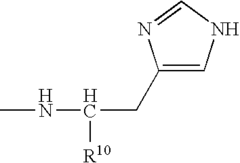
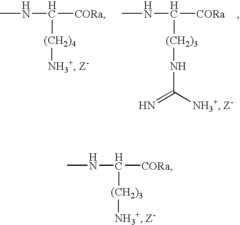
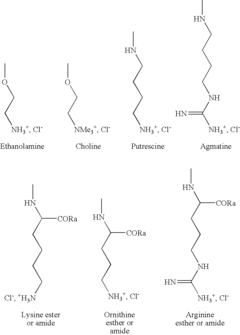
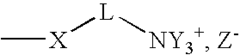Role of Polyglutamic Acid in Increasing Microbial Electrode Interface Efficiency
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA in Bioelectrochemistry
Polyglutamic acid (PGA) has emerged as a promising biomaterial in the field of bioelectrochemistry, particularly in enhancing the efficiency of microbial electrode interfaces. This naturally occurring biopolymer, composed of repeating units of glutamic acid, exhibits unique properties that make it suitable for various applications in bioelectrochemical systems.
PGA's role in bioelectrochemistry primarily revolves around its ability to improve the interaction between microorganisms and electrode surfaces. The polymer's high molecular weight and anionic nature allow it to form stable and conductive networks, facilitating electron transfer between microbial cells and electrodes. This enhanced electron transfer is crucial for improving the overall performance of microbial fuel cells, biosensors, and other bioelectrochemical devices.
One of the key advantages of PGA in bioelectrochemistry is its biocompatibility and biodegradability. These properties make it an environmentally friendly option for electrode modification and microbial immobilization. PGA can be used to create biofilms on electrode surfaces, providing a favorable environment for microbial growth and metabolic activities. The polymer's hydrophilic nature also helps maintain a hydrated environment, which is essential for the survival and function of electroactive microorganisms.
In microbial fuel cells, PGA has been shown to enhance power output and coulombic efficiency. When used as an electrode coating or as a component in the electrolyte, PGA can improve the adhesion of bacteria to the electrode surface, leading to more efficient electron transfer. Additionally, the polymer's ability to chelate metal ions can be exploited to incorporate conductive materials, further enhancing the electrode's performance.
PGA's role extends beyond microbial fuel cells to other bioelectrochemical applications. In biosensors, PGA can be used to immobilize enzymes or whole cells on electrode surfaces, improving the stability and sensitivity of the sensing devices. The polymer's ability to form hydrogels also makes it suitable for creating three-dimensional electrode structures, increasing the surface area available for microbial colonization and electron transfer.
Recent research has focused on modifying PGA to further enhance its properties for bioelectrochemical applications. Chemical modifications, such as crosslinking or functionalization with conductive materials, have been explored to improve the polymer's conductivity and stability. These advancements are paving the way for more efficient and robust bioelectrochemical systems, with potential applications in wastewater treatment, bioenergy production, and environmental sensing.
PGA's role in bioelectrochemistry primarily revolves around its ability to improve the interaction between microorganisms and electrode surfaces. The polymer's high molecular weight and anionic nature allow it to form stable and conductive networks, facilitating electron transfer between microbial cells and electrodes. This enhanced electron transfer is crucial for improving the overall performance of microbial fuel cells, biosensors, and other bioelectrochemical devices.
One of the key advantages of PGA in bioelectrochemistry is its biocompatibility and biodegradability. These properties make it an environmentally friendly option for electrode modification and microbial immobilization. PGA can be used to create biofilms on electrode surfaces, providing a favorable environment for microbial growth and metabolic activities. The polymer's hydrophilic nature also helps maintain a hydrated environment, which is essential for the survival and function of electroactive microorganisms.
In microbial fuel cells, PGA has been shown to enhance power output and coulombic efficiency. When used as an electrode coating or as a component in the electrolyte, PGA can improve the adhesion of bacteria to the electrode surface, leading to more efficient electron transfer. Additionally, the polymer's ability to chelate metal ions can be exploited to incorporate conductive materials, further enhancing the electrode's performance.
