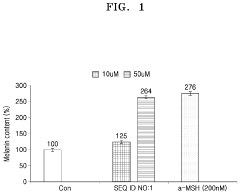
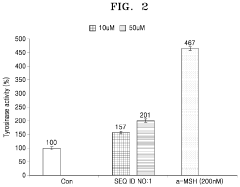
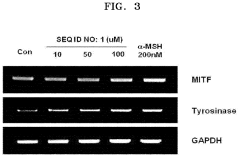
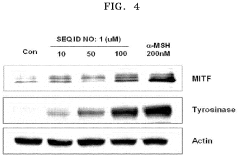
Hydroxyapatite's Role in Modulating Melanin Synthesis for Dermal Applications
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite and Melanin Synthesis: Background and Objectives
Hydroxyapatite (HAp) and melanin synthesis have emerged as crucial areas of research in dermal applications, with significant implications for skin health and cosmetic treatments. The intersection of these two fields represents a promising frontier in biomaterials and dermatology, offering potential solutions for various skin-related issues.
Historically, hydroxyapatite has been primarily associated with bone and dental applications due to its similarity to the mineral component of bones and teeth. However, recent advancements have expanded its potential uses to include dermal applications. This shift in focus has been driven by the discovery of HAp's biocompatibility, its ability to interact with biological systems, and its potential to influence cellular processes in the skin.
Melanin, on the other hand, has long been recognized as the primary pigment responsible for skin color. Its synthesis and regulation play critical roles in protecting the skin from UV radiation and in determining skin tone. The process of melanogenesis, controlled by complex biochemical pathways, has been a subject of intense study in dermatology and cosmetic science.
The convergence of hydroxyapatite research and melanin synthesis studies has opened up new avenues for addressing skin pigmentation disorders, UV protection, and even anti-aging treatments. This interdisciplinary approach aims to leverage the unique properties of HAp to modulate melanin production and distribution in the skin.
The primary objective of this research is to elucidate the mechanisms by which hydroxyapatite can influence melanin synthesis and to explore its potential applications in dermal products. This includes investigating how HAp interacts with melanocytes, the cells responsible for melanin production, and how it may affect the various stages of the melanogenesis process.
Furthermore, this research seeks to understand the broader implications of HAp-melanin interactions on skin health. This encompasses exploring how HAp might be used to address hyperpigmentation disorders, enhance UV protection, or even contribute to more even skin tone distribution.
Another key goal is to develop novel formulations and delivery systems that can effectively incorporate HAp into dermal applications. This involves overcoming challenges related to particle size, stability, and skin penetration to ensure optimal efficacy and safety of HAp-based products.
By focusing on these objectives, researchers aim to bridge the gap between materials science and dermatology, potentially revolutionizing approaches to skin care and treatment. The outcomes of this research could lead to the development of advanced dermal products that offer more targeted and effective solutions for a range of skin concerns, from pigmentation issues to photoaging.
Historically, hydroxyapatite has been primarily associated with bone and dental applications due to its similarity to the mineral component of bones and teeth. However, recent advancements have expanded its potential uses to include dermal applications. This shift in focus has been driven by the discovery of HAp's biocompatibility, its ability to interact with biological systems, and its potential to influence cellular processes in the skin.
Melanin, on the other hand, has long been recognized as the primary pigment responsible for skin color. Its synthesis and regulation play critical roles in protecting the skin from UV radiation and in determining skin tone. The process of melanogenesis, controlled by complex biochemical pathways, has been a subject of intense study in dermatology and cosmetic science.
The convergence of hydroxyapatite research and melanin synthesis studies has opened up new avenues for addressing skin pigmentation disorders, UV protection, and even anti-aging treatments. This interdisciplinary approach aims to leverage the unique properties of HAp to modulate melanin production and distribution in the skin.
The primary objective of this research is to elucidate the mechanisms by which hydroxyapatite can influence melanin synthesis and to explore its potential applications in dermal products. This includes investigating how HAp interacts with melanocytes, the cells responsible for melanin production, and how it may affect the various stages of the melanogenesis process.
Furthermore, this research seeks to understand the broader implications of HAp-melanin interactions on skin health. This encompasses exploring how HAp might be used to address hyperpigmentation disorders, enhance UV protection, or even contribute to more even skin tone distribution.
