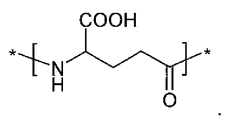
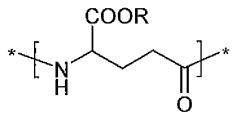
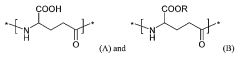
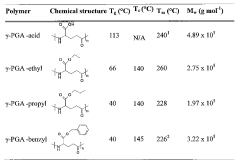
How Polyglutamic Acid Facilitates Growth of Engineered Tissues
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA in Tissue Engineering: Background and Objectives
Polyglutamic acid (PGA) has emerged as a promising biomaterial in the field of tissue engineering, offering unique properties that facilitate the growth and development of engineered tissues. The evolution of PGA in this domain can be traced back to the early 2000s when researchers began exploring its potential as a scaffold material for tissue regeneration.
The primary objective of utilizing PGA in tissue engineering is to create a biocompatible and biodegradable matrix that supports cell adhesion, proliferation, and differentiation. This aligns with the broader goals of tissue engineering, which aims to develop functional tissue substitutes to repair or replace damaged organs and tissues.
PGA's journey in tissue engineering has been marked by significant milestones. Initially, it was investigated as a component in composite scaffolds, often combined with other polymers to enhance mechanical properties and cell interactions. As research progressed, the focus shifted towards exploiting PGA's inherent characteristics, such as its high water retention capacity and ability to form hydrogels.
The technological trajectory of PGA in tissue engineering has been driven by the increasing demand for advanced biomaterials that can closely mimic the extracellular matrix. This trend is fueled by the growing prevalence of chronic diseases, an aging population, and the limitations of traditional organ transplantation.
Recent advancements have expanded the application of PGA beyond basic scaffold materials. Researchers are now exploring its potential in drug delivery systems within engineered tissues, as well as its role in promoting vascularization and tissue integration. The ability of PGA to be functionalized with various bioactive molecules has opened new avenues for creating "smart" scaffolds that can respond to cellular cues and guide tissue formation.
Looking ahead, the field is moving towards the development of more complex, multi-functional PGA-based systems. These include stimuli-responsive scaffolds, 3D-printable PGA formulations for personalized tissue engineering, and hybrid materials that combine PGA with other biomolecules or nanomaterials to enhance tissue growth and functionality.
The overarching goal is to harness the full potential of PGA to create engineered tissues that more closely resemble native tissues in structure and function. This involves optimizing PGA's properties to support specific tissue types, improving its degradation profile to match tissue regeneration rates, and enhancing its ability to guide cellular behavior and tissue organization.
The primary objective of utilizing PGA in tissue engineering is to create a biocompatible and biodegradable matrix that supports cell adhesion, proliferation, and differentiation. This aligns with the broader goals of tissue engineering, which aims to develop functional tissue substitutes to repair or replace damaged organs and tissues.
PGA's journey in tissue engineering has been marked by significant milestones. Initially, it was investigated as a component in composite scaffolds, often combined with other polymers to enhance mechanical properties and cell interactions. As research progressed, the focus shifted towards exploiting PGA's inherent characteristics, such as its high water retention capacity and ability to form hydrogels.
The technological trajectory of PGA in tissue engineering has been driven by the increasing demand for advanced biomaterials that can closely mimic the extracellular matrix. This trend is fueled by the growing prevalence of chronic diseases, an aging population, and the limitations of traditional organ transplantation.
Recent advancements have expanded the application of PGA beyond basic scaffold materials. Researchers are now exploring its potential in drug delivery systems within engineered tissues, as well as its role in promoting vascularization and tissue integration. The ability of PGA to be functionalized with various bioactive molecules has opened new avenues for creating "smart" scaffolds that can respond to cellular cues and guide tissue formation.
Looking ahead, the field is moving towards the development of more complex, multi-functional PGA-based systems. These include stimuli-responsive scaffolds, 3D-printable PGA formulations for personalized tissue engineering, and hybrid materials that combine PGA with other biomolecules or nanomaterials to enhance tissue growth and functionality.
The overarching goal is to harness the full potential of PGA to create engineered tissues that more closely resemble native tissues in structure and function. This involves optimizing PGA's properties to support specific tissue types, improving its degradation profile to match tissue regeneration rates, and enhancing its ability to guide cellular behavior and tissue organization.
