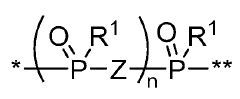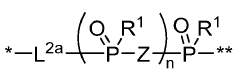Future of NMN in Therapeutic Applications
NMN Background and Objectives
Nicotinamide mononucleotide (NMN) has emerged as a promising molecule in the field of anti-aging and therapeutic applications. The development of NMN as a therapeutic agent traces back to the early 2000s when researchers began to unravel the importance of NAD+ in cellular function and longevity. Subsequent studies in animal models demonstrated NMN's ability to improve various aspects of health, including metabolism, cardiovascular function, and cognitive performance. These findings sparked a wave of research aimed at translating these benefits to human applications.
Looking towards the future of NMN in therapeutic applications, several key objectives have been identified. Firstly, there is a pressing need to conduct large-scale, long-term clinical trials to definitively establish the safety and efficacy of NMN supplementation in humans. While preliminary studies have shown promising results, more robust evidence is required to support its widespread use as a therapeutic agent.
Another critical objective is to optimize NMN delivery methods. Current oral supplementation faces challenges related to bioavailability and stability. Researchers are exploring novel formulations and delivery systems, such as nanoencapsulation and transdermal patches, to enhance NMN's therapeutic potential. Additionally, there is a focus on developing targeted delivery mechanisms to ensure NMN reaches specific tissues or organs where it is most needed.
Understanding the molecular mechanisms underlying NMN's therapeutic effects is another key goal. While its role in NAD+ biosynthesis is well-established, researchers are investigating other potential pathways through which NMN may exert its benefits. This includes exploring its impact on sirtuins, DNA repair mechanisms, and mitochondrial function.
The therapeutic applications of NMN are expanding beyond anti-aging. Researchers are investigating its potential in treating specific age-related diseases, such as Alzheimer's, diabetes, and cardiovascular disorders. The objective is to develop NMN-based interventions that can effectively prevent or mitigate these conditions, potentially revolutionizing the approach to age-related healthcare.
As the field progresses, there is also a growing emphasis on personalized medicine approaches. The goal is to identify genetic, epigenetic, and metabolic factors that influence an individual's response to NMN supplementation. This will enable the development of tailored therapeutic strategies, optimizing the benefits of NMN for each patient.
NMN Market Analysis
The NMN (Nicotinamide Mononucleotide) market has experienced significant growth in recent years, driven by increasing consumer awareness of its potential health benefits and the growing demand for anti-aging supplements. As a key precursor to NAD+ (Nicotinamide Adenine Dinucleotide), NMN has garnered attention for its role in cellular energy production and potential therapeutic applications.
The global NMN market is primarily segmented into dietary supplements, pharmaceuticals, and cosmetics. The dietary supplement segment currently dominates the market, with consumers seeking NMN products for their purported anti-aging and health-promoting effects. The pharmaceutical segment is expected to witness substantial growth in the coming years as research into NMN's therapeutic potential advances.
Geographically, North America and Asia-Pacific regions lead the NMN market, with the United States and Japan being the largest consumers. The market in these regions is characterized by a high level of health consciousness and a willingness to adopt innovative nutritional supplements. Europe is also emerging as a significant market for NMN products, driven by an aging population and increasing interest in preventive healthcare.
The NMN market is highly competitive, with several key players vying for market share. These include established pharmaceutical companies, nutraceutical manufacturers, and specialized anti-aging supplement producers. The market is also seeing an influx of new entrants, particularly in the direct-to-consumer segment, leveraging e-commerce platforms to reach a broader customer base.
Consumer demand for NMN products is influenced by factors such as aging demographics, rising healthcare costs, and a growing emphasis on preventive health measures. The increasing prevalence of age-related diseases and conditions has further fueled interest in NMN as a potential therapeutic agent.