Another key goal is to develop novel formulations and delivery systems that can effectively incorporate HAp into dermal applications. This involves overcoming challenges related to particle size, stability, and skin penetration to ensure optimal efficacy and safety of HAp-based products.
By focusing on these objectives, researchers aim to bridge the gap between materials science and dermatology, potentially revolutionizing approaches to skin care and treatment. The outcomes of this research could lead to the development of advanced dermal products that offer more targeted and effective solutions for a range of skin concerns, from pigmentation issues to photoaging.
Market Analysis for Dermal Melanin Modulation Products
The market for dermal melanin modulation products is experiencing significant growth, driven by increasing consumer demand for skin lightening and pigmentation control solutions. This market segment is part of the broader skincare industry, which is projected to reach $189.3 billion globally by 2025. Within this, products targeting melanin synthesis and regulation are gaining traction due to rising concerns about hyperpigmentation, melasma, and uneven skin tone.
The demand for melanin modulation products is particularly strong in Asia-Pacific regions, where cultural preferences for lighter skin tones persist. However, there is also growing interest in Western markets, driven by concerns about sun damage and age-related pigmentation issues. North America and Europe are seeing increased adoption of these products, especially among aging populations seeking to address age spots and other pigmentation concerns.
Consumer preferences are shifting towards more natural and scientifically-backed solutions, creating opportunities for products incorporating novel ingredients like hydroxyapatite. This trend aligns with the broader move towards "clean beauty" and products perceived as safer and more effective than traditional chemical-based lighteners.
The market is segmented into various product types, including topical creams, serums, oral supplements, and professional treatments. Topical products currently dominate the market, but there is growing interest in systemic approaches and combination therapies that address melanin production from multiple angles.
Key market drivers include increasing disposable incomes in emerging markets, growing awareness of skin health, and advancements in formulation technologies. The rise of social media and influencer marketing has also contributed to increased consumer awareness and demand for these products.
Regulatory landscapes vary significantly across regions, with some countries imposing strict regulations on skin lightening ingredients. This has led to a push for safer, more natural alternatives, creating opportunities for innovative technologies like hydroxyapatite-based solutions.
The competitive landscape is characterized by a mix of large multinational corporations and niche players. Major cosmetic companies are investing heavily in R&D to develop novel melanin-modulating technologies, while smaller companies are focusing on specialized, science-driven approaches.
Looking ahead, the market is expected to continue its growth trajectory, with a CAGR of 5.9% projected through 2027. Emerging trends include personalized skincare solutions, the integration of AI and machine learning for product development, and increased focus on multifunctional products that address multiple skin concerns simultaneously.
The demand for melanin modulation products is particularly strong in Asia-Pacific regions, where cultural preferences for lighter skin tones persist. However, there is also growing interest in Western markets, driven by concerns about sun damage and age-related pigmentation issues. North America and Europe are seeing increased adoption of these products, especially among aging populations seeking to address age spots and other pigmentation concerns.
Consumer preferences are shifting towards more natural and scientifically-backed solutions, creating opportunities for products incorporating novel ingredients like hydroxyapatite. This trend aligns with the broader move towards "clean beauty" and products perceived as safer and more effective than traditional chemical-based lighteners.
The market is segmented into various product types, including topical creams, serums, oral supplements, and professional treatments. Topical products currently dominate the market, but there is growing interest in systemic approaches and combination therapies that address melanin production from multiple angles.
Key market drivers include increasing disposable incomes in emerging markets, growing awareness of skin health, and advancements in formulation technologies. The rise of social media and influencer marketing has also contributed to increased consumer awareness and demand for these products.
Regulatory landscapes vary significantly across regions, with some countries imposing strict regulations on skin lightening ingredients. This has led to a push for safer, more natural alternatives, creating opportunities for innovative technologies like hydroxyapatite-based solutions.
The competitive landscape is characterized by a mix of large multinational corporations and niche players. Major cosmetic companies are investing heavily in R&D to develop novel melanin-modulating technologies, while smaller companies are focusing on specialized, science-driven approaches.
Looking ahead, the market is expected to continue its growth trajectory, with a CAGR of 5.9% projected through 2027. Emerging trends include personalized skincare solutions, the integration of AI and machine learning for product development, and increased focus on multifunctional products that address multiple skin concerns simultaneously.
