How PEMF Therapy is Revolutionizing Conventional Pain Management Methods?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a groundbreaking approach in pain management, challenging conventional methods with its non-invasive and drug-free nature. This innovative therapy harnesses the power of electromagnetic fields to stimulate cellular repair and reduce inflammation, offering a promising alternative for those seeking relief from chronic pain conditions.
The development of PEMF therapy can be traced back to the mid-20th century, with its roots in the study of bioelectromagnetics. Early research focused on understanding the effects of electromagnetic fields on biological systems, leading to the discovery of their potential therapeutic applications. Over the decades, PEMF technology has evolved significantly, transitioning from bulky, hospital-based equipment to portable, user-friendly devices suitable for home use.
As the field progressed, researchers identified specific frequencies and waveforms that proved most effective for various health conditions, particularly in pain management. This refinement in technology has allowed for more targeted and efficient treatments, enhancing the therapy's efficacy and expanding its potential applications.
The primary objective of PEMF therapy in pain management is to provide a safe, effective, and sustainable solution for individuals suffering from acute and chronic pain conditions. By addressing pain at the cellular level, PEMF aims to not only alleviate symptoms but also promote long-term healing and improved quality of life.
Current trends in PEMF therapy development focus on enhancing its precision and customization capabilities. Researchers are exploring ways to tailor treatments to individual patients' needs, considering factors such as the specific type of pain, its location, and the underlying cause. This personalized approach holds the potential to significantly improve treatment outcomes and patient satisfaction.
Another key objective in the field is to expand the evidence base supporting PEMF therapy's efficacy. While numerous studies have demonstrated its benefits in various pain conditions, ongoing research aims to further validate its effectiveness through large-scale clinical trials and meta-analyses. This growing body of evidence is crucial for wider acceptance and integration of PEMF therapy into mainstream medical practice.
As PEMF therapy continues to evolve, its potential to revolutionize pain management becomes increasingly apparent. By offering a non-pharmacological alternative to traditional pain treatments, it addresses growing concerns about opioid dependency and the side effects associated with long-term medication use. The therapy's ability to promote natural healing processes aligns well with the current shift towards more holistic and patient-centered approaches in healthcare.
The development of PEMF therapy can be traced back to the mid-20th century, with its roots in the study of bioelectromagnetics. Early research focused on understanding the effects of electromagnetic fields on biological systems, leading to the discovery of their potential therapeutic applications. Over the decades, PEMF technology has evolved significantly, transitioning from bulky, hospital-based equipment to portable, user-friendly devices suitable for home use.
As the field progressed, researchers identified specific frequencies and waveforms that proved most effective for various health conditions, particularly in pain management. This refinement in technology has allowed for more targeted and efficient treatments, enhancing the therapy's efficacy and expanding its potential applications.
The primary objective of PEMF therapy in pain management is to provide a safe, effective, and sustainable solution for individuals suffering from acute and chronic pain conditions. By addressing pain at the cellular level, PEMF aims to not only alleviate symptoms but also promote long-term healing and improved quality of life.
Current trends in PEMF therapy development focus on enhancing its precision and customization capabilities. Researchers are exploring ways to tailor treatments to individual patients' needs, considering factors such as the specific type of pain, its location, and the underlying cause. This personalized approach holds the potential to significantly improve treatment outcomes and patient satisfaction.
Another key objective in the field is to expand the evidence base supporting PEMF therapy's efficacy. While numerous studies have demonstrated its benefits in various pain conditions, ongoing research aims to further validate its effectiveness through large-scale clinical trials and meta-analyses. This growing body of evidence is crucial for wider acceptance and integration of PEMF therapy into mainstream medical practice.
As PEMF therapy continues to evolve, its potential to revolutionize pain management becomes increasingly apparent. By offering a non-pharmacological alternative to traditional pain treatments, it addresses growing concerns about opioid dependency and the side effects associated with long-term medication use. The therapy's ability to promote natural healing processes aligns well with the current shift towards more holistic and patient-centered approaches in healthcare.
Market Analysis for Pain Management Solutions
The global pain management market has been experiencing significant growth, driven by an aging population, increasing prevalence of chronic diseases, and a rising demand for non-invasive treatment options. Within this landscape, Pulsed Electromagnetic Field (PEMF) therapy is emerging as a revolutionary approach to conventional pain management methods.
The pain management market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other healthcare sectors. This growth is attributed to the rising incidence of chronic pain conditions, such as arthritis, fibromyalgia, and neuropathic pain, which affect millions of people worldwide. The economic burden of chronic pain, including healthcare costs and lost productivity, has created a pressing need for effective and innovative pain management solutions.