However, the NMN market faces challenges, including regulatory uncertainties, high production costs, and the need for more extensive clinical research to substantiate health claims. The lack of standardization in product quality and dosage across the industry also presents concerns for consumers and healthcare professionals alike.
Despite these challenges, the future outlook for the NMN market remains positive. Ongoing research into NMN's therapeutic applications, particularly in areas such as cardiovascular health, metabolic disorders, and neurodegenerative diseases, is expected to open new avenues for market growth. Additionally, advancements in production technologies may lead to reduced costs and improved product quality, potentially expanding market accessibility.
NMN Research Challenges
Despite the promising potential of Nicotinamide Mononucleotide (NMN) in therapeutic applications, several significant research challenges persist. These challenges span various aspects of NMN research, from fundamental biological mechanisms to clinical applications.
One of the primary challenges is fully elucidating the molecular mechanisms by which NMN exerts its beneficial effects. While it is known that NMN is a precursor to NAD+, the complete pathway of its action, including potential off-target effects and interactions with other cellular processes, remains to be fully understood. This gap in knowledge hinders the development of targeted therapies and optimal dosing strategies.
Another major hurdle is the limited bioavailability of NMN. Oral administration, while convenient, faces challenges in terms of absorption and distribution. The compound's stability in the gastrointestinal tract and its ability to cross cellular membranes effectively are areas that require further investigation. Developing novel delivery methods or chemical modifications to enhance bioavailability is crucial for maximizing NMN's therapeutic potential.
The long-term effects of NMN supplementation also present a significant research challenge. While short-term studies have shown promising results, the consequences of prolonged NMN use, particularly in diverse populations and age groups, remain unclear. This includes potential side effects, drug interactions, and the impact on various physiological systems over extended periods.
Translating preclinical findings to human applications poses another substantial challenge. The efficacy and safety profiles observed in animal models may not directly translate to human subjects due to differences in metabolism, genetic factors, and environmental influences. Conducting well-designed, large-scale clinical trials to validate NMN's therapeutic effects in humans is essential but faces logistical and financial hurdles.
Furthermore, the development of standardized, high-quality NMN production methods presents a technical challenge. Ensuring consistent purity, stability, and potency of NMN for research and therapeutic use is crucial for reliable results and potential commercialization. This involves optimizing synthesis processes, developing robust quality control measures, and establishing regulatory standards.
Lastly, the complex interplay between NMN and various age-related diseases presents a multifaceted research challenge. Understanding how NMN supplementation affects different pathological conditions, and whether its effects are uniform across various diseases or tissue-specific, requires extensive investigation. This complexity is further compounded by individual variations in metabolism and genetic predispositions, necessitating personalized approaches to NMN-based therapies.
Current NMN Applications
01 NMN synthesis and production methods
Various methods for synthesizing and producing Nicotinamide Mononucleotide (NMN) are described. These include enzymatic synthesis, chemical synthesis, and biotechnological approaches. The methods aim to improve yield, purity, and cost-effectiveness of NMN production for use in supplements and pharmaceutical applications.- NMN synthesis and production methods: Various methods for synthesizing and producing Nicotinamide Mononucleotide (NMN) are described. These include enzymatic synthesis, chemical synthesis, and biotechnological approaches. The methods aim to improve yield, purity, and cost-effectiveness of NMN production for use in supplements and pharmaceuticals.
- NMN formulations and delivery systems: Different formulations and delivery systems for NMN are developed to enhance its bioavailability and stability. These include oral formulations, transdermal patches, sublingual tablets, and novel drug delivery systems. The formulations aim to improve the absorption and efficacy of NMN in the body.
- NMN applications in anti-aging and health: NMN is investigated for its potential anti-aging effects and health benefits. Research focuses on its role in improving cellular energy metabolism, DNA repair, and overall longevity. Applications include treatments for age-related diseases, metabolic disorders, and cognitive decline.
- NMN combination therapies: Combination therapies involving NMN and other compounds are explored to enhance therapeutic effects. These combinations may include other NAD+ precursors, antioxidants, or complementary nutrients. The aim is to create synergistic effects for improved health outcomes and disease prevention.
