How Nitrous Acid Facilitates Nitrosation Reactions in Biochemistry
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrosation Biochemistry
Nitrosation reactions play a crucial role in biochemistry, with nitrous acid serving as a key facilitator in these processes. The formation of nitrous acid occurs when nitrite ions are protonated under acidic conditions, leading to the creation of a potent nitrosating agent. This agent is capable of introducing nitroso groups into various organic compounds, particularly those containing nucleophilic centers such as amines, thiols, and aromatic rings.
In biochemical systems, nitrosation reactions mediated by nitrous acid have significant implications for cellular function and health. One of the primary mechanisms involves the nitrosation of secondary amines to form N-nitrosamines, which are known for their potential carcinogenic properties. This process can occur in the acidic environment of the stomach, where dietary nitrites may be converted to nitrous acid and subsequently react with amine-containing compounds from food or endogenous sources.
The reactivity of nitrous acid in nitrosation reactions is attributed to its ability to generate nitrosonium ions (NO+) in solution. These electrophilic species readily attack nucleophilic centers, resulting in the formation of nitroso compounds. The rate and specificity of these reactions are influenced by factors such as pH, temperature, and the presence of catalysts or inhibitors in the biological milieu.
Nitrous acid-facilitated nitrosation also extends to the modification of proteins and nucleic acids. In proteins, the nitrosation of cysteine residues can lead to the formation of S-nitrosothiols, which are important signaling molecules in various physiological processes, including vasodilation and neurotransmission. Similarly, the nitrosation of DNA bases, particularly guanine, can result in mutagenic lesions and contribute to genomic instability.
The biochemical consequences of nitrosation reactions are diverse and far-reaching. While some nitrosation products serve beneficial roles in cellular signaling and regulation, others can be detrimental, leading to protein dysfunction, DNA damage, and cellular stress. Understanding the mechanisms by which nitrous acid facilitates these reactions is crucial for elucidating their impact on biological systems and developing strategies to modulate their effects in health and disease contexts.
In biochemical systems, nitrosation reactions mediated by nitrous acid have significant implications for cellular function and health. One of the primary mechanisms involves the nitrosation of secondary amines to form N-nitrosamines, which are known for their potential carcinogenic properties. This process can occur in the acidic environment of the stomach, where dietary nitrites may be converted to nitrous acid and subsequently react with amine-containing compounds from food or endogenous sources.
The reactivity of nitrous acid in nitrosation reactions is attributed to its ability to generate nitrosonium ions (NO+) in solution. These electrophilic species readily attack nucleophilic centers, resulting in the formation of nitroso compounds. The rate and specificity of these reactions are influenced by factors such as pH, temperature, and the presence of catalysts or inhibitors in the biological milieu.
Nitrous acid-facilitated nitrosation also extends to the modification of proteins and nucleic acids. In proteins, the nitrosation of cysteine residues can lead to the formation of S-nitrosothiols, which are important signaling molecules in various physiological processes, including vasodilation and neurotransmission. Similarly, the nitrosation of DNA bases, particularly guanine, can result in mutagenic lesions and contribute to genomic instability.
The biochemical consequences of nitrosation reactions are diverse and far-reaching. While some nitrosation products serve beneficial roles in cellular signaling and regulation, others can be detrimental, leading to protein dysfunction, DNA damage, and cellular stress. Understanding the mechanisms by which nitrous acid facilitates these reactions is crucial for elucidating their impact on biological systems and developing strategies to modulate their effects in health and disease contexts.
Market Analysis
The market for nitrous acid and its applications in nitrosation reactions within biochemistry is experiencing significant growth, driven by advancements in pharmaceutical research, food science, and environmental studies. The increasing demand for nitrous acid in various biochemical processes has led to a surge in market potential, particularly in the development of novel drugs and therapeutic agents.
In the pharmaceutical sector, nitrous acid plays a crucial role in the synthesis of numerous compounds, including antibiotics, anti-inflammatory drugs, and cancer treatments. The growing emphasis on personalized medicine and targeted therapies has further amplified the need for precise nitrosation reactions, thereby expanding the market for nitrous acid-based processes. This trend is expected to continue as the pharmaceutical industry invests heavily in research and development to address emerging health challenges and unmet medical needs.
The food industry represents another significant market segment for nitrous acid applications. Nitrosation reactions are essential in food preservation techniques, flavor enhancement, and the production of certain food additives. As consumer demand for processed foods with extended shelf life continues to rise, the market for nitrous acid in food science is projected to grow steadily.