Current Challenges in Melanin Synthesis Modulation
Despite significant advancements in understanding melanin synthesis, several challenges persist in effectively modulating this process for dermal applications, particularly when considering the role of hydroxyapatite. One of the primary obstacles is the complex nature of melanogenesis itself, which involves multiple enzymatic steps and regulatory pathways. This complexity makes it difficult to precisely target and control melanin production without affecting other cellular processes.
The bioavailability and stability of hydroxyapatite in dermal formulations present another significant challenge. While hydroxyapatite has shown promise in modulating melanin synthesis, ensuring its effective delivery to melanocytes in the skin remains problematic. The skin's natural barrier function often impedes the penetration of topically applied compounds, limiting their efficacy in reaching the target cells.
Furthermore, the interaction between hydroxyapatite and the various factors involved in melanogenesis is not fully understood. This knowledge gap hinders the development of optimized formulations that can effectively leverage hydroxyapatite's potential in modulating melanin synthesis. Researchers are still working to elucidate the exact mechanisms by which hydroxyapatite influences melanocyte activity and melanin production.
Another challenge lies in achieving consistent and predictable results across different skin types and conditions. The variability in individual skin characteristics, including melanocyte density, baseline melanin levels, and skin permeability, makes it difficult to develop a one-size-fits-all approach to melanin modulation using hydroxyapatite.
The long-term effects and safety profile of hydroxyapatite-based treatments for melanin modulation also require further investigation. While hydroxyapatite is generally considered biocompatible, its prolonged use in dermal applications may have unforeseen consequences on skin health and function. Ensuring the safety and efficacy of these treatments over extended periods remains a critical challenge.
Additionally, the formulation of stable and effective products incorporating hydroxyapatite for melanin modulation poses technical challenges. Issues such as particle size, dispersion, and compatibility with other ingredients in skincare formulations need to be addressed to create products that are both effective and cosmetically appealing.
Lastly, regulatory hurdles and the need for extensive clinical trials present significant obstacles in bringing hydroxyapatite-based melanin modulation treatments to market. The rigorous testing required to demonstrate safety and efficacy, particularly for products that alter skin pigmentation, can be time-consuming and costly, potentially slowing the development and commercialization of innovative solutions in this field.
The bioavailability and stability of hydroxyapatite in dermal formulations present another significant challenge. While hydroxyapatite has shown promise in modulating melanin synthesis, ensuring its effective delivery to melanocytes in the skin remains problematic. The skin's natural barrier function often impedes the penetration of topically applied compounds, limiting their efficacy in reaching the target cells.
Furthermore, the interaction between hydroxyapatite and the various factors involved in melanogenesis is not fully understood. This knowledge gap hinders the development of optimized formulations that can effectively leverage hydroxyapatite's potential in modulating melanin synthesis. Researchers are still working to elucidate the exact mechanisms by which hydroxyapatite influences melanocyte activity and melanin production.
Another challenge lies in achieving consistent and predictable results across different skin types and conditions. The variability in individual skin characteristics, including melanocyte density, baseline melanin levels, and skin permeability, makes it difficult to develop a one-size-fits-all approach to melanin modulation using hydroxyapatite.
The long-term effects and safety profile of hydroxyapatite-based treatments for melanin modulation also require further investigation. While hydroxyapatite is generally considered biocompatible, its prolonged use in dermal applications may have unforeseen consequences on skin health and function. Ensuring the safety and efficacy of these treatments over extended periods remains a critical challenge.
Additionally, the formulation of stable and effective products incorporating hydroxyapatite for melanin modulation poses technical challenges. Issues such as particle size, dispersion, and compatibility with other ingredients in skincare formulations need to be addressed to create products that are both effective and cosmetically appealing.
Lastly, regulatory hurdles and the need for extensive clinical trials present significant obstacles in bringing hydroxyapatite-based melanin modulation treatments to market. The rigorous testing required to demonstrate safety and efficacy, particularly for products that alter skin pigmentation, can be time-consuming and costly, potentially slowing the development and commercialization of innovative solutions in this field.
Existing Hydroxyapatite-based Solutions for Melanin Modulation
01 Hydroxyapatite-based melanin synthesis
Hydroxyapatite can be used as a substrate or catalyst for melanin synthesis. This approach combines the biocompatible properties of hydroxyapatite with the protective and pigmentation properties of melanin, potentially useful in various biomedical and cosmetic applications.- Hydroxyapatite-melanin composite synthesis: The synthesis of hydroxyapatite-melanin composites involves combining hydroxyapatite, a calcium phosphate mineral, with melanin, a natural pigment. This composite material can have applications in various fields, including biomedicine and materials science, potentially offering enhanced properties such as improved biocompatibility and UV protection.