- NMN quality control and analysis methods: Techniques for quality control and analysis of NMN are developed to ensure product purity and efficacy. These include spectroscopic methods, chromatography, and other analytical techniques to detect impurities, assess stability, and verify the concentration of NMN in various formulations.
02 NMN formulations and delivery systems
Different formulations and delivery systems for NMN are developed to enhance its bioavailability and stability. These include oral formulations, transdermal patches, sublingual tablets, and novel drug delivery systems. The formulations aim to improve the absorption and efficacy of NMN in the body.Expand Specific Solutions03 NMN applications in anti-aging and health promotion
NMN is investigated for its potential anti-aging effects and health-promoting properties. Research focuses on its role in improving cellular energy metabolism, DNA repair, and overall longevity. Applications include supplements for healthy aging, sports nutrition, and potential treatments for age-related diseases.Expand Specific Solutions04 NMN combination therapies and synergistic effects
Studies explore the combination of NMN with other compounds to enhance its therapeutic effects. These combinations aim to create synergistic effects for various health applications, including metabolic disorders, cardiovascular health, and cognitive function improvement.Expand Specific Solutions05 NMN quality control and analytical methods
Development of quality control measures and analytical methods for NMN to ensure product purity and consistency. This includes techniques for detecting impurities, quantifying NMN content, and assessing stability in various formulations. These methods are crucial for maintaining high standards in NMN production and research.Expand Specific Solutions
Key NMN Industry Players
The future of NMN in therapeutic applications is in a nascent stage, with the market showing significant growth potential. The industry is transitioning from research to early commercialization, driven by increasing interest in anti-aging and metabolic health solutions. While the market size is expanding, it remains relatively small compared to established pharmaceutical sectors. Technologically, NMN applications are still evolving, with varying levels of maturity across different companies. Key players like Metro International Biotech, Washington University in St. Louis, and Harvard College are at the forefront of research, while companies such as Meiji Holdings and Bontac Bio-Engineering are focusing on commercial development. The competitive landscape is diverse, with academic institutions, biotech startups, and established pharmaceutical companies all vying for breakthroughs in NMN-based therapies.
Metro International Biotech LLC
Meiji Holdings Co., Ltd.
NMN Mechanism Insights
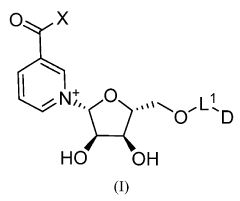
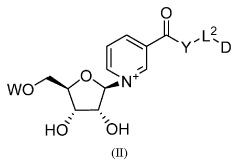
- Administration of nicotinamide mononucleotide (NMN) through various compositions and routes, including oral and intraocular administration, to treat and prevent age-associated degenerative changes by enhancing NAD+ levels and promoting cellular health.
- Development of compounds comprising an NAD moiety linked to an antiviral moiety, specifically represented by certain structures, which can be administered to increase NAD levels and treat viral infections by enhancing the body's natural NAD synthesis and providing antiviral activity.
Regulatory Framework for NMN
The regulatory framework for NMN (Nicotinamide Mononucleotide) is a complex and evolving landscape that significantly impacts its future in therapeutic applications. Currently, the regulatory status of NMN varies across different regions and countries, creating challenges for its widespread adoption in medical treatments.
In the United States, the Food and Drug Administration (FDA) has not yet approved NMN as a drug or dietary supplement. In 2022, the FDA issued a statement indicating that NMN does not meet the definition of a dietary supplement under the Federal Food, Drug, and Cosmetic Act. This decision has created uncertainty in the market and has led to increased scrutiny of NMN-containing products.
Japan, on the other hand, has taken a more progressive approach. The country has approved NMN as a food ingredient, allowing its use in various products. This regulatory environment has made Japan a hub for NMN research and product development, potentially giving it a competitive edge in the global market.