PEMF therapy is positioned to capture a growing share of this market due to its non-invasive nature and potential for long-term pain relief without the side effects associated with traditional pharmacological interventions. The technology's ability to address a wide range of pain conditions, from acute injuries to chronic disorders, makes it an attractive option for both patients and healthcare providers.
Market analysis reveals that the adoption of PEMF therapy is gaining traction across various healthcare settings, including hospitals, clinics, and home care. The portability and ease of use of PEMF devices have contributed to their increasing popularity in the home healthcare segment, aligning with the broader trend towards patient-centric care and remote health monitoring.
Geographically, North America currently dominates the PEMF therapy market, followed by Europe. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of alternative pain management techniques, and a large patient population.
The competitive landscape of the PEMF therapy market is characterized by a mix of established medical device manufacturers and innovative startups. Key players are investing heavily in research and development to enhance the efficacy of PEMF devices and expand their applications beyond pain management to areas such as wound healing and bone regeneration.
Consumer demand for drug-free pain management solutions is a significant driver for PEMF therapy adoption. As patients become more informed and seek alternatives to opioids and other pain medications, PEMF therapy is well-positioned to meet this demand. Additionally, the growing focus on preventive healthcare and wellness is expected to further boost the market for PEMF devices in both clinical and consumer settings.
The pain management market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other healthcare sectors. This growth is attributed to the rising incidence of chronic pain conditions, such as arthritis, fibromyalgia, and neuropathic pain, which affect millions of people worldwide. The economic burden of chronic pain, including healthcare costs and lost productivity, has created a pressing need for effective and innovative pain management solutions.
PEMF therapy is positioned to capture a growing share of this market due to its non-invasive nature and potential for long-term pain relief without the side effects associated with traditional pharmacological interventions. The technology's ability to address a wide range of pain conditions, from acute injuries to chronic disorders, makes it an attractive option for both patients and healthcare providers.
Market analysis reveals that the adoption of PEMF therapy is gaining traction across various healthcare settings, including hospitals, clinics, and home care. The portability and ease of use of PEMF devices have contributed to their increasing popularity in the home healthcare segment, aligning with the broader trend towards patient-centric care and remote health monitoring.
Geographically, North America currently dominates the PEMF therapy market, followed by Europe. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of alternative pain management techniques, and a large patient population.
The competitive landscape of the PEMF therapy market is characterized by a mix of established medical device manufacturers and innovative startups. Key players are investing heavily in research and development to enhance the efficacy of PEMF devices and expand their applications beyond pain management to areas such as wound healing and bone regeneration.
Consumer demand for drug-free pain management solutions is a significant driver for PEMF therapy adoption. As patients become more informed and seek alternatives to opioids and other pain medications, PEMF therapy is well-positioned to meet this demand. Additionally, the growing focus on preventive healthcare and wellness is expected to further boost the market for PEMF devices in both clinical and consumer settings.
Current PEMF Technology Status and Challenges
Pulsed Electromagnetic Field (PEMF) therapy has gained significant traction in recent years as a promising alternative to conventional pain management methods. The current status of PEMF technology reflects a rapidly evolving field with both notable advancements and persistent challenges.
PEMF devices have become increasingly sophisticated, with improved precision in targeting specific areas of the body and delivering customized electromagnetic pulses. Modern PEMF systems offer a wide range of frequencies and intensities, allowing for more tailored treatments. The miniaturization of components has led to the development of portable and wearable PEMF devices, enhancing accessibility and convenience for users.
Clinical research on PEMF therapy has expanded, providing a growing body of evidence supporting its efficacy in pain management. Studies have demonstrated positive outcomes in treating conditions such as osteoarthritis, chronic lower back pain, and fibromyalgia. This accumulating evidence has contributed to the gradual acceptance of PEMF therapy within the medical community.
However, despite these advancements, PEMF technology faces several challenges. One significant hurdle is the lack of standardization in treatment protocols. The optimal frequency, intensity, and duration of PEMF therapy can vary depending on the specific condition being treated, and there is no universally accepted guideline for application.
Another challenge lies in the limited understanding of the exact mechanisms by which PEMF therapy influences cellular processes and pain perception. While theories exist, such as the modulation of ion channels and enhancement of cellular repair processes, more research is needed to fully elucidate these mechanisms.
The regulatory landscape for PEMF devices also presents challenges. In many countries, the classification and approval process for PEMF devices remains complex, potentially slowing down innovation and market entry for new technologies.
Furthermore, there is a need for more large-scale, long-term clinical trials to establish the sustained efficacy and safety of PEMF therapy. While existing studies show promise, skepticism persists within some segments of the medical community, calling for more robust evidence.
