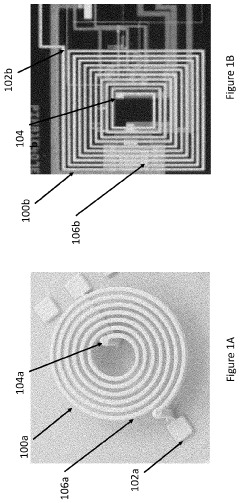
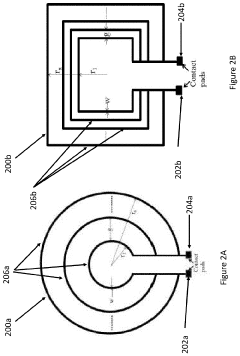
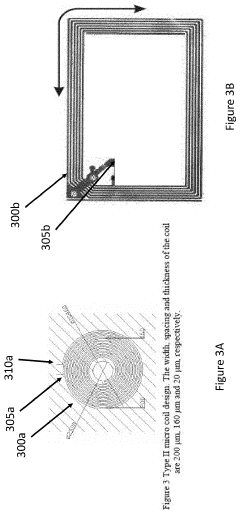
How PEMF Therapy Supports Musculoskeletal Health?
AUG 11, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background
Pulsed Electromagnetic Field (PEMF) therapy is a non-invasive treatment modality that has gained significant attention in recent years for its potential to support musculoskeletal health. The therapy utilizes electromagnetic fields to stimulate cellular repair and regeneration, offering a promising approach to managing various musculoskeletal conditions.
The concept of using electromagnetic fields for therapeutic purposes dates back to the mid-20th century when scientists began exploring the effects of electromagnetic energy on biological systems. However, it wasn't until the 1970s that PEMF therapy started to gain traction in medical applications, particularly in the field of orthopedics.
PEMF therapy works by delivering pulsed electromagnetic fields to targeted areas of the body. These fields penetrate deep into tissues, interacting with cells at a molecular level. The therapy is based on the principle that electromagnetic fields can influence cellular behavior, including ion transport, cell membrane potential, and various metabolic processes.
In the context of musculoskeletal health, PEMF therapy has shown potential in addressing a wide range of conditions, including osteoarthritis, rheumatoid arthritis, osteoporosis, and chronic pain syndromes. The therapy is believed to promote tissue healing, reduce inflammation, and enhance bone and cartilage regeneration.
One of the key mechanisms through which PEMF therapy supports musculoskeletal health is by stimulating the production of growth factors and promoting angiogenesis. This can lead to improved blood circulation and enhanced delivery of nutrients to affected tissues, facilitating the healing process.
Furthermore, PEMF therapy has been associated with increased collagen production, which is crucial for maintaining the structural integrity of connective tissues such as tendons and ligaments. This aspect of PEMF therapy makes it particularly relevant in the treatment and prevention of sports injuries and age-related musculoskeletal degeneration.
Research has also suggested that PEMF therapy may have a positive impact on bone density, making it a potential adjunct therapy for conditions like osteoporosis. By stimulating osteoblast activity and inhibiting osteoclast function, PEMF therapy could contribute to improved bone mineral density and reduced fracture risk.
As the field of regenerative medicine continues to evolve, PEMF therapy has emerged as a promising tool in the management of musculoskeletal disorders. Its non-invasive nature, coupled with the growing body of evidence supporting its efficacy, has led to increased adoption in both clinical and home-based settings.
The concept of using electromagnetic fields for therapeutic purposes dates back to the mid-20th century when scientists began exploring the effects of electromagnetic energy on biological systems. However, it wasn't until the 1970s that PEMF therapy started to gain traction in medical applications, particularly in the field of orthopedics.
PEMF therapy works by delivering pulsed electromagnetic fields to targeted areas of the body. These fields penetrate deep into tissues, interacting with cells at a molecular level. The therapy is based on the principle that electromagnetic fields can influence cellular behavior, including ion transport, cell membrane potential, and various metabolic processes.
In the context of musculoskeletal health, PEMF therapy has shown potential in addressing a wide range of conditions, including osteoarthritis, rheumatoid arthritis, osteoporosis, and chronic pain syndromes. The therapy is believed to promote tissue healing, reduce inflammation, and enhance bone and cartilage regeneration.
One of the key mechanisms through which PEMF therapy supports musculoskeletal health is by stimulating the production of growth factors and promoting angiogenesis. This can lead to improved blood circulation and enhanced delivery of nutrients to affected tissues, facilitating the healing process.
Furthermore, PEMF therapy has been associated with increased collagen production, which is crucial for maintaining the structural integrity of connective tissues such as tendons and ligaments. This aspect of PEMF therapy makes it particularly relevant in the treatment and prevention of sports injuries and age-related musculoskeletal degeneration.
