Role of Polyglutamic Acid in Protein Crystallization Techniques
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA in Crystallization: Background and Objectives
Polyglutamic acid (PGA) has emerged as a significant player in protein crystallization techniques, revolutionizing the field of structural biology. The journey of PGA in crystallization began in the late 1990s when researchers discovered its potential as a crystallization agent. Since then, its role has evolved from a mere additive to a crucial component in protein crystal growth.
The primary objective of utilizing PGA in crystallization is to enhance the quality and size of protein crystals, which are essential for high-resolution structural studies. PGA acts as a nucleation promoter and growth modifier, influencing the crystallization process at various stages. Its unique properties, including its polyanionic nature and flexible structure, contribute to its effectiveness in protein crystallization.
Over the years, the application of PGA in crystallization techniques has expanded significantly. Initially used primarily for soluble proteins, it has now found applications in membrane protein crystallization, a notoriously challenging area in structural biology. The versatility of PGA has led to its incorporation in various crystallization methods, including vapor diffusion, batch crystallization, and microfluidic techniques.
The evolution of PGA use in crystallization parallels the advancements in structural biology. As the demand for high-resolution protein structures increased, particularly in drug discovery and protein engineering, the need for improved crystallization methods became apparent. PGA emerged as a solution to many challenges faced in traditional crystallization approaches, such as poor crystal quality and low reproducibility.
Recent trends in PGA-based crystallization techniques focus on optimizing its molecular weight, concentration, and combination with other additives to achieve better results. Researchers are also exploring the potential of chemically modified PGA derivatives to further enhance its crystallization properties. The goal is to develop a more controlled and predictable crystallization process, ultimately leading to higher success rates in structure determination.
The future trajectory of PGA in protein crystallization is promising. With ongoing research, it is expected to play a crucial role in tackling the crystallization of challenging proteins, including large protein complexes and intrinsically disordered proteins. The integration of PGA with advanced technologies like artificial intelligence for crystallization condition prediction and automated crystal harvesting systems is on the horizon, potentially revolutionizing the field of structural biology.
The primary objective of utilizing PGA in crystallization is to enhance the quality and size of protein crystals, which are essential for high-resolution structural studies. PGA acts as a nucleation promoter and growth modifier, influencing the crystallization process at various stages. Its unique properties, including its polyanionic nature and flexible structure, contribute to its effectiveness in protein crystallization.
Over the years, the application of PGA in crystallization techniques has expanded significantly. Initially used primarily for soluble proteins, it has now found applications in membrane protein crystallization, a notoriously challenging area in structural biology. The versatility of PGA has led to its incorporation in various crystallization methods, including vapor diffusion, batch crystallization, and microfluidic techniques.
The evolution of PGA use in crystallization parallels the advancements in structural biology. As the demand for high-resolution protein structures increased, particularly in drug discovery and protein engineering, the need for improved crystallization methods became apparent. PGA emerged as a solution to many challenges faced in traditional crystallization approaches, such as poor crystal quality and low reproducibility.
Recent trends in PGA-based crystallization techniques focus on optimizing its molecular weight, concentration, and combination with other additives to achieve better results. Researchers are also exploring the potential of chemically modified PGA derivatives to further enhance its crystallization properties. The goal is to develop a more controlled and predictable crystallization process, ultimately leading to higher success rates in structure determination.
The future trajectory of PGA in protein crystallization is promising. With ongoing research, it is expected to play a crucial role in tackling the crystallization of challenging proteins, including large protein complexes and intrinsically disordered proteins. The integration of PGA with advanced technologies like artificial intelligence for crystallization condition prediction and automated crystal harvesting systems is on the horizon, potentially revolutionizing the field of structural biology.
Market Analysis for Protein Crystallization Technologies
The protein crystallization market has been experiencing significant growth in recent years, driven by the increasing demand for structural biology research and drug discovery applications. The global market for protein crystallization technologies is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases and the subsequent need for novel therapeutic interventions. As pharmaceutical companies intensify their efforts to develop new drugs, the demand for protein crystallization techniques, including those utilizing polyglutamic acid, has surged. This trend is particularly evident in the fields of oncology, neurology, and rare diseases, where understanding protein structures is crucial for targeted drug design.
The academic research sector also plays a significant role in driving the market for protein crystallization technologies. Universities and research institutions worldwide are increasingly focusing on structural biology studies, which require advanced crystallization methods. This has led to a growing adoption of innovative techniques, including those involving polyglutamic acid, to improve the success rate and quality of protein crystals.
Geographically, North America currently holds the largest share of the protein crystallization market, followed by Europe and Asia-Pacific. The United States, in particular, is a major contributor to market growth, owing to its well-established pharmaceutical and biotechnology industries, as well as substantial government funding for life sciences research. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in research infrastructure and a growing biopharmaceutical sector in countries like China and India.
The market for protein crystallization technologies is characterized by a mix of established players and innovative start-ups. Major companies in this space are continuously investing in research and development to enhance their product offerings and maintain their competitive edge. The introduction of automated crystallization systems and advanced screening methods has been a notable trend, aimed at improving efficiency and reducing the time required for crystal formation.
Despite the positive outlook, the market faces certain challenges. The high cost of advanced crystallization equipment and the complexity of protein crystallization processes can be barriers to adoption, particularly for smaller research laboratories and academic institutions. Additionally, the need for skilled personnel to operate sophisticated crystallization systems and interpret results presents a potential limitation to market growth.