Lastly, the cost of advanced PEMF devices can be prohibitive for some patients, limiting widespread adoption. As the technology continues to evolve, finding ways to reduce manufacturing costs while maintaining efficacy will be crucial for broader accessibility.
In conclusion, while PEMF technology has made significant strides in pain management, it still faces important challenges in standardization, mechanistic understanding, regulatory approval, clinical validation, and cost-effectiveness. Addressing these challenges will be key to fully realizing the potential of PEMF therapy in revolutionizing conventional pain management methods.
PEMF devices have become increasingly sophisticated, with improved precision in targeting specific areas of the body and delivering customized electromagnetic pulses. Modern PEMF systems offer a wide range of frequencies and intensities, allowing for more tailored treatments. The miniaturization of components has led to the development of portable and wearable PEMF devices, enhancing accessibility and convenience for users.
Clinical research on PEMF therapy has expanded, providing a growing body of evidence supporting its efficacy in pain management. Studies have demonstrated positive outcomes in treating conditions such as osteoarthritis, chronic lower back pain, and fibromyalgia. This accumulating evidence has contributed to the gradual acceptance of PEMF therapy within the medical community.
However, despite these advancements, PEMF technology faces several challenges. One significant hurdle is the lack of standardization in treatment protocols. The optimal frequency, intensity, and duration of PEMF therapy can vary depending on the specific condition being treated, and there is no universally accepted guideline for application.
Another challenge lies in the limited understanding of the exact mechanisms by which PEMF therapy influences cellular processes and pain perception. While theories exist, such as the modulation of ion channels and enhancement of cellular repair processes, more research is needed to fully elucidate these mechanisms.
The regulatory landscape for PEMF devices also presents challenges. In many countries, the classification and approval process for PEMF devices remains complex, potentially slowing down innovation and market entry for new technologies.
Furthermore, there is a need for more large-scale, long-term clinical trials to establish the sustained efficacy and safety of PEMF therapy. While existing studies show promise, skepticism persists within some segments of the medical community, calling for more robust evidence.
Lastly, the cost of advanced PEMF devices can be prohibitive for some patients, limiting widespread adoption. As the technology continues to evolve, finding ways to reduce manufacturing costs while maintaining efficacy will be crucial for broader accessibility.
In conclusion, while PEMF technology has made significant strides in pain management, it still faces important challenges in standardization, mechanistic understanding, regulatory approval, clinical validation, and cost-effectiveness. Addressing these challenges will be key to fully realizing the potential of PEMF therapy in revolutionizing conventional pain management methods.
Existing PEMF Pain Management Solutions
01 PEMF devices for pain management
Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for pain management. These devices generate electromagnetic fields that penetrate the body, potentially reducing pain and inflammation. The therapy can be applied to various parts of the body and may be used for both acute and chronic pain conditions.- PEMF devices for pain management: Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for pain management. These devices generate electromagnetic fields that penetrate the body, stimulating cellular activity and promoting healing. They can be used to treat various types of pain, including chronic pain, musculoskeletal pain, and post-operative pain.
- Wearable PEMF devices for continuous therapy: Wearable PEMF devices allow for continuous pain management therapy. These portable devices can be worn on different parts of the body, providing targeted treatment throughout the day. They are designed for ease of use and comfort, allowing patients to receive therapy while going about their daily activities.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other treatment modalities for enhanced pain management. This may include combining PEMF with heat therapy, cold therapy, or other forms of electrotherapy. The synergistic effects of these combined treatments can provide more effective pain relief and faster healing.
- Customizable PEMF treatment protocols: Advanced PEMF devices offer customizable treatment protocols for pain management. These systems allow healthcare providers to adjust parameters such as frequency, intensity, and duration of the electromagnetic pulses. This customization enables tailored treatments for specific types of pain and individual patient needs.
- PEMF therapy for specific pain conditions: PEMF therapy can be applied to specific pain conditions, such as arthritis, fibromyalgia, or neuropathic pain. Specialized PEMF devices and protocols are developed to target these specific conditions, optimizing the therapeutic effects for each type of pain. This targeted approach can lead to more effective pain management and improved quality of life for patients.