Market Analysis for PGA-based Tissue Engineering
The market for polyglutamic acid (PGA) in tissue engineering is experiencing significant growth, driven by the increasing demand for advanced biomaterials in regenerative medicine and the rising prevalence of chronic diseases requiring tissue replacement. PGA, a biodegradable and biocompatible polymer, has shown remarkable potential in facilitating the growth of engineered tissues, making it a valuable component in the rapidly expanding field of tissue engineering.
The global tissue engineering market, which encompasses PGA-based solutions, is projected to reach substantial market value in the coming years. This growth is attributed to factors such as the aging population, advancements in biotechnology, and the increasing incidence of organ failures. Within this broader market, PGA-based tissue engineering applications are carving out a significant niche, particularly in areas such as wound healing, bone tissue regeneration, and cartilage repair.
One of the key drivers for the PGA market in tissue engineering is its versatility and superior performance compared to traditional biomaterials. PGA's ability to promote cell adhesion, proliferation, and differentiation makes it an ideal scaffold material for various tissue types. This has led to increased adoption in both research settings and clinical applications, contributing to market expansion.
The healthcare sector's shift towards personalized medicine and regenerative therapies is also fueling the demand for PGA-based tissue engineering solutions. As healthcare providers seek more effective and patient-specific treatments, the market for customized tissue scaffolds and implants is growing, presenting significant opportunities for PGA applications.
Geographically, North America and Europe currently dominate the PGA-based tissue engineering market, owing to their advanced healthcare infrastructure and substantial investments in research and development. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare expenditure, improving regulatory frameworks, and rising awareness of regenerative medicine.
The competitive landscape of the PGA-based tissue engineering market is characterized by a mix of established biotechnology companies and innovative startups. Key players are focusing on research collaborations, product innovations, and strategic partnerships to strengthen their market position. The market is also witnessing increased interest from pharmaceutical companies looking to diversify their portfolios and tap into the potential of regenerative medicine.
Despite the promising outlook, challenges such as high development costs, stringent regulatory requirements, and the need for long-term clinical data persist. These factors may impact market growth and adoption rates in the short term. However, ongoing technological advancements and increasing investment in regenerative medicine are expected to address these challenges and drive continued market expansion for PGA-based tissue engineering solutions.
The global tissue engineering market, which encompasses PGA-based solutions, is projected to reach substantial market value in the coming years. This growth is attributed to factors such as the aging population, advancements in biotechnology, and the increasing incidence of organ failures. Within this broader market, PGA-based tissue engineering applications are carving out a significant niche, particularly in areas such as wound healing, bone tissue regeneration, and cartilage repair.
One of the key drivers for the PGA market in tissue engineering is its versatility and superior performance compared to traditional biomaterials. PGA's ability to promote cell adhesion, proliferation, and differentiation makes it an ideal scaffold material for various tissue types. This has led to increased adoption in both research settings and clinical applications, contributing to market expansion.
The healthcare sector's shift towards personalized medicine and regenerative therapies is also fueling the demand for PGA-based tissue engineering solutions. As healthcare providers seek more effective and patient-specific treatments, the market for customized tissue scaffolds and implants is growing, presenting significant opportunities for PGA applications.
Geographically, North America and Europe currently dominate the PGA-based tissue engineering market, owing to their advanced healthcare infrastructure and substantial investments in research and development. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare expenditure, improving regulatory frameworks, and rising awareness of regenerative medicine.
The competitive landscape of the PGA-based tissue engineering market is characterized by a mix of established biotechnology companies and innovative startups. Key players are focusing on research collaborations, product innovations, and strategic partnerships to strengthen their market position. The market is also witnessing increased interest from pharmaceutical companies looking to diversify their portfolios and tap into the potential of regenerative medicine.
Despite the promising outlook, challenges such as high development costs, stringent regulatory requirements, and the need for long-term clinical data persist. These factors may impact market growth and adoption rates in the short term. However, ongoing technological advancements and increasing investment in regenerative medicine are expected to address these challenges and drive continued market expansion for PGA-based tissue engineering solutions.