PGA's role extends beyond microbial fuel cells to other bioelectrochemical applications. In biosensors, PGA can be used to immobilize enzymes or whole cells on electrode surfaces, improving the stability and sensitivity of the sensing devices. The polymer's ability to form hydrogels also makes it suitable for creating three-dimensional electrode structures, increasing the surface area available for microbial colonization and electron transfer.
Recent research has focused on modifying PGA to further enhance its properties for bioelectrochemical applications. Chemical modifications, such as crosslinking or functionalization with conductive materials, have been explored to improve the polymer's conductivity and stability. These advancements are paving the way for more efficient and robust bioelectrochemical systems, with potential applications in wastewater treatment, bioenergy production, and environmental sensing.
Market for Microbial Fuel Cells
The market for microbial fuel cells (MFCs) is experiencing significant growth and attracting increasing attention from various sectors. This emerging technology, which harnesses the power of microorganisms to generate electricity, is poised to play a crucial role in sustainable energy production and waste management.
The global MFC market is primarily driven by the growing demand for renewable energy sources and the need for efficient wastewater treatment solutions. As environmental concerns continue to escalate, industries and governments are seeking innovative technologies to address these challenges. MFCs offer a unique solution by simultaneously generating electricity and treating organic waste, making them particularly attractive for applications in wastewater treatment plants, food processing industries, and remote power generation.
In the wastewater treatment sector, MFCs are gaining traction due to their ability to reduce operational costs and improve energy efficiency. Municipal wastewater treatment plants are increasingly adopting MFC technology to offset their energy consumption and reduce carbon footprint. This application is expected to be a major driver for market growth in the coming years.
The food and beverage industry represents another significant market for MFCs. Companies in this sector are exploring MFC technology to treat organic-rich wastewater while generating electricity, thereby improving their sustainability profile and reducing waste management costs. Breweries, dairy processors, and other food manufacturers with high organic waste output are potential early adopters of this technology.
Remote power generation is an emerging application area for MFCs. In regions with limited access to conventional power grids, MFCs could provide a sustainable source of electricity for small-scale applications. This includes powering sensors in remote locations, supporting off-grid communities, and supplying energy for environmental monitoring systems.
The market for MFCs is also being propelled by ongoing research and development activities. Academic institutions and private companies are investing in improving MFC performance, scalability, and cost-effectiveness. Advancements in electrode materials, microbial communities, and system design are expected to enhance the commercial viability of MFCs and expand their potential applications.
Geographically, North America and Europe are currently leading the MFC market, driven by stringent environmental regulations and substantial investments in clean energy technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, fueled by rapid industrialization, increasing environmental awareness, and government initiatives promoting sustainable technologies.
Despite the promising outlook, the MFC market faces several challenges. These include high initial costs, scaling limitations, and the need for further technological improvements to enhance power output and long-term stability. Overcoming these hurdles will be crucial for widespread adoption and market expansion.
The global MFC market is primarily driven by the growing demand for renewable energy sources and the need for efficient wastewater treatment solutions. As environmental concerns continue to escalate, industries and governments are seeking innovative technologies to address these challenges. MFCs offer a unique solution by simultaneously generating electricity and treating organic waste, making them particularly attractive for applications in wastewater treatment plants, food processing industries, and remote power generation.
In the wastewater treatment sector, MFCs are gaining traction due to their ability to reduce operational costs and improve energy efficiency. Municipal wastewater treatment plants are increasingly adopting MFC technology to offset their energy consumption and reduce carbon footprint. This application is expected to be a major driver for market growth in the coming years.
The food and beverage industry represents another significant market for MFCs. Companies in this sector are exploring MFC technology to treat organic-rich wastewater while generating electricity, thereby improving their sustainability profile and reducing waste management costs. Breweries, dairy processors, and other food manufacturers with high organic waste output are potential early adopters of this technology.