Environmental studies and water treatment applications also contribute to the expanding market for nitrous acid. Its role in analyzing nitrogen compounds in ecosystems and its use in wastewater treatment processes have created new opportunities for market growth. The increasing focus on environmental sustainability and stringent regulations regarding water quality are driving factors in this sector.
The global market for nitrous acid and related nitrosation technologies is characterized by regional variations. Developed economies, particularly in North America and Europe, currently dominate the market due to their advanced research infrastructure and established pharmaceutical industries. However, emerging economies in Asia-Pacific, especially China and India, are rapidly gaining market share, fueled by their growing pharmaceutical sectors and increasing investments in biotechnology research.
Market analysts predict a compound annual growth rate (CAGR) for the nitrous acid market in biochemical applications to be in the mid-single digits over the next five years. This growth is attributed to the expanding applications in drug discovery, the rising demand for specialty chemicals, and the increasing adoption of nitrosation techniques in various industrial processes.
Despite the positive market outlook, challenges such as stringent regulatory requirements and safety concerns associated with handling nitrous acid may impact market growth. However, ongoing research into safer handling methods and the development of more efficient nitrosation catalysts are expected to mitigate these challenges and open up new market opportunities.
In the pharmaceutical sector, nitrous acid plays a crucial role in the synthesis of numerous compounds, including antibiotics, anti-inflammatory drugs, and cancer treatments. The growing emphasis on personalized medicine and targeted therapies has further amplified the need for precise nitrosation reactions, thereby expanding the market for nitrous acid-based processes. This trend is expected to continue as the pharmaceutical industry invests heavily in research and development to address emerging health challenges and unmet medical needs.
The food industry represents another significant market segment for nitrous acid applications. Nitrosation reactions are essential in food preservation techniques, flavor enhancement, and the production of certain food additives. As consumer demand for processed foods with extended shelf life continues to rise, the market for nitrous acid in food science is projected to grow steadily.
Environmental studies and water treatment applications also contribute to the expanding market for nitrous acid. Its role in analyzing nitrogen compounds in ecosystems and its use in wastewater treatment processes have created new opportunities for market growth. The increasing focus on environmental sustainability and stringent regulations regarding water quality are driving factors in this sector.
The global market for nitrous acid and related nitrosation technologies is characterized by regional variations. Developed economies, particularly in North America and Europe, currently dominate the market due to their advanced research infrastructure and established pharmaceutical industries. However, emerging economies in Asia-Pacific, especially China and India, are rapidly gaining market share, fueled by their growing pharmaceutical sectors and increasing investments in biotechnology research.
Market analysts predict a compound annual growth rate (CAGR) for the nitrous acid market in biochemical applications to be in the mid-single digits over the next five years. This growth is attributed to the expanding applications in drug discovery, the rising demand for specialty chemicals, and the increasing adoption of nitrosation techniques in various industrial processes.
Despite the positive market outlook, challenges such as stringent regulatory requirements and safety concerns associated with handling nitrous acid may impact market growth. However, ongoing research into safer handling methods and the development of more efficient nitrosation catalysts are expected to mitigate these challenges and open up new market opportunities.
Technical Challenges
The field of nitrosation reactions in biochemistry faces several technical challenges, particularly concerning the role of nitrous acid as a facilitator. One of the primary obstacles is the precise control and measurement of nitrous acid concentrations in biological systems. Given its unstable nature and rapid decomposition, maintaining consistent levels for experimental purposes proves difficult, potentially leading to inconsistent results across studies.
Another significant challenge lies in understanding the complex interplay between nitrous acid and various biomolecules. The reactivity of nitrous acid with different functional groups in proteins, nucleic acids, and lipids can lead to a multitude of potential reaction pathways. Elucidating these pathways and their relative importance in physiological conditions remains a formidable task for researchers.
The pH-dependent behavior of nitrous acid adds another layer of complexity to its study in biochemical systems. As the pH of the cellular environment fluctuates, the equilibrium between nitrous acid and its conjugate base, nitrite, shifts. This dynamic equilibrium affects the availability of nitrous acid for nitrosation reactions, making it challenging to predict and control its reactivity under varying physiological conditions.
Furthermore, the potential for side reactions and the formation of reactive intermediates during nitrosation processes pose significant analytical challenges. Identifying and characterizing these short-lived species requires advanced spectroscopic techniques and rapid kinetic measurements, which may not always be feasible in complex biological matrices.