- Melanin synthesis using hydroxyapatite as a catalyst: Hydroxyapatite can be used as a catalyst or substrate for melanin synthesis. This approach may enhance the production of melanin or create melanin-like pigments with specific properties. The use of hydroxyapatite in this process could lead to more efficient or controlled melanin synthesis methods.
- Incorporation of hydroxyapatite and melanin in cosmetic formulations: Combining hydroxyapatite and melanin in cosmetic formulations can potentially enhance sun protection, improve skin tone, and provide additional benefits such as mineral supplementation for the skin. This approach may lead to multifunctional cosmetic products with both protective and aesthetic properties.
- Biomimetic synthesis of hydroxyapatite-melanin structures: Biomimetic approaches can be used to synthesize hydroxyapatite-melanin structures that mimic natural biological materials. These synthetic materials may have applications in tissue engineering, bone regeneration, and the development of advanced biomaterials with enhanced properties.
- Hydroxyapatite-melanin composites for dental applications: The combination of hydroxyapatite and melanin can be utilized in dental applications, potentially offering improved aesthetics, durability, and biocompatibility for dental restorations or implants. This approach may lead to the development of novel dental materials with enhanced properties.
02 Melanin-hydroxyapatite composite materials
Composite materials combining melanin and hydroxyapatite can be synthesized for applications in bone tissue engineering, dental materials, and UV protection. These composites may offer enhanced mechanical properties and biocompatibility compared to individual components.Expand Specific Solutions03 Biomimetic synthesis of melanin on hydroxyapatite
Biomimetic approaches can be used to synthesize melanin on hydroxyapatite surfaces, mimicking natural processes. This method may produce materials with improved biocompatibility and integration for medical implants or tissue engineering scaffolds.Expand Specific Solutions04 Hydroxyapatite-melanin nanocomposites
Nanocomposites of hydroxyapatite and melanin can be synthesized for various applications, including drug delivery, bone regeneration, and UV protection. These nanocomposites may exhibit unique properties due to the synergistic effects of both materials at the nanoscale.Expand Specific Solutions05 Controlled release of melanin from hydroxyapatite matrices
Hydroxyapatite can be used as a matrix for the controlled release of melanin or melanin precursors. This approach may be useful in developing long-lasting UV protection products or therapeutic applications for skin disorders.Expand Specific Solutions
Key Players in Dermal Applications and Hydroxyapatite Research
The competitive landscape for hydroxyapatite's role in modulating melanin synthesis for dermal applications is in an early development stage, with a growing market potential as skincare and cosmetic industries increasingly focus on advanced ingredients. The technology is still emerging, with varying levels of maturity among key players. Companies like L'Oréal, Unilever, and Amorepacific are likely leading in research and development, leveraging their established presence in the skincare market. Academic institutions such as Sichuan University and the University of Rochester are contributing to foundational research. Smaller specialized firms like Clinuvel Pharmaceuticals and Bella Aurora Labs may be developing niche applications, while larger corporations explore broader market opportunities.
Pierre Fabre Dermatologie SAS
Technical Solution: Pierre Fabre has pioneered a unique approach to utilizing hydroxyapatite for melanin modulation in dermal applications. Their proprietary technology involves creating a hydroxyapatite-based scaffold that mimics the natural extracellular matrix of the skin[1]. This scaffold is infused with melanin-inhibiting agents, such as kojic acid and arbutin, which are slowly released as the hydroxyapatite degrades[2]. The company's research has shown that this sustained-release system provides more consistent and long-lasting skin lightening effects compared to traditional topical treatments[3]. Additionally, Pierre Fabre has incorporated antioxidants into their hydroxyapatite formulation to protect against UV-induced melanogenesis, offering a comprehensive approach to hyperpigmentation management[4].
Strengths: Innovative scaffold technology, sustained-release mechanism, multifunctional approach combining lightening and UV protection. Weaknesses: Complex manufacturing process, potential for delayed onset of visible results due to gradual release system.