Research has also suggested that PEMF therapy may have a positive impact on bone density, making it a potential adjunct therapy for conditions like osteoporosis. By stimulating osteoblast activity and inhibiting osteoclast function, PEMF therapy could contribute to improved bone mineral density and reduced fracture risk.
As the field of regenerative medicine continues to evolve, PEMF therapy has emerged as a promising tool in the management of musculoskeletal disorders. Its non-invasive nature, coupled with the growing body of evidence supporting its efficacy, has led to increased adoption in both clinical and home-based settings.
Market Analysis
The market for PEMF (Pulsed Electromagnetic Field) therapy in musculoskeletal health is experiencing significant growth, driven by increasing awareness of non-invasive treatment options and the rising prevalence of musculoskeletal disorders. The global PEMF therapy devices market is projected to expand at a compound annual growth rate of 7.2% from 2021 to 2028, reflecting the growing demand for alternative pain management solutions.
Musculoskeletal conditions, including arthritis, osteoporosis, and sports injuries, affect millions of people worldwide. The aging population and sedentary lifestyles contribute to the increasing incidence of these disorders, creating a substantial market opportunity for PEMF therapy. In the United States alone, musculoskeletal conditions affect more than one in two adults, with chronic low back pain being the most common complaint.
The market for PEMF therapy devices is segmented into home-use devices and clinical-grade equipment. Home-use devices are gaining popularity due to their convenience and affordability, while clinical-grade equipment continues to be in high demand among healthcare providers. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and growing awareness of alternative therapies.
Key market players in the PEMF therapy industry include OMI PLC, Orthofix Medical Inc., and Bemer Group. These companies are investing heavily in research and development to improve device efficacy and expand their product portfolios. Technological advancements, such as the integration of smart features and improved portability, are expected to further drive market growth.
The COVID-19 pandemic has had a mixed impact on the PEMF therapy market. While it initially disrupted supply chains and reduced access to clinical treatments, it also accelerated the adoption of home-use devices as patients sought alternative ways to manage their musculoskeletal health. This shift towards home-based care is likely to persist, creating new opportunities for market expansion.
Regulatory approvals and reimbursement policies play a crucial role in market growth. As more clinical evidence supporting the efficacy of PEMF therapy emerges, it is expected that insurance coverage and regulatory acceptance will improve, further driving market expansion. However, the lack of standardized treatment protocols and limited awareness among some healthcare professionals remain challenges that need to be addressed to fully realize the market potential of PEMF therapy in musculoskeletal health.
Musculoskeletal conditions, including arthritis, osteoporosis, and sports injuries, affect millions of people worldwide. The aging population and sedentary lifestyles contribute to the increasing incidence of these disorders, creating a substantial market opportunity for PEMF therapy. In the United States alone, musculoskeletal conditions affect more than one in two adults, with chronic low back pain being the most common complaint.
The market for PEMF therapy devices is segmented into home-use devices and clinical-grade equipment. Home-use devices are gaining popularity due to their convenience and affordability, while clinical-grade equipment continues to be in high demand among healthcare providers. North America currently holds the largest market share, followed by Europe and Asia-Pacific. The Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure and growing awareness of alternative therapies.
Key market players in the PEMF therapy industry include OMI PLC, Orthofix Medical Inc., and Bemer Group. These companies are investing heavily in research and development to improve device efficacy and expand their product portfolios. Technological advancements, such as the integration of smart features and improved portability, are expected to further drive market growth.
The COVID-19 pandemic has had a mixed impact on the PEMF therapy market. While it initially disrupted supply chains and reduced access to clinical treatments, it also accelerated the adoption of home-use devices as patients sought alternative ways to manage their musculoskeletal health. This shift towards home-based care is likely to persist, creating new opportunities for market expansion.
Regulatory approvals and reimbursement policies play a crucial role in market growth. As more clinical evidence supporting the efficacy of PEMF therapy emerges, it is expected that insurance coverage and regulatory acceptance will improve, further driving market expansion. However, the lack of standardized treatment protocols and limited awareness among some healthcare professionals remain challenges that need to be addressed to fully realize the market potential of PEMF therapy in musculoskeletal health.
Technical Challenges
Despite the growing popularity of Pulsed Electromagnetic Field (PEMF) therapy for musculoskeletal health, several technical challenges persist in its development and application. One of the primary obstacles is the lack of standardization in PEMF devices and treatment protocols. The wide variety of devices available in the market, each with different frequencies, intensities, and waveforms, makes it difficult to establish consistent treatment guidelines and compare efficacy across studies.