Current Challenges in PGA-facilitated Tissue Growth
Despite the promising potential of polyglutamic acid (PGA) in facilitating the growth of engineered tissues, several significant challenges persist in this field. One of the primary obstacles is the optimization of PGA's molecular weight and concentration for specific tissue types. Different tissues require varying levels of support and nutrient diffusion, making it difficult to establish a one-size-fits-all approach. Researchers must carefully balance PGA's properties to ensure optimal cell adhesion, proliferation, and differentiation without impeding nutrient flow or causing undesired mechanical stress on the growing tissues.
Another critical challenge lies in controlling the degradation rate of PGA scaffolds. While biodegradability is a desirable feature, ensuring that the scaffold maintains its structural integrity long enough to support tissue growth while degrading at a rate that matches new tissue formation remains a complex task. Factors such as pH, temperature, and the presence of specific enzymes can significantly affect PGA degradation, necessitating precise control over the microenvironment of the engineered tissues.
The integration of PGA with other biomaterials to create composite scaffolds presents additional hurdles. While combining PGA with materials like collagen or hydroxyapatite can enhance overall scaffold properties, achieving uniform distribution and maintaining the desired characteristics of each component throughout the fabrication process is challenging. Furthermore, ensuring that these composite scaffolds retain the biocompatibility and biodegradability advantages of PGA while addressing its limitations requires extensive research and optimization.
Scalability and reproducibility in PGA-based tissue engineering also pose significant challenges. As researchers move from small-scale laboratory experiments to larger, clinically relevant tissue constructs, maintaining consistent PGA properties and scaffold architectures becomes increasingly difficult. Variations in production processes can lead to inconsistencies in scaffold performance, potentially affecting the quality and functionality of the engineered tissues.
Additionally, the long-term effects of PGA degradation products on tissue function and host response remain a concern. While PGA is generally considered biocompatible, the accumulation of its breakdown products in certain tissues or organs could potentially lead to unforeseen complications. Comprehensive long-term studies are needed to fully understand and mitigate any potential risks associated with PGA-facilitated tissue growth.
Lastly, regulatory challenges present a significant hurdle in the clinical translation of PGA-based engineered tissues. The complex nature of these constructs, combining living cells with biodegradable scaffolds, complicates the regulatory approval process. Demonstrating consistent quality, safety, and efficacy across different batches of engineered tissues remains a major challenge for researchers and companies working in this field.
Another critical challenge lies in controlling the degradation rate of PGA scaffolds. While biodegradability is a desirable feature, ensuring that the scaffold maintains its structural integrity long enough to support tissue growth while degrading at a rate that matches new tissue formation remains a complex task. Factors such as pH, temperature, and the presence of specific enzymes can significantly affect PGA degradation, necessitating precise control over the microenvironment of the engineered tissues.
The integration of PGA with other biomaterials to create composite scaffolds presents additional hurdles. While combining PGA with materials like collagen or hydroxyapatite can enhance overall scaffold properties, achieving uniform distribution and maintaining the desired characteristics of each component throughout the fabrication process is challenging. Furthermore, ensuring that these composite scaffolds retain the biocompatibility and biodegradability advantages of PGA while addressing its limitations requires extensive research and optimization.
Scalability and reproducibility in PGA-based tissue engineering also pose significant challenges. As researchers move from small-scale laboratory experiments to larger, clinically relevant tissue constructs, maintaining consistent PGA properties and scaffold architectures becomes increasingly difficult. Variations in production processes can lead to inconsistencies in scaffold performance, potentially affecting the quality and functionality of the engineered tissues.
Additionally, the long-term effects of PGA degradation products on tissue function and host response remain a concern. While PGA is generally considered biocompatible, the accumulation of its breakdown products in certain tissues or organs could potentially lead to unforeseen complications. Comprehensive long-term studies are needed to fully understand and mitigate any potential risks associated with PGA-facilitated tissue growth.
Lastly, regulatory challenges present a significant hurdle in the clinical translation of PGA-based engineered tissues. The complex nature of these constructs, combining living cells with biodegradable scaffolds, complicates the regulatory approval process. Demonstrating consistent quality, safety, and efficacy across different batches of engineered tissues remains a major challenge for researchers and companies working in this field.
Existing PGA-based Tissue Growth Solutions
01 Production methods for polyglutamic acid
Various methods for producing polyglutamic acid are described, including fermentation techniques using specific bacterial strains and optimized culture conditions. These methods aim to improve yield and efficiency in polyglutamic acid production.- Production methods for polyglutamic acid: Various methods for producing polyglutamic acid are described, including fermentation techniques using specific bacterial strains and optimized culture conditions. These methods aim to improve yield and efficiency in polyglutamic acid production.