Remote power generation is an emerging application area for MFCs. In regions with limited access to conventional power grids, MFCs could provide a sustainable source of electricity for small-scale applications. This includes powering sensors in remote locations, supporting off-grid communities, and supplying energy for environmental monitoring systems.
The market for MFCs is also being propelled by ongoing research and development activities. Academic institutions and private companies are investing in improving MFC performance, scalability, and cost-effectiveness. Advancements in electrode materials, microbial communities, and system design are expected to enhance the commercial viability of MFCs and expand their potential applications.
Geographically, North America and Europe are currently leading the MFC market, driven by stringent environmental regulations and substantial investments in clean energy technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, fueled by rapid industrialization, increasing environmental awareness, and government initiatives promoting sustainable technologies.
Despite the promising outlook, the MFC market faces several challenges. These include high initial costs, scaling limitations, and the need for further technological improvements to enhance power output and long-term stability. Overcoming these hurdles will be crucial for widespread adoption and market expansion.
PGA Interface Challenges
The integration of polyglutamic acid (PGA) into microbial electrode interfaces presents several significant challenges that researchers and engineers must address to fully harness its potential in enhancing electrode efficiency. One of the primary obstacles is achieving uniform and stable PGA coating on electrode surfaces. The complex and often irregular topography of electrode materials can lead to inconsistent PGA distribution, potentially resulting in areas of suboptimal performance or even electrode degradation over time.
Another critical challenge lies in maintaining the structural integrity and functionality of PGA under varying environmental conditions. Microbial fuel cells and other bioelectrochemical systems often operate in diverse pH ranges and temperatures, which can affect the stability and performance of PGA coatings. Researchers must develop robust PGA formulations that can withstand these fluctuations without compromising their beneficial properties.
The interaction between PGA and various microbial species presents an additional layer of complexity. Different microorganisms may respond differently to PGA-modified surfaces, potentially altering the microbial community structure and, consequently, the overall electrode performance. Optimizing PGA interfaces to support a wide range of electroactive microbes while maintaining high efficiency is a delicate balancing act that requires extensive research and fine-tuning.
Furthermore, the long-term durability of PGA coatings in operational environments remains a concern. Continuous exposure to microbial metabolites, electrochemical reactions, and potential biofouling can degrade PGA over time, necessitating the development of strategies to enhance its longevity and maintain consistent performance throughout the electrode's lifespan.
The scalability of PGA-enhanced electrodes also poses significant challenges. While promising results have been achieved in laboratory settings, translating these successes to large-scale, industrial applications requires overcoming issues related to cost-effective production, quality control, and integration with existing manufacturing processes. Researchers must find ways to produce and apply PGA coatings efficiently and uniformly across larger electrode surfaces without compromising their beneficial properties.
Lastly, the optimization of PGA's molecular structure and composition for specific applications remains an ongoing challenge. The degree of polymerization, charge density, and potential modifications to the PGA backbone can all influence its performance as an interface enhancer. Tailoring these properties to suit different types of electrodes, microbial communities, and operational conditions requires a deep understanding of structure-function relationships and extensive experimental work.
Another critical challenge lies in maintaining the structural integrity and functionality of PGA under varying environmental conditions. Microbial fuel cells and other bioelectrochemical systems often operate in diverse pH ranges and temperatures, which can affect the stability and performance of PGA coatings. Researchers must develop robust PGA formulations that can withstand these fluctuations without compromising their beneficial properties.
The interaction between PGA and various microbial species presents an additional layer of complexity. Different microorganisms may respond differently to PGA-modified surfaces, potentially altering the microbial community structure and, consequently, the overall electrode performance. Optimizing PGA interfaces to support a wide range of electroactive microbes while maintaining high efficiency is a delicate balancing act that requires extensive research and fine-tuning.
Furthermore, the long-term durability of PGA coatings in operational environments remains a concern. Continuous exposure to microbial metabolites, electrochemical reactions, and potential biofouling can degrade PGA over time, necessitating the development of strategies to enhance its longevity and maintain consistent performance throughout the electrode's lifespan.