Another significant challenge lies in the optimization of PEMF parameters for specific musculoskeletal conditions. While research has shown promising results for various applications, determining the ideal frequency, intensity, and duration of treatment for each condition remains a complex task. This is further complicated by individual patient factors such as age, overall health status, and the severity of the musculoskeletal issue.
The development of more targeted and localized PEMF delivery systems presents another technical hurdle. Current devices often provide broad-field exposure, which may limit their effectiveness for treating specific anatomical regions or deep-seated musculoskeletal problems. Enhancing the precision of PEMF application could potentially improve treatment outcomes and reduce the risk of unintended effects on surrounding tissues.
Ensuring the long-term safety of PEMF therapy is an ongoing challenge. While short-term studies have generally shown a favorable safety profile, more extensive research is needed to evaluate the potential cumulative effects of prolonged PEMF exposure on various body systems, including the musculoskeletal, nervous, and endocrine systems.
The integration of PEMF therapy with other treatment modalities and technologies poses both opportunities and challenges. Developing synergistic approaches that combine PEMF with other therapies, such as physical rehabilitation or pharmacological interventions, requires careful consideration of potential interactions and optimal timing of treatments.
Lastly, the miniaturization and portability of PEMF devices for home use present technical challenges in maintaining treatment efficacy while ensuring user-friendly design and affordability. Balancing these factors is crucial for expanding the accessibility of PEMF therapy for musculoskeletal health management outside clinical settings.
Another significant challenge lies in the optimization of PEMF parameters for specific musculoskeletal conditions. While research has shown promising results for various applications, determining the ideal frequency, intensity, and duration of treatment for each condition remains a complex task. This is further complicated by individual patient factors such as age, overall health status, and the severity of the musculoskeletal issue.
The development of more targeted and localized PEMF delivery systems presents another technical hurdle. Current devices often provide broad-field exposure, which may limit their effectiveness for treating specific anatomical regions or deep-seated musculoskeletal problems. Enhancing the precision of PEMF application could potentially improve treatment outcomes and reduce the risk of unintended effects on surrounding tissues.
Ensuring the long-term safety of PEMF therapy is an ongoing challenge. While short-term studies have generally shown a favorable safety profile, more extensive research is needed to evaluate the potential cumulative effects of prolonged PEMF exposure on various body systems, including the musculoskeletal, nervous, and endocrine systems.
The integration of PEMF therapy with other treatment modalities and technologies poses both opportunities and challenges. Developing synergistic approaches that combine PEMF with other therapies, such as physical rehabilitation or pharmacological interventions, requires careful consideration of potential interactions and optimal timing of treatments.
Lastly, the miniaturization and portability of PEMF devices for home use present technical challenges in maintaining treatment efficacy while ensuring user-friendly design and affordability. Balancing these factors is crucial for expanding the accessibility of PEMF therapy for musculoskeletal health management outside clinical settings.
Current PEMF Solutions
01 PEMF devices for musculoskeletal treatment
Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating musculoskeletal conditions. These devices generate electromagnetic fields that penetrate the body, stimulating cellular repair and reducing inflammation in muscles, bones, and joints. The therapy can be applied to various body parts and is often used for pain management and improving overall musculoskeletal health.- PEMF devices for musculoskeletal treatment: Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating musculoskeletal conditions. These devices generate electromagnetic fields that penetrate the body, stimulating cellular repair and reducing inflammation in muscles, bones, and joints. The therapy can be applied to various body parts and is often used for pain management and accelerating healing in musculoskeletal injuries.
- Wearable PEMF devices for continuous therapy: Wearable PEMF devices allow for continuous therapy application, improving patient compliance and treatment efficacy. These devices are designed to be compact, portable, and can be worn during daily activities. They often target specific body areas such as the back, knees, or shoulders, providing localized treatment for musculoskeletal issues without interrupting the patient's routine.
- Combination of PEMF with other therapies: PEMF therapy is often combined with other treatment modalities to enhance musculoskeletal health outcomes. This may include integration with physical therapy exercises, heat therapy, or other forms of electromagnetic stimulation. The synergistic effect of combined therapies can lead to improved pain relief, increased range of motion, and faster recovery from musculoskeletal injuries.
- Customizable PEMF treatment protocols: Advanced PEMF devices offer customizable treatment protocols to address specific musculoskeletal conditions. These systems allow for adjustments in frequency, intensity, and duration of the electromagnetic pulses. Customization enables healthcare providers to tailor treatments to individual patient needs, optimizing the therapeutic effects for various musculoskeletal disorders such as osteoarthritis, tendinitis, or fracture healing.