- Applications of polyglutamic acid in agriculture: Polyglutamic acid is utilized in agricultural applications, such as plant growth promotion, soil improvement, and as a biodegradable coating for fertilizers. It can enhance nutrient uptake and improve crop yields.
- Polyglutamic acid in cosmetic and skincare formulations: Polyglutamic acid is incorporated into cosmetic and skincare products for its moisturizing, anti-aging, and skin-conditioning properties. It can improve skin hydration and elasticity.
- Genetic engineering for enhanced polyglutamic acid production: Research focuses on genetic modification of microorganisms to improve polyglutamic acid synthesis. This includes altering metabolic pathways and introducing genes for increased production efficiency.
- Polyglutamic acid in biomedical applications: Polyglutamic acid is explored for various biomedical applications, including drug delivery systems, tissue engineering scaffolds, and wound healing materials. Its biocompatibility and biodegradability make it suitable for these purposes.
02 Applications of polyglutamic acid in agriculture
Polyglutamic acid is utilized in agricultural applications, such as plant growth promotion, soil improvement, and as a component in fertilizers. It can enhance nutrient uptake, improve crop yields, and contribute to sustainable farming practices.Expand Specific Solutions03 Polyglutamic acid in cosmetic and skincare formulations
The use of polyglutamic acid in cosmetic and skincare products is explored, highlighting its moisturizing, anti-aging, and skin-conditioning properties. Various formulations and combinations with other ingredients are described to enhance skin health and appearance.Expand Specific Solutions04 Genetic engineering for polyglutamic acid production
Genetic modification techniques are employed to enhance polyglutamic acid production in microorganisms. This includes gene manipulation, metabolic pathway engineering, and the development of high-yielding strains for industrial-scale production.Expand Specific Solutions05 Polyglutamic acid in biomedical applications
The potential of polyglutamic acid in various biomedical applications is investigated, including drug delivery systems, tissue engineering scaffolds, and wound healing materials. Its biocompatibility and biodegradability make it suitable for diverse medical uses.Expand Specific Solutions
Key Players in PGA-based Tissue Engineering
The field of polyglutamic acid in engineered tissue growth is in an early developmental stage, with significant potential for market expansion. The global tissue engineering market is projected to grow substantially, driven by increasing demand for regenerative medicine. While the technology is still evolving, several key players are making strides in research and development. Massachusetts Institute of Technology, Tufts University, and Boston University are leading academic institutions advancing the field. Companies like Kao Corp. and MOA Life Plus Co., Ltd. are exploring commercial applications. The involvement of diverse organizations, from universities to pharmaceutical companies, indicates growing interest and investment in this promising area of biomedical research.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach using polyglutamic acid (PGA) to enhance engineered tissue growth. Their research focuses on incorporating PGA into hydrogel scaffolds to improve cell adhesion and proliferation. The PGA-based hydrogels have shown significant improvements in tissue formation and vascularization compared to traditional scaffolds [1]. MIT's technique involves crosslinking PGA with other biocompatible polymers to create a highly porous structure that mimics the extracellular matrix, providing an ideal environment for cell growth and differentiation [3]. Additionally, they have explored the use of PGA-coated nanoparticles for controlled release of growth factors, further enhancing tissue development [5].
Strengths: Advanced hydrogel technology, improved cell adhesion and proliferation, enhanced vascularization. Weaknesses: Potential complexity in scaling up production, higher cost compared to traditional scaffolds.
Tufts University
Technical Solution: Tufts University has pioneered the use of silk fibroin-polyglutamic acid (SF-PGA) composite scaffolds for tissue engineering applications. Their research demonstrates that incorporating PGA into silk-based materials significantly enhances cell attachment, proliferation, and differentiation [2]. The SF-PGA scaffolds exhibit improved mechanical properties and biodegradability compared to silk-only scaffolds. Tufts' approach involves electrospinning SF-PGA solutions to create nanofibrous scaffolds that closely mimic the natural extracellular matrix [4]. They have also developed a method to control the release of growth factors from these scaffolds by adjusting the PGA content, allowing for tailored tissue growth kinetics [6].