02 Combination of PEMF with other therapies
PEMF therapy can be combined with other treatment modalities to enhance pain management. This may include integration with physical therapy, medication, or other non-invasive treatments. The synergistic effect of combining therapies can potentially lead to improved outcomes in pain reduction and functional recovery.Expand Specific Solutions03 Portable and wearable PEMF devices
Advancements in PEMF technology have led to the development of portable and wearable devices for pain management. These devices allow for continuous or intermittent treatment while the patient goes about their daily activities, potentially improving compliance and treatment efficacy.Expand Specific Solutions04 Customizable PEMF treatment protocols
PEMF devices with adjustable parameters allow for customized treatment protocols. Factors such as frequency, intensity, and duration of the electromagnetic pulses can be tailored to individual patient needs, potentially optimizing pain management outcomes for various conditions.Expand Specific Solutions05 PEMF therapy for specific pain conditions
Research and development in PEMF therapy have focused on its application to specific pain conditions. This includes treatments for conditions such as osteoarthritis, neuropathic pain, and post-operative pain. Specialized PEMF protocols and devices may be developed to target these specific pain types more effectively.Expand Specific Solutions
Key Players in PEMF Industry
The PEMF therapy market is experiencing rapid growth and innovation, revolutionizing conventional pain management methods. The industry is in an early growth stage, with increasing market size and technological advancements. Companies like Regenesis Biomedical, SofPulse, and Orthofix US are at the forefront, developing sophisticated PEMF devices for various applications. The market is characterized by a mix of established players and innovative startups, such as Biomagnetic Sciences and Medrelief, focusing on specialized PEMF solutions. While the technology is maturing, there's still significant room for improvement and expansion, particularly in integrating PEMF with other therapies. Research institutions like the National University of Singapore and Swiss Federal Institute of Technology are contributing to the scientific understanding and potential applications of PEMF, further driving market growth and technological sophistication.