- PEMF therapy for post-surgical recovery: PEMF therapy is utilized in post-surgical recovery for musculoskeletal procedures. The treatment helps reduce inflammation, accelerate tissue repair, and manage pain following surgeries such as joint replacements or spinal interventions. By promoting faster healing and reducing the need for pain medication, PEMF therapy can contribute to improved outcomes and shorter recovery times for patients undergoing musculoskeletal surgeries.
02 Wearable PEMF devices for continuous therapy
Wearable PEMF devices allow for continuous therapy throughout the day. These portable units can be worn on specific body parts, providing targeted treatment for musculoskeletal issues. The wearable nature of these devices enables patients to receive therapy while going about their daily activities, potentially improving treatment efficacy and patient compliance.Expand Specific Solutions03 Combination of PEMF with other therapies
PEMF therapy can be combined with other treatment modalities to enhance musculoskeletal health outcomes. This may include integration with physical therapy exercises, heat therapy, or other forms of electromagnetic stimulation. The synergistic effect of combined therapies can potentially lead to improved pain relief, faster healing, and better overall musculoskeletal function.Expand Specific Solutions04 Customizable PEMF treatment protocols
Advanced PEMF devices offer customizable treatment protocols to address specific musculoskeletal conditions. These systems allow for adjustments in frequency, intensity, and duration of electromagnetic pulses based on the patient's needs. Customization can target different tissue types and conditions, potentially improving the effectiveness of the therapy for various musculoskeletal health issues.Expand Specific Solutions05 PEMF therapy for post-surgical recovery
PEMF therapy is utilized in post-surgical recovery for musculoskeletal procedures. The therapy can help reduce inflammation, accelerate tissue healing, and manage pain following surgeries such as joint replacements or bone fracture repairs. This application of PEMF may lead to faster recovery times and improved outcomes for patients undergoing musculoskeletal surgeries.Expand Specific Solutions
Key Industry Players
The PEMF therapy market for musculoskeletal health is in a growth phase, with increasing adoption and technological advancements. The global market size is expanding, driven by rising awareness of non-invasive treatments and an aging population. Technologically, PEMF therapy is maturing, with companies like Regenesis Biomedical, Venus Concept, and SofPulse leading innovation. These firms are developing more sophisticated devices, improving efficacy and user experience. Academic institutions such as the National University of Singapore and Swiss Federal Institute of Technology are contributing to the scientific understanding of PEMF, while companies like Biomagnetic Sciences and EMKinetics are pushing the boundaries of application. The competitive landscape is diverse, with established medical device manufacturers and specialized PEMF companies vying for market share.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed a proprietary PEMF therapy system called Provant Therapy. This system utilizes a specific pulsed electromagnetic field to stimulate cellular repair and reduce inflammation in musculoskeletal tissues. The Provant device delivers a precise 27.12 MHz radio frequency signal in short pulses, which has been shown to activate the body's natural healing processes[1]. Clinical studies have demonstrated that this therapy can significantly reduce pain and improve joint function in conditions such as osteoarthritis and chronic low back pain[2]. The company's approach focuses on non-invasive, drug-free treatment options that can be used in both clinical settings and at home.
Strengths: Targeted frequency for optimal cellular response, FDA-cleared for multiple indications, non-invasive treatment option. Weaknesses: May require multiple sessions for optimal results, effectiveness can vary among individuals.
Venus Concept Ltd.
Technical Solution: Venus Concept has developed the Venus Pulse technology, which combines PEMF with other modalities such as radio frequency (RF) for comprehensive musculoskeletal health support. Their devices, like the Venus Legacy, use Multi-Polar Radio Frequency and Pulsed Electro Magnetic Fields to penetrate deeper into the tissue layers, promoting collagen and elastin production[3]. This synergistic approach aims to not only alleviate pain but also improve tissue quality and function. The PEMF component is designed to increase blood flow and oxygenation to the treated areas, enhancing the overall healing process. Venus Concept's technology is particularly noted for its ability to target both superficial and deep tissue layers, making it versatile for various musculoskeletal conditions[4].
Strengths: Combines PEMF with other therapeutic modalities, targets multiple tissue layers, versatile application. Weaknesses: Higher cost due to multi-modal approach, may require specialized training for optimal use.
Core PEMF Innovations
Pulsed Electromagnetic Field Devices Integrated into Adjustable Clothing
PatentPendingUS20230104434A1
Innovation
- A pulsed electromagnetic field device integrated into wearable clothing, using arrays of planar microcoils that generate controlled, homogenous magnetic fields, allowing for comfortable, long-term use and targeted treatment of various brain-related disorders and conditions.