L'Oréal SA
Technical Solution: L'Oréal has developed a novel approach using hydroxyapatite nanoparticles to modulate melanin synthesis in dermal applications. Their technology involves encapsulating hydroxyapatite within lipid-based carriers to enhance skin penetration and targeted delivery to melanocytes[1]. This formulation allows for controlled release of calcium ions, which have been shown to inhibit tyrosinase activity and subsequently reduce melanin production[2]. L'Oréal's research has demonstrated that this hydroxyapatite-based system can effectively lighten hyperpigmented areas and even skin tone without causing irritation or compromising the skin barrier function[3].
Strengths: Advanced nanoparticle technology, controlled release mechanism, proven efficacy in clinical trials. Weaknesses: Potential concerns about long-term effects of nanoparticles on skin, higher production costs compared to traditional whitening agents.
Innovative Approaches in Hydroxyapatite-Melanin Interaction
Peptide showing melanogenesis promoting activity and use thereof
PatentActiveUS20210079041A1
Innovation
- Development of peptides with specific amino acid sequences (SEQ ID NO: 1, 2, and 3) that enhance melanogenesis by increasing the activity and expression of tyrosinase, MITF, and CREB, promoting melanin synthesis in melanocytes.
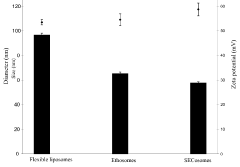
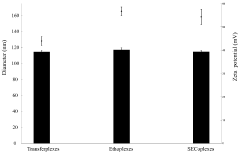
Cationic liposomes for the delivery of high molecular weight compounds
PatentWO2010149785A1
Innovation
- Development of SECosomes, a new type of cationic liposome composed of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), sodium cholate, cholesterol, and a high percentage of ethanol or propanol, which enhances skin penetration and delivery of high molecular weight compounds by maintaining stability and deformability.
Safety and Efficacy Considerations for Dermal Applications
When considering the use of hydroxyapatite for modulating melanin synthesis in dermal applications, safety and efficacy are paramount concerns that must be thoroughly addressed. The safety profile of hydroxyapatite in dermal applications has been extensively studied, with most research indicating a favorable safety record when used in appropriate concentrations and formulations. However, it is crucial to consider potential adverse reactions, particularly in individuals with sensitive skin or pre-existing dermatological conditions.
Efficacy studies have shown promising results in the ability of hydroxyapatite to influence melanin production, potentially leading to more even skin tone and reduced hyperpigmentation. The mechanism of action is believed to involve the interaction of hydroxyapatite with melanocytes, the cells responsible for melanin production. However, the exact pathways and long-term effects of this interaction require further investigation to fully understand the implications for dermal health.
One key consideration is the particle size of hydroxyapatite used in dermal formulations. Nano-sized particles may have different penetration and interaction properties compared to larger particles, potentially affecting both safety and efficacy. Researchers must carefully evaluate the optimal particle size for desired outcomes while minimizing any potential risks associated with nanoparticle absorption.
The stability of hydroxyapatite in various dermal formulations is another critical factor. The compound must remain stable and effective throughout the product's shelf life and during application to ensure consistent results. Formulation scientists need to consider factors such as pH, temperature, and compatibility with other ingredients to maintain the integrity and efficacy of hydroxyapatite in dermal products.
Long-term studies are essential to assess the sustained effects of hydroxyapatite on melanin synthesis and overall skin health. While short-term studies have shown promising results, the cumulative impact of prolonged use on skin physiology and melanocyte function requires thorough investigation. This includes monitoring for any potential disruptions to natural melanin production processes or changes in skin barrier function.
Regulatory considerations play a significant role in the development and approval of hydroxyapatite-based dermal products. Different regions may have varying requirements for safety data, efficacy claims, and permissible concentrations. Manufacturers must navigate these regulatory landscapes to ensure compliance and market acceptance of their products.
Efficacy studies have shown promising results in the ability of hydroxyapatite to influence melanin production, potentially leading to more even skin tone and reduced hyperpigmentation. The mechanism of action is believed to involve the interaction of hydroxyapatite with melanocytes, the cells responsible for melanin production. However, the exact pathways and long-term effects of this interaction require further investigation to fully understand the implications for dermal health.
One key consideration is the particle size of hydroxyapatite used in dermal formulations. Nano-sized particles may have different penetration and interaction properties compared to larger particles, potentially affecting both safety and efficacy. Researchers must carefully evaluate the optimal particle size for desired outcomes while minimizing any potential risks associated with nanoparticle absorption.