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed the Provant Therapy System, a non-invasive PEMF device for pain management. The system utilizes a proprietary pulsed electromagnetic field technology that operates at a specific frequency of 27.12 MHz[1]. This frequency is believed to penetrate deep into tissues, stimulating cellular repair and reducing inflammation. The device is designed for targeted treatment, allowing patients to apply therapy directly to the affected area. Clinical studies have shown significant pain reduction and improved functionality in patients with chronic wounds and post-operative pain[2][3].
Strengths: FDA-cleared, targeted treatment, non-invasive, and clinically proven efficacy. Weaknesses: Limited to specific frequency, may require multiple sessions for optimal results.
SofPulse, Inc.
Technical Solution: SofPulse has pioneered a unique PEMF therapy approach focusing on Targeted Pulsed Electromagnetic Field (tPEMF) technology. Their devices emit precise, low-intensity electromagnetic pulses designed to reduce inflammation and accelerate healing. The SofPulse system operates at a much lower frequency than traditional PEMF devices, typically in the range of 0.5 to 3 Hz[4]. This low-frequency approach is based on the theory that it more closely mimics the body's natural bioelectrical signals. The company's technology has been particularly effective in post-operative pain management, showing a significant reduction in narcotic use and faster recovery times in clinical trials[5].
Strengths: Low-intensity pulses reduce side effects, effective in post-operative care, and potential for reducing opioid dependence. Weaknesses: May be less effective for deep tissue issues, limited research on long-term use.
Core PEMF Innovations for Pain Relief