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
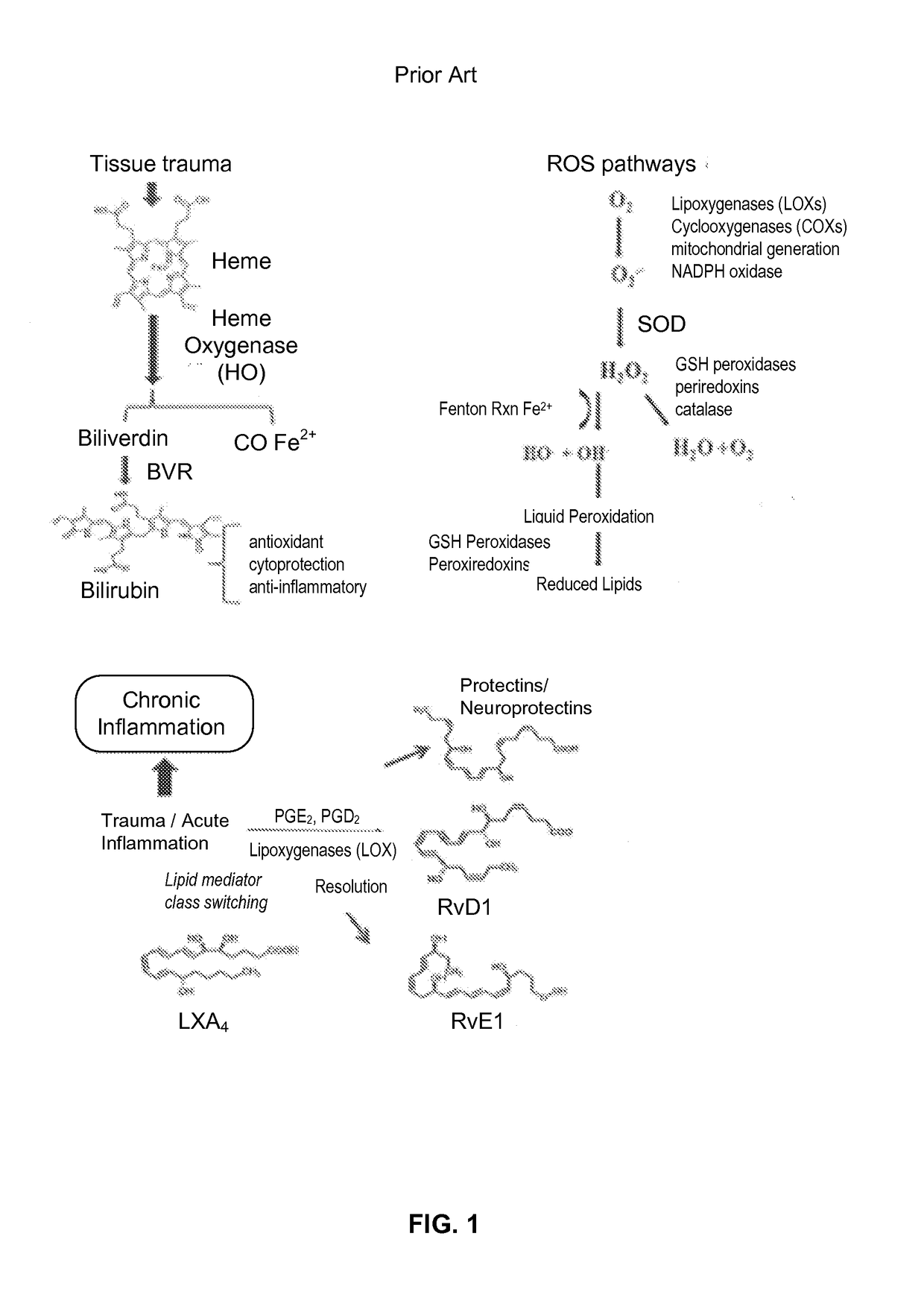
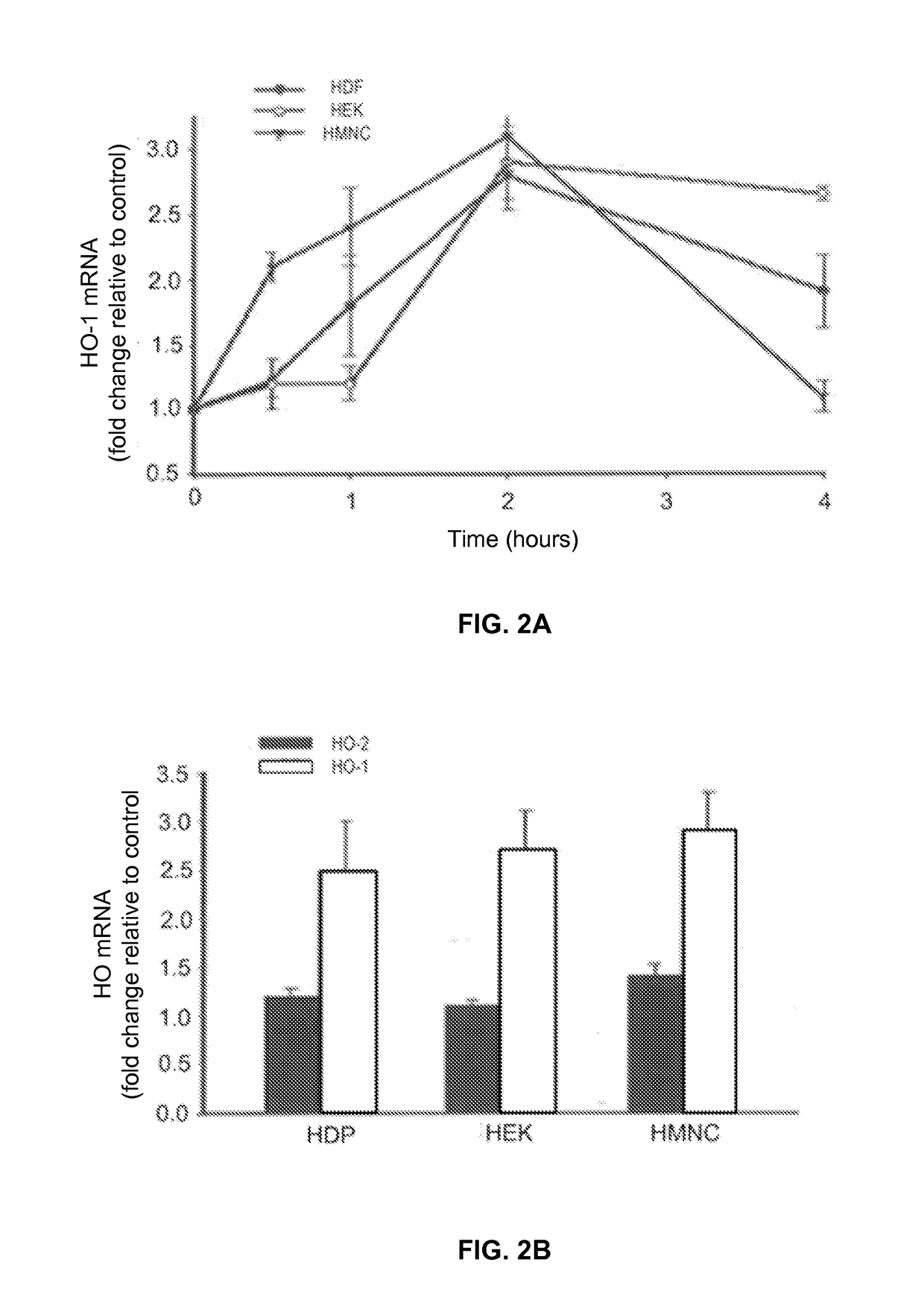
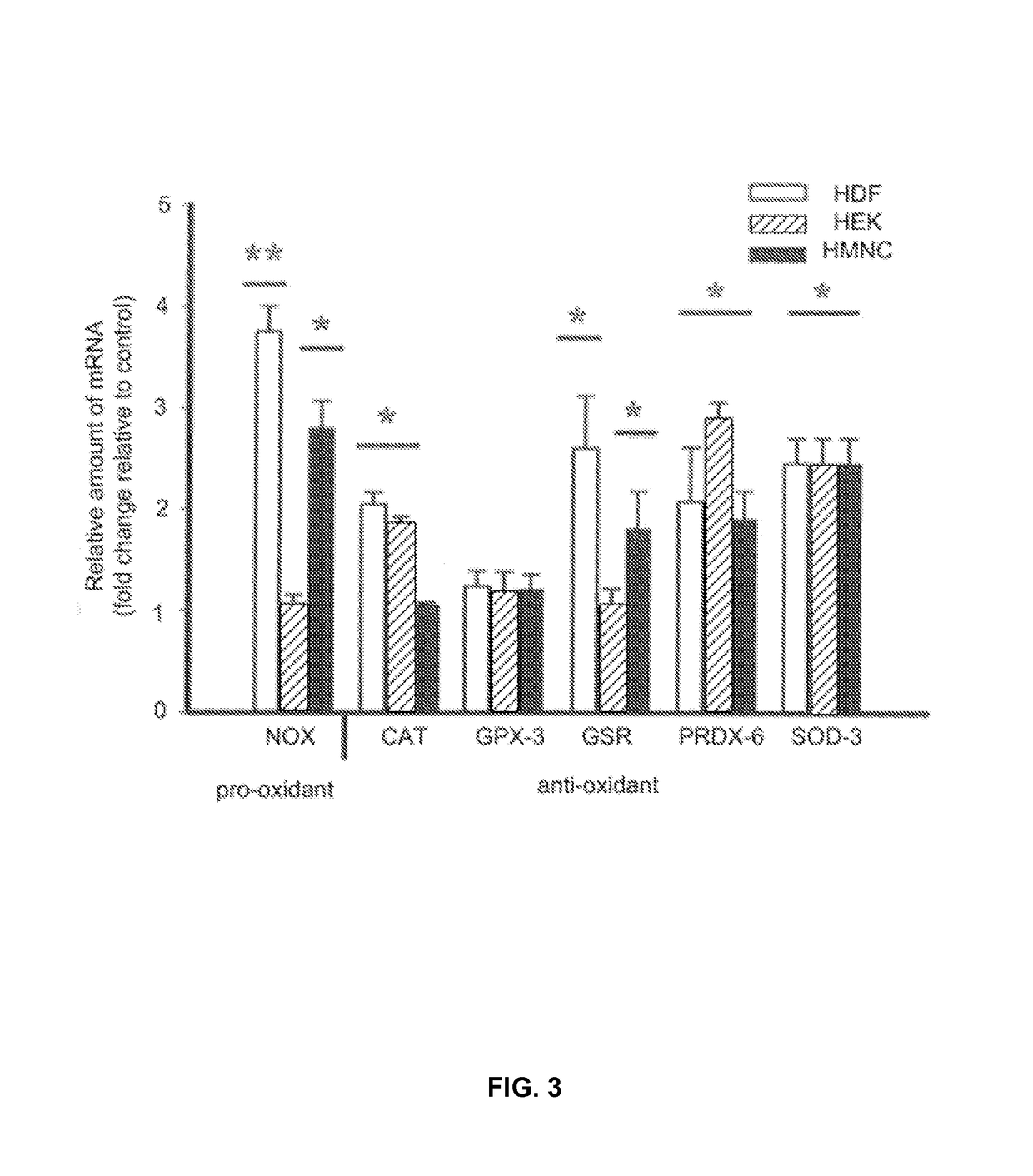
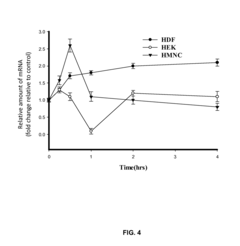
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Clinical Efficacy Studies
Clinical efficacy studies play a crucial role in validating the effectiveness of PEMF therapy for musculoskeletal health. Numerous randomized controlled trials have demonstrated positive outcomes across various conditions, including osteoarthritis, chronic low back pain, and bone fracture healing.
In osteoarthritis studies, PEMF therapy has shown significant improvements in pain reduction and functional capacity. A meta-analysis of 14 trials involving 823 patients revealed that PEMF treatment led to substantial decreases in pain scores and enhanced physical function compared to placebo groups. The most pronounced effects were observed in knee osteoarthritis patients, with improvements sustained for up to 12 weeks post-treatment.