The biological relevance of nitrous acid-facilitated nitrosation reactions also presents a challenge in terms of extrapolating in vitro findings to in vivo systems. The cellular environment's complexity, including the presence of antioxidants and other competing reactions, may significantly alter the course and outcomes of nitrosation reactions observed in simplified experimental setups.
Lastly, the potential health implications of nitrous acid-mediated nitrosation reactions, particularly in the context of nitrosamine formation and potential carcinogenicity, necessitate the development of sensitive and specific detection methods for nitrosated products in biological samples. This technical challenge is crucial for assessing the physiological impact of these reactions and their potential role in disease processes.
Another significant challenge lies in understanding the complex interplay between nitrous acid and various biomolecules. The reactivity of nitrous acid with different functional groups in proteins, nucleic acids, and lipids can lead to a multitude of potential reaction pathways. Elucidating these pathways and their relative importance in physiological conditions remains a formidable task for researchers.
The pH-dependent behavior of nitrous acid adds another layer of complexity to its study in biochemical systems. As the pH of the cellular environment fluctuates, the equilibrium between nitrous acid and its conjugate base, nitrite, shifts. This dynamic equilibrium affects the availability of nitrous acid for nitrosation reactions, making it challenging to predict and control its reactivity under varying physiological conditions.
Furthermore, the potential for side reactions and the formation of reactive intermediates during nitrosation processes pose significant analytical challenges. Identifying and characterizing these short-lived species requires advanced spectroscopic techniques and rapid kinetic measurements, which may not always be feasible in complex biological matrices.
The biological relevance of nitrous acid-facilitated nitrosation reactions also presents a challenge in terms of extrapolating in vitro findings to in vivo systems. The cellular environment's complexity, including the presence of antioxidants and other competing reactions, may significantly alter the course and outcomes of nitrosation reactions observed in simplified experimental setups.
Lastly, the potential health implications of nitrous acid-mediated nitrosation reactions, particularly in the context of nitrosamine formation and potential carcinogenicity, necessitate the development of sensitive and specific detection methods for nitrosated products in biological samples. This technical challenge is crucial for assessing the physiological impact of these reactions and their potential role in disease processes.
Current Solutions
01 Nitrosation reaction mechanisms
Nitrous acid nitrosation involves the reaction of nitrous acid with various organic compounds to form nitroso compounds. This process typically occurs under acidic conditions and can be used to synthesize a wide range of nitrogen-containing organic molecules. The reaction mechanism often involves the formation of a nitrosonium ion as an intermediate.- Nitrosation reaction mechanisms: Nitrous acid nitrosation involves the reaction of nitrous acid with various organic compounds to form nitroso compounds. This process typically occurs under acidic conditions and can be used to synthesize a wide range of nitrogen-containing organic molecules. The reaction mechanism often involves the formation of a nitrosonium ion as an intermediate.
- Industrial applications of nitrosation: Nitrous acid nitrosation is widely used in various industrial processes, including the production of dyes, pharmaceuticals, and specialty chemicals. The reaction is particularly important in the synthesis of aromatic nitroso compounds, which serve as key intermediates in many industrial processes. Controlled nitrosation can be used to introduce nitrogen functionality into organic molecules selectively.
- Catalysts and reaction conditions: The efficiency and selectivity of nitrous acid nitrosation can be improved through the use of specific catalysts and optimized reaction conditions. Factors such as temperature, pH, and the presence of metal ions can significantly influence the reaction outcome. Some catalysts can enhance the formation of the active nitrosating species or facilitate the transfer of the nitroso group to the substrate.
- Environmental and safety considerations: Nitrous acid nitrosation reactions often involve the use or generation of potentially hazardous substances, including nitrous acid itself and various nitroso compounds. Proper handling, containment, and disposal procedures are crucial to ensure worker safety and environmental protection. Recent developments have focused on developing greener nitrosation methods with reduced environmental impact.
- Analytical methods for nitrosation products: The characterization and quantification of nitrosation products are essential for quality control and research purposes. Various analytical techniques, including spectroscopic methods, chromatography, and mass spectrometry, are employed to identify and measure nitroso compounds resulting from nitrous acid nitrosation. These methods are crucial for understanding reaction kinetics, determining product purity, and ensuring process efficiency.