The scalability of PGA-enhanced electrodes also poses significant challenges. While promising results have been achieved in laboratory settings, translating these successes to large-scale, industrial applications requires overcoming issues related to cost-effective production, quality control, and integration with existing manufacturing processes. Researchers must find ways to produce and apply PGA coatings efficiently and uniformly across larger electrode surfaces without compromising their beneficial properties.
Lastly, the optimization of PGA's molecular structure and composition for specific applications remains an ongoing challenge. The degree of polymerization, charge density, and potential modifications to the PGA backbone can all influence its performance as an interface enhancer. Tailoring these properties to suit different types of electrodes, microbial communities, and operational conditions requires a deep understanding of structure-function relationships and extensive experimental work.
Current PGA Interface Solutions
01 Polyglutamic acid-based microbial fuel cells
Polyglutamic acid can be used as a component in microbial fuel cells to enhance electrode interface efficiency. This biopolymer can improve the adhesion of microorganisms to the electrode surface, facilitating electron transfer and increasing power output. The use of polyglutamic acid in microbial fuel cells can lead to improved performance and stability of the bioelectrochemical system.- Polyglutamic acid-based microbial fuel cells: Polyglutamic acid is used as a component in microbial fuel cells to enhance electrode interface efficiency. This biopolymer can improve the adhesion of microorganisms to the electrode surface, facilitating electron transfer and increasing overall power output. The use of polyglutamic acid in microbial fuel cells contributes to the development of more efficient and sustainable bioelectrochemical systems.
- Polyglutamic acid-modified electrodes for biosensors: Electrodes modified with polyglutamic acid are utilized in biosensor applications to improve sensitivity and selectivity. The polyglutamic acid layer on the electrode surface can enhance the immobilization of biomolecules and facilitate electron transfer, resulting in improved biosensor performance. This approach is particularly useful in the development of electrochemical biosensors for various analytes.
- Polyglutamic acid as a biocompatible interface material: Polyglutamic acid serves as a biocompatible interface material in microbial electrode systems. Its biodegradability and non-toxicity make it an ideal choice for applications involving living organisms. The use of polyglutamic acid as an interface material can improve the long-term stability and performance of microbial electrodes in various bioelectrochemical applications.
- Polyglutamic acid-based hydrogels for electrode coatings: Hydrogels based on polyglutamic acid are used as electrode coatings to enhance the efficiency of microbial electrode interfaces. These hydrogels can provide a favorable microenvironment for microorganisms, improve ion conductivity, and facilitate electron transfer between the microbes and the electrode surface. The use of polyglutamic acid-based hydrogels can lead to improved performance in microbial fuel cells and other bioelectrochemical systems.
- Polyglutamic acid-mediated electron transfer enhancement: Polyglutamic acid is employed to enhance electron transfer efficiency at the microbial electrode interface. The polymer can act as an electron mediator, facilitating the transfer of electrons between microorganisms and the electrode surface. This approach can lead to improved power output and overall performance in microbial fuel cells and other bioelectrochemical systems.