For chronic low back pain, several studies have reported promising results. A double-blind, placebo-controlled trial involving 100 patients with persistent lumbar pain showed that PEMF therapy resulted in a 40% reduction in pain intensity and a 30% improvement in overall functionality after eight weeks of treatment. These benefits were maintained at the three-month follow-up, suggesting long-term efficacy.
In the realm of bone fracture healing, PEMF therapy has demonstrated accelerated recovery times and improved bone density. A systematic review of 13 studies encompassing 924 patients with long bone fractures found that PEMF treatment reduced healing time by an average of 37% compared to standard care alone. Additionally, radiographic assessments revealed higher bone mineral density in PEMF-treated groups, indicating enhanced bone quality.
Clinical studies have also explored PEMF therapy's efficacy in treating other musculoskeletal conditions. Research on fibromyalgia patients has shown significant reductions in pain scores and improvements in sleep quality following PEMF treatment. Similarly, studies on rotator cuff tendinopathy have reported decreased pain levels and increased range of motion in patients receiving PEMF therapy compared to control groups.
While the majority of clinical trials support the efficacy of PEMF therapy, it is important to note that some studies have produced mixed results. Factors such as treatment duration, frequency, and intensity may influence outcomes. Furthermore, the optimal parameters for PEMF therapy may vary depending on the specific musculoskeletal condition being treated.
Overall, the body of clinical evidence suggests that PEMF therapy holds significant promise as a non-invasive treatment modality for various musculoskeletal disorders. However, further large-scale, long-term studies are needed to establish standardized protocols and elucidate the mechanisms underlying its therapeutic effects.
In osteoarthritis studies, PEMF therapy has shown significant improvements in pain reduction and functional capacity. A meta-analysis of 14 trials involving 823 patients revealed that PEMF treatment led to substantial decreases in pain scores and enhanced physical function compared to placebo groups. The most pronounced effects were observed in knee osteoarthritis patients, with improvements sustained for up to 12 weeks post-treatment.
