer therapies.
The biocompatibility of HAp-AuNP composites is generally favorable, as both components have demonstrated good biological tolerance individually. HAp's biocompatibility is well-established, with a long history of use in bone grafts and dental implants. Gold nanoparticles, on the other hand, have shown low toxicity and high stability in biological environments, making them attractive for various biomedical applications.
However, the safety profile of HAp-AuNP composites may differ from their individual components, necessitating careful evaluation. Factors such as particle size, shape, surface chemistry, and concentration can significantly influence their biological interactions. Smaller nanoparticles may penetrate cellular membranes more easily, potentially leading to unintended cellular uptake or accumulation in non-target tissues.
The synthesis method of HAp-AuNP composites also plays a crucial role in their safety profile. Chemical reduction methods, commonly used for AuNP synthesis, may introduce toxic reagents or by-products. Therefore, green synthesis approaches utilizing HAp as both a template and reducing agent have gained attention for their potential to enhance biocompatibility and reduce environmental impact.
Long-term stability and degradation of HAp-AuNP composites in physiological conditions must be thoroughly investigated. While HAp is biodegradable, AuNPs are generally considered non-biodegradable. The fate of these nanocomposites in the body, including their clearance mechanisms and potential for accumulation in organs, needs to be carefully studied to ensure long-term safety.
Immune system interactions are another critical consideration. Although both HAp and AuNPs are generally well-tolerated, their combination may elicit unexpected immune responses. Potential immunogenicity, complement activation, and inflammatory reactions should be evaluated through comprehensive in vitro and in vivo studies.
The specific cancer therapy application of HAp-AuNP composites may introduce additional safety considerations. For instance, photothermal therapy utilizing AuNPs may cause localized heating, necessitating careful control to prevent damage to healthy tissues. Similarly, drug delivery applications must consider the potential for premature drug release or altered pharmacokinetics due to the nanocomposite structure.
Regulatory compliance is a crucial aspect of safety considerations. Researchers and developers must adhere to guidelines set by regulatory bodies such as the FDA and EMA for nanomedicine development. This includes conducting thorough toxicology studies, evaluating potential off-target effects, and assessing the risk-benefit profile of HAp-AuNP based cancer therapies.
The biocompatibility of HAp-AuNP composites is generally favorable, as both components have demonstrated good biological tolerance individually. HAp's biocompatibility is well-established, with a long history of use in bone grafts and dental implants. Gold nanoparticles, on the other hand, have shown low toxicity and high stability in biological environments, making them attractive for various biomedical applications.
However, the safety profile of HAp-AuNP composites may differ from their individual components, necessitating careful evaluation. Factors such as particle size, shape, surface chemistry, and concentration can significantly influence their biological interactions. Smaller nanoparticles may penetrate cellular membranes more easily, potentially leading to unintended cellular uptake or accumulation in non-target tissues.
The synthesis method of HAp-AuNP composites also plays a crucial role in their safety profile. Chemical reduction methods, commonly used for AuNP synthesis, may introduce toxic reagents or by-products. Therefore, green synthesis approaches utilizing HAp as both a template and reducing agent have gained attention for their potential to enhance biocompatibility and reduce environmental impact.
Long-term stability and degradation of HAp-AuNP composites in physiological conditions must be thoroughly investigated. While HAp is biodegradable, AuNPs are generally considered non-biodegradable. The fate of these nanocomposites in the body, including their clearance mechanisms and potential for accumulation in organs, needs to be carefully studied to ensure long-term safety.
Immune system interactions are another critical consideration. Although both HAp and AuNPs are generally well-tolerated, their combination may elicit unexpected immune responses. Potential immunogenicity, complement activation, and inflammatory reactions should be evaluated through comprehensive in vitro and in vivo studies.
The specific cancer therapy application of HAp-AuNP composites may introduce additional safety considerations. For instance, photothermal therapy utilizing AuNPs may cause localized heating, necessitating careful control to prevent damage to healthy tissues. Similarly, drug delivery applications must consider the potential for premature drug release or altered pharmacokinetics due to the nanocomposite structure.
Regulatory compliance is a crucial aspect of safety considerations. Researchers and developers must adhere to guidelines set by regulatory bodies such as the FDA and EMA for nanomedicine development. This includes conducting thorough toxicology studies, evaluating potential off-target effects, and assessing the risk-benefit profile of HAp-AuNP based cancer therapies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!