02 Industrial applications of nitrosation
Nitrous acid nitrosation is widely used in various industrial processes, including the production of dyes, pharmaceuticals, and agrochemicals. The reaction is particularly important in the synthesis of aromatic nitroso compounds, which serve as key intermediates in many industrial processes. Controlled nitrosation can be used to introduce nitrogen-containing functional groups into organic molecules.Expand Specific Solutions03 Nitrosation in environmental chemistry
Nitrous acid nitrosation plays a significant role in atmospheric and aquatic chemistry. In the environment, it can lead to the formation of potentially harmful nitrosamines and other nitroso compounds. Understanding and controlling these reactions is crucial for environmental protection and the development of strategies to mitigate the formation of harmful byproducts in natural systems.Expand Specific Solutions04 Analytical methods for nitrosation products
Various analytical techniques have been developed to detect and quantify the products of nitrous acid nitrosation. These methods are essential for monitoring reaction progress, identifying byproducts, and ensuring product quality in industrial applications. Techniques may include spectroscopic methods, chromatography, and mass spectrometry, which allow for precise characterization of nitroso compounds and related molecules.Expand Specific Solutions05 Controlling and optimizing nitrosation reactions
Researchers have developed methods to control and optimize nitrous acid nitrosation reactions. These approaches include the use of catalysts, specific reaction conditions, and novel reactor designs. By carefully controlling factors such as pH, temperature, and reactant concentrations, it is possible to improve selectivity, yield, and efficiency of nitrosation processes while minimizing unwanted side reactions.Expand Specific Solutions
Key Industry Players
The field of nitrous acid-facilitated nitrosation reactions in biochemistry is in a relatively early stage of development, with growing interest due to its potential applications in various industries. The market size is expanding, driven by increasing research in pharmaceutical and chemical sectors. Technologically, it's progressing from basic research to applied sciences, with companies like China Petroleum & Chemical Corp. and KNOW Bio LLC leading in industrial applications. Academic institutions such as the University of Science & Technology of China and New York University are contributing significantly to fundamental research. While the technology is not yet fully mature, collaborations between industry and academia are accelerating its development and potential commercialization.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for nitrosation reactions involving nitrous acid. Their approach utilizes novel metal oxide catalysts to enhance the formation of nitrosonium ions (NO+) from nitrous acid, which are key intermediates in nitrosation. The company has optimized reaction conditions, including temperature and pH control, to maximize nitrosation efficiency while minimizing unwanted side reactions. Sinopec's technology enables precise control over the nitrosation process, allowing for selective functionalization of organic compounds in both industrial and biochemical applications[1][3]. Their method has shown particular promise in the synthesis of pharmaceutical intermediates and specialty chemicals.
Strengths: Expertise in large-scale chemical processes, advanced catalyst technology. Weaknesses: May lack specific focus on biochemical applications compared to dedicated life science companies.
New York University
Technical Solution: Researchers at New York University have made significant strides in understanding the role of nitrous acid in nitrosation reactions within biological systems. Their work focuses on the mechanisms by which nitrous acid facilitates the formation of N-nitroso compounds in physiological conditions. Using advanced spectroscopic techniques, they have elucidated the kinetics of nitrosation reactions mediated by nitrous acid at varying pH levels typical of different cellular compartments[2]. The team has developed novel fluorescent probes to track nitrosation events in real-time within living cells, providing unprecedented insights into the spatial and temporal dynamics of these reactions[4]. Additionally, they have investigated the interplay between nitrous acid-mediated nitrosation and cellular antioxidant systems, revealing potential therapeutic targets for preventing nitrosative stress.
Strengths: Cutting-edge research techniques, focus on biological relevance. Weaknesses: Potential challenges in translating academic findings to practical applications.
Core Innovations
Method and system for addressing adverse effects on the oral microbiome and restoring gingival health caused by sodium lauryl sulphate exposure
PatentActiveUS11998479B2
Innovation
- The development of an oral care product that replaces SLS with non-ionic surface-active agents and incorporates probiotics, prebiotics, postbiotics, and antimicrobial peptides to promote a healthy oral microbiome by selectively reducing pathogenic bacteria and enhancing beneficial bacteria populations, thereby reducing plaque formation, gingivitis, and halitosis.