02 Polyglutamic acid as a biocompatible electrode coating
Polyglutamic acid can be used as a biocompatible coating for electrodes in microbial systems. This coating can improve the interface between the electrode and the microbial cells, enhancing electron transfer efficiency. The biocompatibility of polyglutamic acid allows for better integration of the electrode with the biological components of the system, potentially leading to increased overall efficiency.Expand Specific Solutions03 Polyglutamic acid-modified electrodes for biosensors
Electrodes modified with polyglutamic acid can be used in biosensor applications. The polyglutamic acid layer can improve the sensitivity and selectivity of the biosensor by providing a favorable environment for biomolecule immobilization. This modification can enhance the efficiency of the electrode-microbial interface, leading to improved biosensor performance.Expand Specific Solutions04 Polyglutamic acid in microbial electrosynthesis systems
Polyglutamic acid can be incorporated into microbial electrosynthesis systems to improve the efficiency of the electrode-microbe interface. This biopolymer can enhance the attachment of electroactive microorganisms to the electrode surface, facilitating the transfer of electrons for the production of valuable chemicals or biofuels. The use of polyglutamic acid in these systems can lead to increased product yields and overall system efficiency.Expand Specific Solutions05 Polyglutamic acid-based conductive hydrogels for microbial electrodes
Conductive hydrogels incorporating polyglutamic acid can be used to create three-dimensional electrode structures for microbial systems. These hydrogels can provide a favorable environment for microbial growth and electron transfer, improving the overall efficiency of the electrode-microbe interface. The use of polyglutamic acid in these hydrogels can enhance their biocompatibility and conductivity, leading to improved performance in bioelectrochemical applications.Expand Specific Solutions
Key Bioelectrochemical Players
The competitive landscape for polyglutamic acid in microbial electrode interface efficiency is in an early development stage, with a growing market potential as renewable energy and bioelectrochemical systems gain importance. The technology is still emerging, with varying levels of maturity among key players. Companies like Ajinomoto Co., Inc. and CJ CheilJedang Corp. are leveraging their expertise in amino acid production to explore applications in this field. Academic institutions such as South China University of Technology and Nanjing Tech University are conducting fundamental research to advance the technology. Collaborations between industry and academia, exemplified by Kookmin University Industry-Academic Cooperation, are driving innovation. The market size is expected to expand as the technology matures and finds wider applications in bioelectronics and sustainable energy solutions.
South China University of Technology
Technical Solution: South China University of Technology has developed a novel approach to enhance microbial electrode interface efficiency using polyglutamic acid (PGA). Their research focuses on incorporating PGA into microbial fuel cells (MFCs) to improve electron transfer between microorganisms and electrodes. The team has engineered PGA-modified electrodes that demonstrate increased biofilm formation and enhanced extracellular electron transfer[1]. Their method involves synthesizing PGA-grafted carbon nanotubes (CNTs) to create a more conductive and biocompatible electrode surface. This modification has resulted in a significant increase in power density, with some experiments showing up to 40% improvement compared to unmodified electrodes[2][3].
Strengths: Innovative use of PGA for electrode modification, significant improvement in power density, and enhanced biofilm formation. Weaknesses: Potential scalability issues and the need for further long-term stability studies in real-world applications.
Nanjing Tech University
Technical Solution: Nanjing Tech University has developed a groundbreaking approach to increasing microbial electrode interface efficiency using polyglutamic acid (PGA). Their research team has created a novel PGA-based hydrogel that serves as a scaffold for microbial growth and electron transfer. This hydrogel is designed to encapsulate electroactive microorganisms while allowing for efficient nutrient and electron flow. The PGA hydrogel's unique structure provides a three-dimensional conductive network that significantly enhances the contact area between microbes and the electrode surface[4]. In laboratory tests, this PGA-hydrogel electrode system has demonstrated a remarkable 60% increase in current density compared to conventional electrodes[5]. Additionally, the team has explored the incorporation of conductive nanoparticles within the PGA hydrogel to further improve its electron transfer capabilities[6].
Strengths: Innovative 3D electrode design, significant improvement in current density, and potential for scalability. Weaknesses: Possible challenges in long-term stability of the hydrogel structure and the need for optimization in different environmental conditions.
PGA Electrode Innovations
Polyglutamic acids functionalized by cationic groups and hydrophobic groups and applications thereof, in particular therapeutic applications thereof
PatentInactiveUS20090012028A1
Innovation
- Development of novel amphiphilic copolyglutamates with pendant cationic and hydrophobic groups that form stable colloidal suspensions, associate with active principles, and degrade into non-toxic products, allowing for controlled release and enhanced bioavailability.