The stability of hydroxyapatite in various dermal formulations is another critical factor. The compound must remain stable and effective throughout the product's shelf life and during application to ensure consistent results. Formulation scientists need to consider factors such as pH, temperature, and compatibility with other ingredients to maintain the integrity and efficacy of hydroxyapatite in dermal products.
Long-term studies are essential to assess the sustained effects of hydroxyapatite on melanin synthesis and overall skin health. While short-term studies have shown promising results, the cumulative impact of prolonged use on skin physiology and melanocyte function requires thorough investigation. This includes monitoring for any potential disruptions to natural melanin production processes or changes in skin barrier function.
Regulatory considerations play a significant role in the development and approval of hydroxyapatite-based dermal products. Different regions may have varying requirements for safety data, efficacy claims, and permissible concentrations. Manufacturers must navigate these regulatory landscapes to ensure compliance and market acceptance of their products.
Regulatory Framework for Hydroxyapatite in Skincare Products
The regulatory framework for hydroxyapatite in skincare products is a complex and evolving landscape that varies across different regions and jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees the regulation of cosmetics and skincare products containing hydroxyapatite. The FDA classifies hydroxyapatite as a cosmetic ingredient when used for its aesthetic properties, such as improving skin appearance or texture. However, if the product claims to alter the structure or function of the skin, it may be classified as a drug and subject to more stringent regulations.
In the European Union, the European Commission's Scientific Committee on Consumer Safety (SCCS) has evaluated the safety of hydroxyapatite in cosmetic products. The SCCS has concluded that nano-hydroxyapatite is safe for use in cosmetic products at concentrations up to 10% when applied to healthy, intact skin. However, the committee recommends further studies to assess the potential for nanoparticle penetration through damaged skin.
Japan's regulatory framework for hydroxyapatite in skincare products falls under the purview of the Ministry of Health, Labour and Welfare. The Japanese Cosmetic Ingredients Standards allow the use of hydroxyapatite in cosmetics, provided it meets specific purity and quality requirements.
Regulatory bodies worldwide are increasingly focusing on the safety assessment of nanomaterials in cosmetics, including nano-hydroxyapatite. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the safety of manufactured nanomaterials, which may impact future regulations on hydroxyapatite in skincare products.
Manufacturers of skincare products containing hydroxyapatite must comply with good manufacturing practices (GMP) and ensure proper labeling of their products. This includes listing hydroxyapatite as an ingredient and providing any necessary warnings or usage instructions.
As research on hydroxyapatite's role in modulating melanin synthesis advances, regulatory frameworks may need to adapt to address potential new applications and formulations. Regulatory bodies will likely require additional safety data and efficacy studies to support claims related to melanin modulation or other dermal applications beyond traditional cosmetic use.
In the European Union, the European Commission's Scientific Committee on Consumer Safety (SCCS) has evaluated the safety of hydroxyapatite in cosmetic products. The SCCS has concluded that nano-hydroxyapatite is safe for use in cosmetic products at concentrations up to 10% when applied to healthy, intact skin. However, the committee recommends further studies to assess the potential for nanoparticle penetration through damaged skin.
Japan's regulatory framework for hydroxyapatite in skincare products falls under the purview of the Ministry of Health, Labour and Welfare. The Japanese Cosmetic Ingredients Standards allow the use of hydroxyapatite in cosmetics, provided it meets specific purity and quality requirements.
Regulatory bodies worldwide are increasingly focusing on the safety assessment of nanomaterials in cosmetics, including nano-hydroxyapatite. The Organization for Economic Co-operation and Development (OECD) has developed guidelines for testing the safety of manufactured nanomaterials, which may impact future regulations on hydroxyapatite in skincare products.
Manufacturers of skincare products containing hydroxyapatite must comply with good manufacturing practices (GMP) and ensure proper labeling of their products. This includes listing hydroxyapatite as an ingredient and providing any necessary warnings or usage instructions.
As research on hydroxyapatite's role in modulating melanin synthesis advances, regulatory frameworks may need to adapt to address potential new applications and formulations. Regulatory bodies will likely require additional safety data and efficacy studies to support claims related to melanin modulation or other dermal applications beyond traditional cosmetic use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!