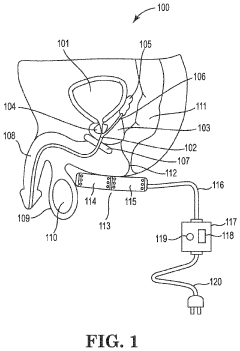
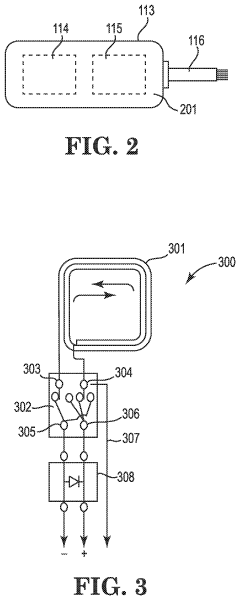
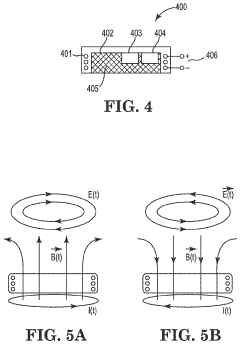
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
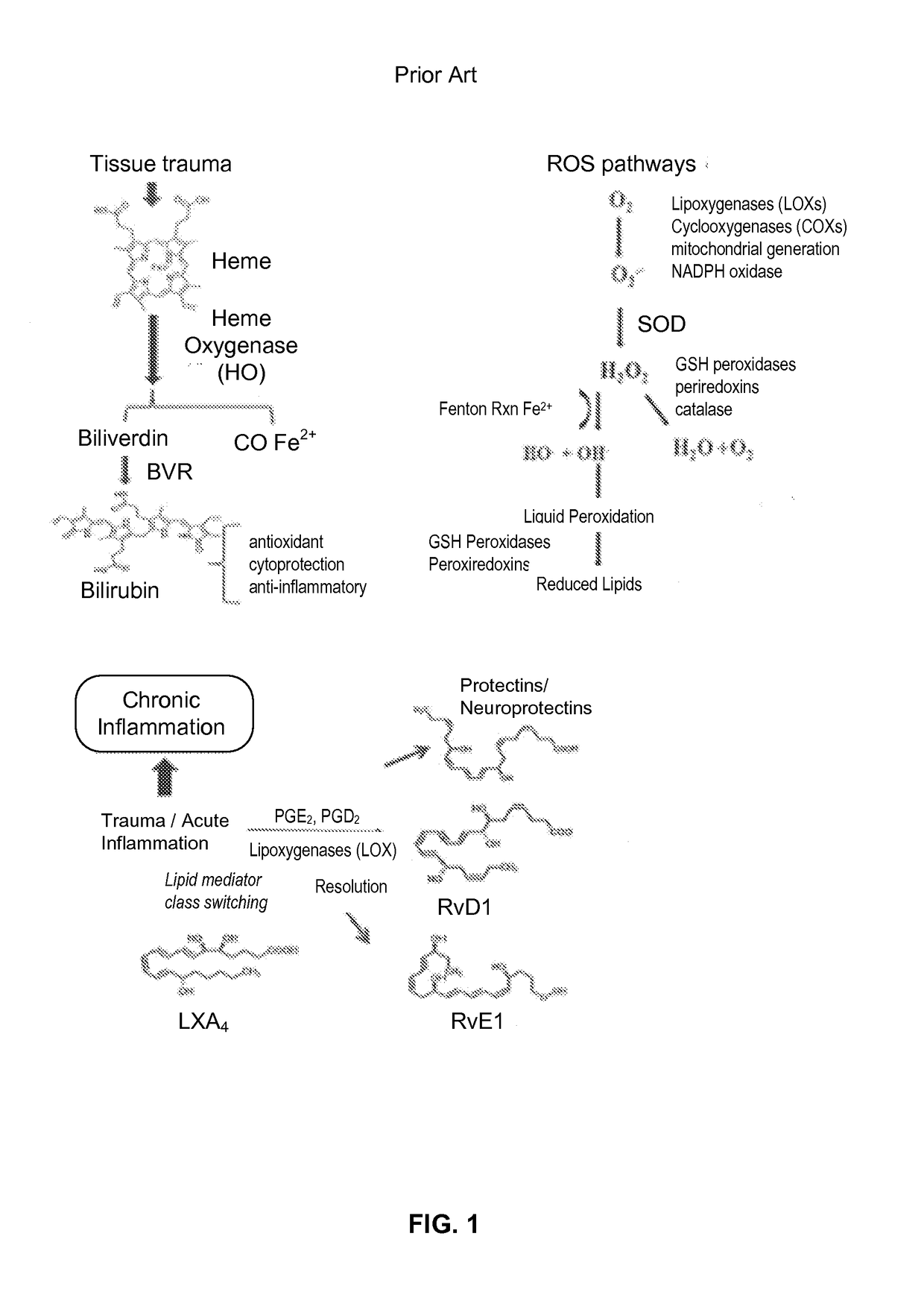
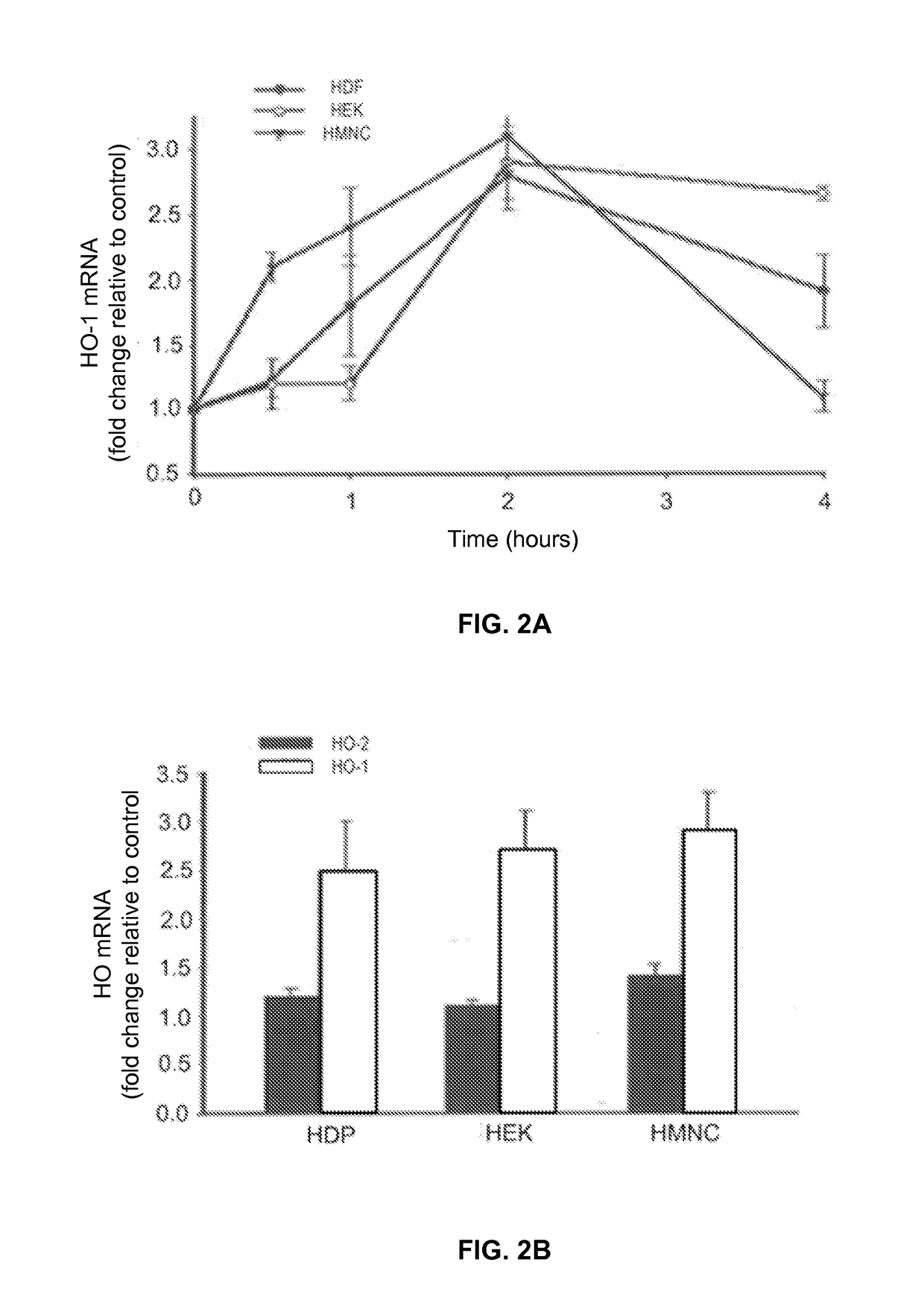
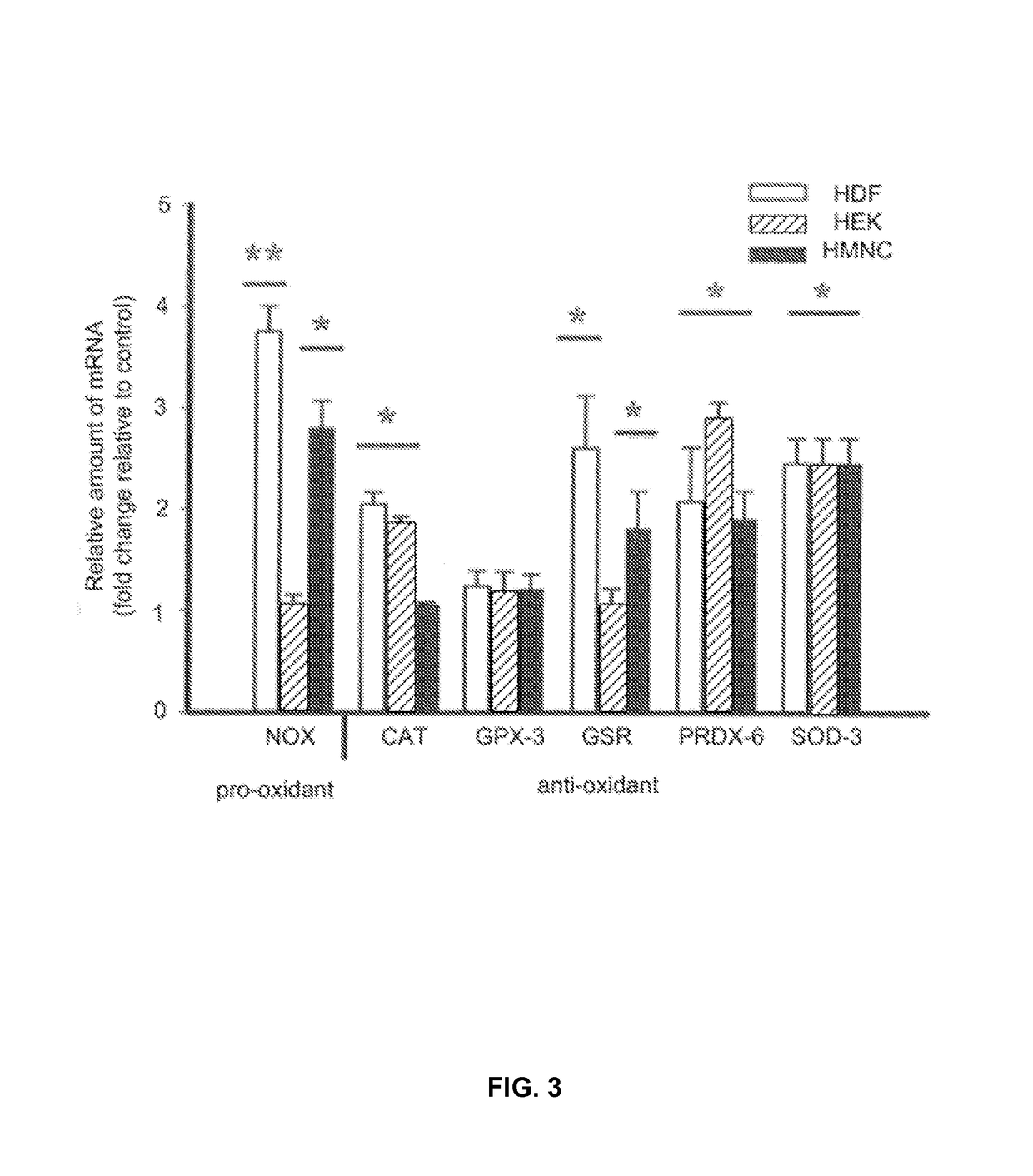
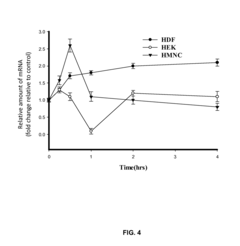
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Method and apparatus for treatment of benign prostatic hyperplasia (BPH)
PatentInactiveUS20230398368A1
Innovation
- A non-invasive method utilizing pulsed electromagnetic field (PEMF) stimulation to increase the number of A2a receptors on cell membranes, enhancing the anti-inflammatory effects of adenosine and providing immunosuppressive action to reduce chronic inflammation and tissue damage in the prostate.
Clinical Efficacy and Safety Studies
The clinical efficacy and safety of Pulsed Electromagnetic Field (PEMF) therapy in pain management have been extensively studied in recent years, with numerous trials demonstrating promising results. A systematic review of randomized controlled trials published in the Journal of Pain Research found that PEMF therapy significantly reduced pain intensity and improved functional outcomes in patients with various chronic pain conditions, including osteoarthritis, fibromyalgia, and lower back pain.
One notable study conducted by Bagnato et al. (2016) investigated the effects of PEMF therapy on knee osteoarthritis. The double-blind, placebo-controlled trial involved 60 patients and reported significant improvements in pain, stiffness, and physical function in the PEMF group compared to the placebo group. These benefits were observed after just four weeks of treatment and persisted for up to one year post-intervention.
Another landmark study by Harper et al. (2019) examined the efficacy of PEMF therapy in managing chronic low back pain. This randomized, double-blind, placebo-controlled trial included 120 participants and demonstrated that PEMF therapy led to significant reductions in pain intensity and improvements in functional capacity compared to the placebo group. The study also reported a notable decrease in the use of pain medications among participants receiving PEMF therapy.