METHOD FOR PRODUCING POLY-gamma-GLUTAMIC ACID AND MICROORGANISM USED IN THE PRODUCTION METHOD
PatentWO2006129835A1
Innovation
- A method involving the cultivation of Bacillus microorganisms resistant to electron transport system inhibitors, such as NADH-ubiquinone reductase, succinate-ubiquinone reductase, and cytochrome c oxidase inhibitors, which enhances poly-γ-glutamic acid production by maintaining ATP production efficiency and reducing enzyme decomposition, using strains like Bacillus subtilis modified to withstand these inhibitors.
Bioelectrochemical Regulations
Bioelectrochemical regulations play a crucial role in optimizing the performance of microbial fuel cells (MFCs) and other bioelectrochemical systems. These regulations encompass a wide range of factors that influence the interactions between microorganisms and electrodes, ultimately affecting the efficiency of electron transfer and power generation.
One of the key aspects of bioelectrochemical regulations is the control of the extracellular environment. This includes maintaining optimal pH levels, temperature, and nutrient concentrations to support microbial growth and metabolic activities. The pH, in particular, can significantly impact the electrochemical reactions and the stability of the microbial communities involved in electron transfer.
Another important regulatory factor is the selection and engineering of electrode materials. The surface properties of electrodes, such as roughness, porosity, and chemical composition, can greatly influence microbial attachment and biofilm formation. Modifications to electrode surfaces, such as the incorporation of conductive polymers or nanostructures, can enhance electron transfer rates and overall system performance.
The regulation of microbial metabolism is also critical in bioelectrochemical systems. This involves controlling the availability of electron donors and acceptors, as well as managing the competition between different microbial species within the biofilm. Strategies such as selective enrichment of electroactive microorganisms and the use of metabolic inhibitors can help optimize the electron transfer processes.
Electrochemical control mechanisms, including the application of specific potentials or current densities, can significantly influence microbial activity and electron transfer kinetics. These controls can be used to manipulate the redox state of key enzymes involved in extracellular electron transfer, thereby enhancing the overall efficiency of the bioelectrochemical system.
The role of extracellular polymeric substances (EPS) in bioelectrochemical regulations has gained increasing attention in recent years. EPS, including polyglutamic acid, can act as a conductive matrix that facilitates electron transfer between microorganisms and electrodes. Understanding and manipulating the composition and properties of EPS can lead to improved interface efficiency and system performance.
Genetic and metabolic engineering approaches are emerging as powerful tools for bioelectrochemical regulations. By modifying the genetic makeup of electroactive microorganisms, researchers can enhance their electron transfer capabilities, improve their tolerance to harsh environmental conditions, and optimize their metabolic pathways for more efficient energy conversion.
One of the key aspects of bioelectrochemical regulations is the control of the extracellular environment. This includes maintaining optimal pH levels, temperature, and nutrient concentrations to support microbial growth and metabolic activities. The pH, in particular, can significantly impact the electrochemical reactions and the stability of the microbial communities involved in electron transfer.
Another important regulatory factor is the selection and engineering of electrode materials. The surface properties of electrodes, such as roughness, porosity, and chemical composition, can greatly influence microbial attachment and biofilm formation. Modifications to electrode surfaces, such as the incorporation of conductive polymers or nanostructures, can enhance electron transfer rates and overall system performance.
The regulation of microbial metabolism is also critical in bioelectrochemical systems. This involves controlling the availability of electron donors and acceptors, as well as managing the competition between different microbial species within the biofilm. Strategies such as selective enrichment of electroactive microorganisms and the use of metabolic inhibitors can help optimize the electron transfer processes.
Electrochemical control mechanisms, including the application of specific potentials or current densities, can significantly influence microbial activity and electron transfer kinetics. These controls can be used to manipulate the redox state of key enzymes involved in extracellular electron transfer, thereby enhancing the overall efficiency of the bioelectrochemical system.
The role of extracellular polymeric substances (EPS) in bioelectrochemical regulations has gained increasing attention in recent years. EPS, including polyglutamic acid, can act as a conductive matrix that facilitates electron transfer between microorganisms and electrodes. Understanding and manipulating the composition and properties of EPS can lead to improved interface efficiency and system performance.