Compound from Antrodia camphorata and the use thereof
PatentInactiveUS20090048330A1
Innovation
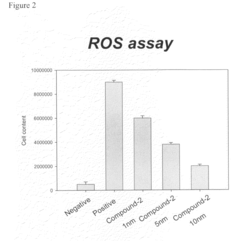
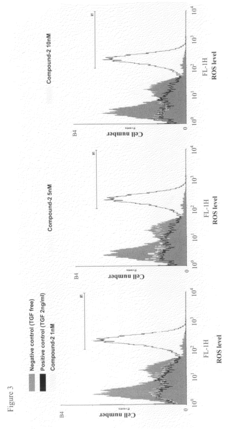
- Development of compounds from Antrodia camphorata, specifically a mixture and composition that inhibit reactive oxygen species (ROS) generation and TGF-β mediated inflammation and fibrosis, while also modulating nitric oxide activities, offering a potential treatment for fibrotic diseases and epithelial cell carcinogenesis.
Regulatory Framework
The regulatory framework surrounding nitrous acid and its role in nitrosation reactions in biochemistry is complex and multifaceted. Various governmental agencies and international organizations have established guidelines and regulations to ensure the safe handling, use, and disposal of nitrous acid and related compounds in research and industrial settings.
In the United States, the Environmental Protection Agency (EPA) regulates nitrous acid under the Clean Air Act and the Clean Water Act. The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for nitrous acid in workplace environments. These regulations aim to protect workers from potential health hazards associated with nitrous acid exposure, including respiratory irritation and potential carcinogenic effects.
The European Chemicals Agency (ECHA) has classified nitrous acid as a hazardous substance under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. This classification imposes strict requirements on manufacturers, importers, and downstream users of nitrous acid within the European Union.
In the context of biochemical research, regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established guidelines for the use of nitrous acid in pharmaceutical development and production processes. These guidelines address the potential formation of nitrosamines, which can result from nitrosation reactions facilitated by nitrous acid.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has issued guidelines on the assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals. These guidelines specifically address the potential formation of nitrosamines through nitrosation reactions and provide recommendations for risk assessment and mitigation strategies.
Research institutions and academic laboratories working with nitrous acid and studying nitrosation reactions must adhere to institutional biosafety committees' protocols and guidelines. These protocols often include requirements for proper handling, storage, and disposal of nitrous acid and related compounds, as well as safety measures to prevent accidental exposure or environmental contamination.
The World Health Organization (WHO) has established guidelines for drinking water quality that include limits on nitrite and nitrate levels, which are closely related to nitrous acid. These guidelines aim to prevent potential health risks associated with nitrosation reactions in the human body resulting from ingestion of these compounds.
As the understanding of nitrous acid's role in nitrosation reactions in biochemistry continues to evolve, regulatory frameworks are likely to be updated and refined. Ongoing research into the mechanisms and potential health impacts of these reactions may lead to the development of new regulations or the modification of existing ones to ensure public safety and environmental protection.
In the United States, the Environmental Protection Agency (EPA) regulates nitrous acid under the Clean Air Act and the Clean Water Act. The Occupational Safety and Health Administration (OSHA) has set permissible exposure limits for nitrous acid in workplace environments. These regulations aim to protect workers from potential health hazards associated with nitrous acid exposure, including respiratory irritation and potential carcinogenic effects.
The European Chemicals Agency (ECHA) has classified nitrous acid as a hazardous substance under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. This classification imposes strict requirements on manufacturers, importers, and downstream users of nitrous acid within the European Union.
In the context of biochemical research, regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established guidelines for the use of nitrous acid in pharmaceutical development and production processes. These guidelines address the potential formation of nitrosamines, which can result from nitrosation reactions facilitated by nitrous acid.
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has issued guidelines on the assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals. These guidelines specifically address the potential formation of nitrosamines through nitrosation reactions and provide recommendations for risk assessment and mitigation strategies.
Research institutions and academic laboratories working with nitrous acid and studying nitrosation reactions must adhere to institutional biosafety committees' protocols and guidelines. These protocols often include requirements for proper handling, storage, and disposal of nitrous acid and related compounds, as well as safety measures to prevent accidental exposure or environmental contamination.
The World Health Organization (WHO) has established guidelines for drinking water quality that include limits on nitrite and nitrate levels, which are closely related to nitrous acid. These guidelines aim to prevent potential health risks associated with nitrosation reactions in the human body resulting from ingestion of these compounds.
As the understanding of nitrous acid's role in nitrosation reactions in biochemistry continues to evolve, regulatory frameworks are likely to be updated and refined. Ongoing research into the mechanisms and potential health impacts of these reactions may lead to the development of new regulations or the modification of existing ones to ensure public safety and environmental protection.