The European Union (EU) has yet to establish a clear regulatory framework for NMN. Currently, it falls under the Novel Food Regulation, requiring safety assessments before market authorization. This process can be lengthy and costly, potentially slowing down the adoption of NMN-based therapies in EU countries.
China has shown increasing interest in NMN research and development. While specific regulations for NMN are still evolving, the country's focus on anti-aging therapies may lead to a more favorable regulatory environment in the future.
As research continues to demonstrate the potential health benefits of NMN, regulatory bodies worldwide are likely to face increasing pressure to establish clear guidelines. The future regulatory framework for NMN will need to balance safety concerns with the potential therapeutic benefits, considering factors such as dosage, long-term effects, and quality control measures.
To advance the regulatory status of NMN, more extensive clinical trials and safety studies will be crucial. These studies will need to address concerns about potential side effects, interactions with other medications, and long-term impacts on health. Additionally, standardization of NMN production and quality control measures will be essential to ensure consistency and safety across different products and manufacturers.
The development of a harmonized international regulatory framework for NMN could significantly accelerate its adoption in therapeutic applications. This would involve collaboration between regulatory agencies, researchers, and industry stakeholders to establish common standards and approval processes.
NMN Safety and Efficacy
The safety and efficacy of Nicotinamide Mononucleotide (NMN) are crucial factors in determining its future therapeutic applications. Extensive research has been conducted to evaluate the potential benefits and risks associated with NMN supplementation.
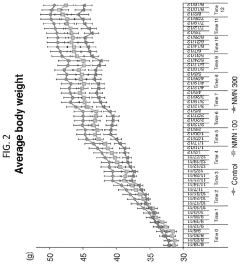
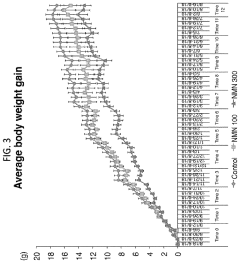
Numerous preclinical studies have demonstrated the safety profile of NMN in various animal models. These studies have shown that NMN administration, even at high doses, does not produce significant adverse effects. Long-term studies in mice have reported no observable toxicity or negative impacts on overall health, suggesting a favorable safety profile for chronic use.
Human clinical trials, although limited in number, have also provided encouraging results regarding NMN safety. Short-term studies have shown that oral NMN supplementation is well-tolerated, with no serious adverse events reported. However, more extensive long-term human studies are needed to fully establish the safety of NMN for prolonged use in diverse populations.
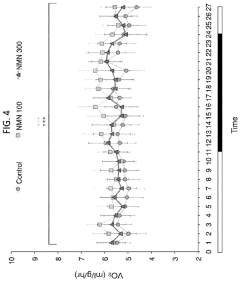
Regarding efficacy, NMN has shown promising results in preclinical models for various age-related conditions. Studies have demonstrated improvements in metabolic health, cardiovascular function, and cognitive performance in aged animals treated with NMN. These findings suggest potential therapeutic applications in age-related diseases and disorders.
Human clinical trials, while still in early stages, have begun to explore NMN's efficacy in various health conditions. Initial results indicate potential benefits in areas such as insulin sensitivity, muscle strength, and cognitive function. However, larger, well-controlled clinical trials are necessary to confirm these preliminary findings and establish optimal dosing regimens.
It is important to note that the long-term effects of NMN supplementation in humans remain unclear. While animal studies suggest sustained benefits without apparent side effects, human data on extended use is limited. This gap in knowledge emphasizes the need for continued research to fully understand the long-term safety and efficacy of NMN in therapeutic applications.
As research progresses, regulatory considerations will play a crucial role in determining the future of NMN as a therapeutic agent. Current regulatory status varies by country, with some classifying NMN as a dietary supplement and others considering it a novel food ingredient. Establishing a clear regulatory framework for NMN will be essential for its development as a potential therapeutic intervention.