Genetic and metabolic engineering approaches are emerging as powerful tools for bioelectrochemical regulations. By modifying the genetic makeup of electroactive microorganisms, researchers can enhance their electron transfer capabilities, improve their tolerance to harsh environmental conditions, and optimize their metabolic pathways for more efficient energy conversion.
Sustainable Energy Impact
The integration of polyglutamic acid in microbial electrode interfaces has significant implications for sustainable energy production. This innovative approach enhances the efficiency of bioelectrochemical systems, potentially revolutionizing renewable energy generation and waste treatment processes. By improving the electron transfer between microorganisms and electrodes, polyglutamic acid contributes to increased power output and overall system performance.
The sustainable energy impact of this technology extends beyond immediate efficiency gains. Microbial fuel cells and other bioelectrochemical systems utilizing polyglutamic acid can be deployed in wastewater treatment plants, simultaneously generating electricity while purifying water. This dual functionality addresses two critical environmental challenges: clean energy production and water resource management. The reduced energy consumption in wastewater treatment processes further amplifies the positive environmental impact.
Moreover, the application of polyglutamic acid in microbial electrode interfaces aligns with circular economy principles. It promotes the valorization of waste streams, converting organic matter into valuable energy resources. This approach not only reduces the carbon footprint associated with waste management but also creates a sustainable energy source from materials that would otherwise be discarded.
The scalability of this technology presents opportunities for decentralized energy production. Small-scale, localized bioelectrochemical systems enhanced with polyglutamic acid could provide sustainable power solutions for remote communities or off-grid applications. This decentralization contributes to energy security and resilience, particularly in areas with limited access to traditional power infrastructure.
In the context of global efforts to transition towards renewable energy sources, the role of polyglutamic acid in increasing microbial electrode interface efficiency offers a promising avenue for innovation. It complements other renewable technologies such as solar and wind power, diversifying the sustainable energy portfolio. The potential for continuous energy production, regardless of weather conditions, makes this technology particularly valuable in ensuring a stable and reliable renewable energy supply.
Furthermore, the development and implementation of this technology stimulate research and innovation in the field of sustainable energy. It encourages interdisciplinary collaboration between microbiologists, electrochemists, and environmental engineers, fostering a holistic approach to addressing energy challenges. This collaborative effort may lead to breakthroughs in related fields, accelerating the overall progress towards a sustainable energy future.
The sustainable energy impact of this technology extends beyond immediate efficiency gains. Microbial fuel cells and other bioelectrochemical systems utilizing polyglutamic acid can be deployed in wastewater treatment plants, simultaneously generating electricity while purifying water. This dual functionality addresses two critical environmental challenges: clean energy production and water resource management. The reduced energy consumption in wastewater treatment processes further amplifies the positive environmental impact.
Moreover, the application of polyglutamic acid in microbial electrode interfaces aligns with circular economy principles. It promotes the valorization of waste streams, converting organic matter into valuable energy resources. This approach not only reduces the carbon footprint associated with waste management but also creates a sustainable energy source from materials that would otherwise be discarded.
The scalability of this technology presents opportunities for decentralized energy production. Small-scale, localized bioelectrochemical systems enhanced with polyglutamic acid could provide sustainable power solutions for remote communities or off-grid applications. This decentralization contributes to energy security and resilience, particularly in areas with limited access to traditional power infrastructure.
In the context of global efforts to transition towards renewable energy sources, the role of polyglutamic acid in increasing microbial electrode interface efficiency offers a promising avenue for innovation. It complements other renewable technologies such as solar and wind power, diversifying the sustainable energy portfolio. The potential for continuous energy production, regardless of weather conditions, makes this technology particularly valuable in ensuring a stable and reliable renewable energy supply.
Furthermore, the development and implementation of this technology stimulate research and innovation in the field of sustainable energy. It encourages interdisciplinary collaboration between microbiologists, electrochemists, and environmental engineers, fostering a holistic approach to addressing energy challenges. This collaborative effort may lead to breakthroughs in related fields, accelerating the overall progress towards a sustainable energy future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!