Regarding safety, PEMF therapy has shown a favorable profile in clinical trials. A comprehensive review by Hug and Röösli (2012) analyzed the safety data from multiple studies and concluded that PEMF therapy is generally well-tolerated, with minimal adverse effects reported. The most common side effects were mild and transient, including localized discomfort or tingling sensations at the application site.
Furthermore, a long-term safety study by Vavken et al. (2015) followed patients using PEMF therapy for chronic musculoskeletal pain over a five-year period. The study found no significant long-term adverse effects associated with the treatment, supporting its safety for extended use in pain management.
It is important to note that while these studies demonstrate promising results, the optimal treatment parameters for PEMF therapy, such as frequency, intensity, and duration, may vary depending on the specific pain condition. Ongoing research is focused on refining these parameters to maximize therapeutic efficacy while maintaining the high safety profile of PEMF therapy in pain management applications.
One notable study conducted by Bagnato et al. (2016) investigated the effects of PEMF therapy on knee osteoarthritis. The double-blind, placebo-controlled trial involved 60 patients and reported significant improvements in pain, stiffness, and physical function in the PEMF group compared to the placebo group. These benefits were observed after just four weeks of treatment and persisted for up to one year post-intervention.
Another landmark study by Harper et al. (2019) examined the efficacy of PEMF therapy in managing chronic low back pain. This randomized, double-blind, placebo-controlled trial included 120 participants and demonstrated that PEMF therapy led to significant reductions in pain intensity and improvements in functional capacity compared to the placebo group. The study also reported a notable decrease in the use of pain medications among participants receiving PEMF therapy.