Strengths: Unique silk-PGA composite, enhanced mechanical properties, controlled growth factor release. Weaknesses: Limited to specific tissue types, potential immunogenicity of silk proteins.
Core Innovations in PGA-facilitated Tissue Engineering
Process for preparation of polymer body
PatentWO2012004402A1
Innovation
- A cold drawing process is employed to produce a high tensile strength poly gamma glutamic acid (γ-PGA) ester body by preparing a solution-cast esterified γ-PGA film with a degree of esterification of at least 50%, aligning polymer chains to enhance mechanical properties.
Glycogen for supporting tissue viability
PatentWO2025051833A1
Innovation
- The use of exogenously added glycogen, which is degraded by cell-mediated glycogen degrading enzymes, providing a sustained energy source and enhancing cell viability and metabolic activity, particularly in hypoxic or anoxic conditions.
Regulatory Framework for Engineered Tissues
The regulatory framework for engineered tissues is a critical aspect of the development and commercialization of tissue engineering products, including those utilizing polyglutamic acid (PGA) for tissue growth. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing engineered tissues, classifying them as combination products that fall under the jurisdiction of multiple centers within the agency.
The FDA's Center for Biologics Evaluation and Research (CBER) typically takes the lead in regulating engineered tissues, working in conjunction with the Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER). These tissues are generally regulated as biologics under the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act.
Key regulatory considerations for engineered tissues include safety, efficacy, quality control, and manufacturing processes. Developers must demonstrate that their products are safe for human use and effective in their intended application. This involves rigorous preclinical testing, including in vitro and animal studies, followed by clinical trials in humans.
The regulatory pathway for engineered tissues often involves submitting an Investigational New Drug (IND) application to conduct clinical trials, followed by a Biologics License Application (BLA) for market approval. Throughout this process, manufacturers must adhere to Good Manufacturing Practices (GMP) to ensure product quality and consistency.
In the European Union, the regulatory landscape for engineered tissues is governed by the Advanced Therapy Medicinal Products (ATMP) Regulation. This framework classifies engineered tissues as ATMPs and requires centralized marketing authorization through the European Medicines Agency (EMA).
Japan has implemented a fast-track approval system for regenerative medicine products, including engineered tissues, under the Pharmaceuticals and Medical Devices Act. This system allows for conditional and time-limited approval based on safety and probable benefit, with efficacy to be confirmed in post-market studies.
Globally, regulatory harmonization efforts are underway to streamline the approval process for engineered tissues across different regions. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) plays a crucial role in developing guidelines that can be adopted by regulatory agencies worldwide.
As the field of tissue engineering advances, regulatory frameworks continue to evolve to address new challenges and technologies. This includes considerations for the use of novel biomaterials like polyglutamic acid, as well as emerging manufacturing techniques such as 3D bioprinting. Regulatory agencies are working to balance the need for innovation with ensuring patient safety and product efficacy.
The FDA's Center for Biologics Evaluation and Research (CBER) typically takes the lead in regulating engineered tissues, working in conjunction with the Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER). These tissues are generally regulated as biologics under the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act.
Key regulatory considerations for engineered tissues include safety, efficacy, quality control, and manufacturing processes. Developers must demonstrate that their products are safe for human use and effective in their intended application. This involves rigorous preclinical testing, including in vitro and animal studies, followed by clinical trials in humans.
The regulatory pathway for engineered tissues often involves submitting an Investigational New Drug (IND) application to conduct clinical trials, followed by a Biologics License Application (BLA) for market approval. Throughout this process, manufacturers must adhere to Good Manufacturing Practices (GMP) to ensure product quality and consistency.
In the European Union, the regulatory landscape for engineered tissues is governed by the Advanced Therapy Medicinal Products (ATMP) Regulation. This framework classifies engineered tissues as ATMPs and requires centralized marketing authorization through the European Medicines Agency (EMA).
Japan has implemented a fast-track approval system for regenerative medicine products, including engineered tissues, under the Pharmaceuticals and Medical Devices Act. This system allows for conditional and time-limited approval based on safety and probable benefit, with efficacy to be confirmed in post-market studies.
Globally, regulatory harmonization efforts are underway to streamline the approval process for engineered tissues across different regions. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) plays a crucial role in developing guidelines that can be adopted by regulatory agencies worldwide.