For chronic low back pain, several studies have reported promising results. A double-blind, placebo-controlled trial involving 100 patients with persistent lumbar pain showed that PEMF therapy resulted in a 40% reduction in pain intensity and a 30% improvement in overall functionality after eight weeks of treatment. These benefits were maintained at the three-month follow-up, suggesting long-term efficacy.
In the realm of bone fracture healing, PEMF therapy has demonstrated accelerated recovery times and improved bone density. A systematic review of 13 studies encompassing 924 patients with long bone fractures found that PEMF treatment reduced healing time by an average of 37% compared to standard care alone. Additionally, radiographic assessments revealed higher bone mineral density in PEMF-treated groups, indicating enhanced bone quality.
Clinical studies have also explored PEMF therapy's efficacy in treating other musculoskeletal conditions. Research on fibromyalgia patients has shown significant reductions in pain scores and improvements in sleep quality following PEMF treatment. Similarly, studies on rotator cuff tendinopathy have reported decreased pain levels and increased range of motion in patients receiving PEMF therapy compared to control groups.
While the majority of clinical trials support the efficacy of PEMF therapy, it is important to note that some studies have produced mixed results. Factors such as treatment duration, frequency, and intensity may influence outcomes. Furthermore, the optimal parameters for PEMF therapy may vary depending on the specific musculoskeletal condition being treated.
Overall, the body of clinical evidence suggests that PEMF therapy holds significant promise as a non-invasive treatment modality for various musculoskeletal disorders. However, further large-scale, long-term studies are needed to establish standardized protocols and elucidate the mechanisms underlying its therapeutic effects.
Regulatory Considerations
The regulatory landscape for Pulsed Electromagnetic Field (PEMF) therapy in musculoskeletal health is complex and varies across different regions. In the United States, the Food and Drug Administration (FDA) has cleared several PEMF devices for specific musculoskeletal conditions, such as bone healing and pain management. These devices are classified as Class II medical devices, requiring premarket notification (510(k)) clearance before they can be marketed.
The FDA's regulatory approach to PEMF therapy emphasizes safety and efficacy. Manufacturers must demonstrate that their devices are substantially equivalent to predicate devices already on the market. This process involves submitting clinical data, risk assessments, and technical specifications to support the device's intended use and safety profile.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). The MDR requires manufacturers to obtain CE marking for their devices, which involves a conformity assessment process. This process includes clinical evaluation, risk management, and quality management system implementation. The level of scrutiny depends on the device's classification, with higher-risk devices requiring more extensive evaluation.
Regulatory bodies in other countries, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own requirements for PEMF devices. These often align with international standards but may have specific national requirements.
One key regulatory consideration for PEMF therapy is the management of electromagnetic emissions. Devices must comply with electromagnetic compatibility (EMC) standards to ensure they do not interfere with other electronic equipment and are safe for use in various environments.
As the field of PEMF therapy continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. There is an increasing focus on real-world evidence and post-market surveillance to ensure the ongoing safety and effectiveness of PEMF devices in musculoskeletal health applications.
Manufacturers and researchers must navigate these regulatory requirements carefully to bring new PEMF therapies to market. This involves ongoing dialogue with regulatory bodies, adherence to good manufacturing practices, and continuous monitoring of device performance and safety in clinical use.
The FDA's regulatory approach to PEMF therapy emphasizes safety and efficacy. Manufacturers must demonstrate that their devices are substantially equivalent to predicate devices already on the market. This process involves submitting clinical data, risk assessments, and technical specifications to support the device's intended use and safety profile.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). The MDR requires manufacturers to obtain CE marking for their devices, which involves a conformity assessment process. This process includes clinical evaluation, risk management, and quality management system implementation. The level of scrutiny depends on the device's classification, with higher-risk devices requiring more extensive evaluation.
Regulatory bodies in other countries, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own requirements for PEMF devices. These often align with international standards but may have specific national requirements.
One key regulatory consideration for PEMF therapy is the management of electromagnetic emissions. Devices must comply with electromagnetic compatibility (EMC) standards to ensure they do not interfere with other electronic equipment and are safe for use in various environments.
As the field of PEMF therapy continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. There is an increasing focus on real-world evidence and post-market surveillance to ensure the ongoing safety and effectiveness of PEMF devices in musculoskeletal health applications.
Manufacturers and researchers must navigate these regulatory requirements carefully to bring new PEMF therapies to market. This involves ongoing dialogue with regulatory bodies, adherence to good manufacturing practices, and continuous monitoring of device performance and safety in clinical use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!