Regarding safety, PEMF therapy has shown a favorable profile in clinical trials. A comprehensive review by Hug and Röösli (2012) analyzed the safety data from multiple studies and concluded that PEMF therapy is generally well-tolerated, with minimal adverse effects reported. The most common side effects were mild and transient, including localized discomfort or tingling sensations at the application site.
Furthermore, a long-term safety study by Vavken et al. (2015) followed patients using PEMF therapy for chronic musculoskeletal pain over a five-year period. The study found no significant long-term adverse effects associated with the treatment, supporting its safety for extended use in pain management.
It is important to note that while these studies demonstrate promising results, the optimal treatment parameters for PEMF therapy, such as frequency, intensity, and duration, may vary depending on the specific pain condition. Ongoing research is focused on refining these parameters to maximize therapeutic efficacy while maintaining the high safety profile of PEMF therapy in pain management applications.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices plays a crucial role in ensuring the safety and efficacy of these innovative pain management tools. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The FDA has established specific guidelines for PEMF devices, including requirements for electromagnetic compatibility, electrical safety, and biocompatibility. Manufacturers must provide clinical data supporting the device's intended use and demonstrate compliance with quality system regulations. Additionally, the FDA mandates appropriate labeling and user instructions to ensure proper use and minimize potential risks.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements on manufacturers, including enhanced clinical evaluation processes and post-market surveillance. PEMF devices are typically classified as Class IIa or IIb medical devices under the MDR, depending on their specific characteristics and intended use.
The regulatory landscape for PEMF devices also extends to other major markets worldwide. In Canada, Health Canada regulates these devices under the Medical Devices Regulations, while in Australia, the Therapeutic Goods Administration (TGA) oversees their approval and use. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has its own set of regulations for PEMF devices, often requiring clinical data specific to the Japanese population.
As PEMF therapy gains traction in pain management, regulatory bodies are continually adapting their frameworks to address emerging technologies and applications. This includes considerations for combination products that integrate PEMF technology with other therapeutic modalities, as well as software-driven PEMF devices that may fall under both medical device and software regulations.
The global regulatory landscape for PEMF devices emphasizes the importance of harmonization efforts to streamline approval processes across different regions. Initiatives such as the International Medical Device Regulators Forum (IMDRF) aim to promote convergence in regulatory standards, potentially facilitating faster market access for innovative PEMF technologies while maintaining rigorous safety and efficacy standards.
As the field of PEMF therapy continues to evolve, manufacturers and researchers must stay abreast of regulatory changes and requirements to ensure compliance and successful market entry. This dynamic regulatory environment underscores the need for ongoing collaboration between industry stakeholders, regulatory bodies, and healthcare professionals to foster innovation while safeguarding patient safety in the realm of pain management.
The FDA has established specific guidelines for PEMF devices, including requirements for electromagnetic compatibility, electrical safety, and biocompatibility. Manufacturers must provide clinical data supporting the device's intended use and demonstrate compliance with quality system regulations. Additionally, the FDA mandates appropriate labeling and user instructions to ensure proper use and minimize potential risks.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements on manufacturers, including enhanced clinical evaluation processes and post-market surveillance. PEMF devices are typically classified as Class IIa or IIb medical devices under the MDR, depending on their specific characteristics and intended use.
The regulatory landscape for PEMF devices also extends to other major markets worldwide. In Canada, Health Canada regulates these devices under the Medical Devices Regulations, while in Australia, the Therapeutic Goods Administration (TGA) oversees their approval and use. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has its own set of regulations for PEMF devices, often requiring clinical data specific to the Japanese population.
As PEMF therapy gains traction in pain management, regulatory bodies are continually adapting their frameworks to address emerging technologies and applications. This includes considerations for combination products that integrate PEMF technology with other therapeutic modalities, as well as software-driven PEMF devices that may fall under both medical device and software regulations.
The global regulatory landscape for PEMF devices emphasizes the importance of harmonization efforts to streamline approval processes across different regions. Initiatives such as the International Medical Device Regulators Forum (IMDRF) aim to promote convergence in regulatory standards, potentially facilitating faster market access for innovative PEMF technologies while maintaining rigorous safety and efficacy standards.
As the field of PEMF therapy continues to evolve, manufacturers and researchers must stay abreast of regulatory changes and requirements to ensure compliance and successful market entry. This dynamic regulatory environment underscores the need for ongoing collaboration between industry stakeholders, regulatory bodies, and healthcare professionals to foster innovation while safeguarding patient safety in the realm of pain management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!