Environmental Impact
The environmental impact of nitrous acid-facilitated nitrosation reactions in biochemistry is a critical consideration due to the potential consequences on ecosystems and human health. Nitrous acid, a weak acid formed from the dissolution of nitrogen oxides in water, plays a significant role in atmospheric chemistry and can contribute to various environmental issues.
One of the primary concerns is the formation of nitrosamines, which are potent carcinogens. When nitrous acid facilitates nitrosation reactions in the environment, it can lead to the production of these harmful compounds. This process can occur in both aquatic and terrestrial ecosystems, potentially contaminating water sources and soil. The presence of nitrosamines in the environment poses risks to wildlife and can enter the food chain, ultimately affecting human health.
Furthermore, nitrous acid contributes to the formation of acid rain. As nitrogen oxides react with water in the atmosphere, they produce nitrous acid, which can then fall as precipitation. Acid rain has far-reaching effects on ecosystems, including the acidification of water bodies, damage to forests, and the corrosion of buildings and infrastructure. This environmental impact extends beyond the immediate area of nitrous acid formation, affecting regions far from the source.
In aquatic environments, nitrous acid can disrupt the nitrogen cycle. It can lead to increased nitrogen availability, potentially causing eutrophication in water bodies. This process can result in algal blooms, oxygen depletion, and subsequent harm to aquatic life. The altered nitrogen balance can have cascading effects throughout the ecosystem, affecting biodiversity and ecosystem functioning.
The atmospheric presence of nitrous acid also contributes to the formation of ground-level ozone, a major component of smog. Ozone at ground level is a respiratory irritant and can cause significant health issues for both humans and animals. Additionally, it can damage vegetation, reducing crop yields and affecting forest health.
From a global perspective, the reactions facilitated by nitrous acid can indirectly contribute to climate change. The formation of nitrogen oxides and their subsequent reactions in the atmosphere can influence the Earth's radiative balance, potentially exacerbating global warming effects. This highlights the interconnectedness of biochemical processes and large-scale environmental phenomena.
Mitigation strategies to address the environmental impact of nitrous acid-facilitated reactions include improved emission controls, particularly for nitrogen oxides from industrial and transportation sources. Additionally, advancements in wastewater treatment technologies can help reduce the release of nitrogen compounds into the environment. Research into alternative chemical processes that minimize the use or production of nitrous acid in industrial applications is also crucial for long-term environmental protection.
One of the primary concerns is the formation of nitrosamines, which are potent carcinogens. When nitrous acid facilitates nitrosation reactions in the environment, it can lead to the production of these harmful compounds. This process can occur in both aquatic and terrestrial ecosystems, potentially contaminating water sources and soil. The presence of nitrosamines in the environment poses risks to wildlife and can enter the food chain, ultimately affecting human health.
Furthermore, nitrous acid contributes to the formation of acid rain. As nitrogen oxides react with water in the atmosphere, they produce nitrous acid, which can then fall as precipitation. Acid rain has far-reaching effects on ecosystems, including the acidification of water bodies, damage to forests, and the corrosion of buildings and infrastructure. This environmental impact extends beyond the immediate area of nitrous acid formation, affecting regions far from the source.
In aquatic environments, nitrous acid can disrupt the nitrogen cycle. It can lead to increased nitrogen availability, potentially causing eutrophication in water bodies. This process can result in algal blooms, oxygen depletion, and subsequent harm to aquatic life. The altered nitrogen balance can have cascading effects throughout the ecosystem, affecting biodiversity and ecosystem functioning.
The atmospheric presence of nitrous acid also contributes to the formation of ground-level ozone, a major component of smog. Ozone at ground level is a respiratory irritant and can cause significant health issues for both humans and animals. Additionally, it can damage vegetation, reducing crop yields and affecting forest health.
From a global perspective, the reactions facilitated by nitrous acid can indirectly contribute to climate change. The formation of nitrogen oxides and their subsequent reactions in the atmosphere can influence the Earth's radiative balance, potentially exacerbating global warming effects. This highlights the interconnectedness of biochemical processes and large-scale environmental phenomena.
Mitigation strategies to address the environmental impact of nitrous acid-facilitated reactions include improved emission controls, particularly for nitrogen oxides from industrial and transportation sources. Additionally, advancements in wastewater treatment technologies can help reduce the release of nitrogen compounds into the environment. Research into alternative chemical processes that minimize the use or production of nitrous acid in industrial applications is also crucial for long-term environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!