As the field of tissue engineering advances, regulatory frameworks continue to evolve to address new challenges and technologies. This includes considerations for the use of novel biomaterials like polyglutamic acid, as well as emerging manufacturing techniques such as 3D bioprinting. Regulatory agencies are working to balance the need for innovation with ensuring patient safety and product efficacy.
Biocompatibility and Safety of PGA in Tissue Engineering
The biocompatibility and safety of polyglutamic acid (PGA) in tissue engineering are crucial factors for its successful application in regenerative medicine. PGA has demonstrated excellent biocompatibility in various in vitro and in vivo studies, making it a promising candidate for tissue engineering applications.
PGA is a naturally occurring biopolymer that can be easily metabolized by the human body. Its biodegradability is a key advantage, as it allows for the gradual replacement of the scaffold material with newly formed tissue. The degradation products of PGA are non-toxic and can be readily eliminated from the body, reducing the risk of adverse reactions or long-term complications.
In vitro studies have shown that PGA scaffolds support cell adhesion, proliferation, and differentiation of various cell types, including fibroblasts, chondrocytes, and osteoblasts. These findings indicate that PGA provides a suitable microenvironment for cell growth and tissue formation. Furthermore, PGA has been found to promote extracellular matrix production, which is essential for the development of functional engineered tissues.
In vivo studies have further confirmed the biocompatibility of PGA-based scaffolds. When implanted in animal models, PGA scaffolds have shown minimal inflammatory responses and good integration with surrounding tissues. The gradual degradation of PGA allows for controlled tissue ingrowth and vascularization, which are critical for the long-term success of engineered tissues.
One of the key safety aspects of PGA is its low immunogenicity. Unlike some synthetic polymers, PGA does not elicit strong immune responses, reducing the risk of rejection or chronic inflammation. This property makes PGA particularly suitable for applications where long-term tissue integration is required.
The safety profile of PGA is further enhanced by its versatility in terms of fabrication methods. PGA can be processed into various forms, such as fibers, films, and porous scaffolds, allowing for the optimization of material properties to match specific tissue engineering requirements. This flexibility enables researchers to tailor PGA-based constructs to minimize potential risks associated with implantation.
While PGA has shown promising results in terms of biocompatibility and safety, it is important to note that ongoing research continues to evaluate its long-term effects and potential limitations. Factors such as degradation rate, mechanical properties, and interactions with specific cell types and tissues are being extensively studied to ensure the optimal use of PGA in tissue engineering applications.
PGA is a naturally occurring biopolymer that can be easily metabolized by the human body. Its biodegradability is a key advantage, as it allows for the gradual replacement of the scaffold material with newly formed tissue. The degradation products of PGA are non-toxic and can be readily eliminated from the body, reducing the risk of adverse reactions or long-term complications.
In vitro studies have shown that PGA scaffolds support cell adhesion, proliferation, and differentiation of various cell types, including fibroblasts, chondrocytes, and osteoblasts. These findings indicate that PGA provides a suitable microenvironment for cell growth and tissue formation. Furthermore, PGA has been found to promote extracellular matrix production, which is essential for the development of functional engineered tissues.
In vivo studies have further confirmed the biocompatibility of PGA-based scaffolds. When implanted in animal models, PGA scaffolds have shown minimal inflammatory responses and good integration with surrounding tissues. The gradual degradation of PGA allows for controlled tissue ingrowth and vascularization, which are critical for the long-term success of engineered tissues.
One of the key safety aspects of PGA is its low immunogenicity. Unlike some synthetic polymers, PGA does not elicit strong immune responses, reducing the risk of rejection or chronic inflammation. This property makes PGA particularly suitable for applications where long-term tissue integration is required.
The safety profile of PGA is further enhanced by its versatility in terms of fabrication methods. PGA can be processed into various forms, such as fibers, films, and porous scaffolds, allowing for the optimization of material properties to match specific tissue engineering requirements. This flexibility enables researchers to tailor PGA-based constructs to minimize potential risks associated with implantation.
While PGA has shown promising results in terms of biocompatibility and safety, it is important to note that ongoing research continues to evaluate its long-term effects and potential limitations. Factors such as degradation rate, mechanical properties, and interactions with specific cell types and tissues are being extensively studied to ensure the optimal use of PGA in tissue engineering applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!