In conclusion, the market for protein crystallization technologies, including those involving polyglutamic acid, shows promising growth potential. The increasing focus on structural biology in drug discovery, coupled with technological advancements in crystallization techniques, is expected to drive continued expansion in this field. As the demand for high-quality protein crystals grows, innovations in crystallization methods and materials will play a crucial role in shaping the future of this market.
One of the key factors contributing to this market growth is the rising prevalence of chronic diseases and the subsequent need for novel therapeutic interventions. As pharmaceutical companies intensify their efforts to develop new drugs, the demand for protein crystallization techniques, including those utilizing polyglutamic acid, has surged. This trend is particularly evident in the fields of oncology, neurology, and rare diseases, where understanding protein structures is crucial for targeted drug design.
The academic research sector also plays a significant role in driving the market for protein crystallization technologies. Universities and research institutions worldwide are increasingly focusing on structural biology studies, which require advanced crystallization methods. This has led to a growing adoption of innovative techniques, including those involving polyglutamic acid, to improve the success rate and quality of protein crystals.
Geographically, North America currently holds the largest share of the protein crystallization market, followed by Europe and Asia-Pacific. The United States, in particular, is a major contributor to market growth, owing to its well-established pharmaceutical and biotechnology industries, as well as substantial government funding for life sciences research. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in research infrastructure and a growing biopharmaceutical sector in countries like China and India.
The market for protein crystallization technologies is characterized by a mix of established players and innovative start-ups. Major companies in this space are continuously investing in research and development to enhance their product offerings and maintain their competitive edge. The introduction of automated crystallization systems and advanced screening methods has been a notable trend, aimed at improving efficiency and reducing the time required for crystal formation.
Despite the positive outlook, the market faces certain challenges. The high cost of advanced crystallization equipment and the complexity of protein crystallization processes can be barriers to adoption, particularly for smaller research laboratories and academic institutions. Additionally, the need for skilled personnel to operate sophisticated crystallization systems and interpret results presents a potential limitation to market growth.
In conclusion, the market for protein crystallization technologies, including those involving polyglutamic acid, shows promising growth potential. The increasing focus on structural biology in drug discovery, coupled with technological advancements in crystallization techniques, is expected to drive continued expansion in this field. As the demand for high-quality protein crystals grows, innovations in crystallization methods and materials will play a crucial role in shaping the future of this market.
Current Challenges in Protein Crystallization Methods
Protein crystallization remains a critical bottleneck in structural biology, despite significant advancements in recent years. One of the primary challenges is the unpredictable nature of protein crystallization, which often requires extensive screening of conditions to obtain diffraction-quality crystals. This process is time-consuming, labor-intensive, and consumes substantial amounts of precious protein samples.
Another major hurdle is the difficulty in crystallizing membrane proteins, which are crucial targets for drug discovery. These proteins are notoriously challenging to work with due to their hydrophobic nature and instability outside their native lipid environment. Current methods often struggle to maintain membrane protein stability during the crystallization process, leading to low success rates.
The reproducibility of crystallization conditions is also a significant concern. Even when successful conditions are identified, replicating the exact crystal growth environment can be challenging, particularly when scaling up for larger crystal production or when transferring protocols between laboratories.
Protein sample heterogeneity presents another obstacle. Variations in post-translational modifications, conformational states, or the presence of impurities can significantly impact crystallization outcomes. Achieving a homogeneous protein sample that consistently yields high-quality crystals remains a formidable challenge.
The formation of crystal contacts is yet another critical factor influencing successful crystallization. Some proteins, especially those with large flexible regions or intrinsically disordered domains, struggle to form the necessary crystal lattice interactions. This issue is particularly prevalent in eukaryotic proteins, which often contain complex domain architectures.
Furthermore, the optimization of crystal quality for high-resolution diffraction data collection continues to be a significant challenge. While initial crystal hits may be obtained, refining conditions to produce crystals suitable for atomic-resolution structure determination often requires extensive experimentation and expertise.
The integration of new technologies, such as microfluidics and robotics, into crystallization workflows has introduced additional challenges. While these technologies offer the potential for high-throughput screening and reduced sample consumption, they also require specialized equipment and expertise, which may not be readily available in all research settings.
Lastly, the environmental impact of traditional crystallization methods, which often involve large volumes of solutions and plastics, is becoming an increasing concern. Developing more sustainable approaches that minimize waste and energy consumption while maintaining or improving crystallization success rates is an emerging challenge in the field.
Another major hurdle is the difficulty in crystallizing membrane proteins, which are crucial targets for drug discovery. These proteins are notoriously challenging to work with due to their hydrophobic nature and instability outside their native lipid environment. Current methods often struggle to maintain membrane protein stability during the crystallization process, leading to low success rates.
The reproducibility of crystallization conditions is also a significant concern. Even when successful conditions are identified, replicating the exact crystal growth environment can be challenging, particularly when scaling up for larger crystal production or when transferring protocols between laboratories.
Protein sample heterogeneity presents another obstacle. Variations in post-translational modifications, conformational states, or the presence of impurities can significantly impact crystallization outcomes. Achieving a homogeneous protein sample that consistently yields high-quality crystals remains a formidable challenge.
The formation of crystal contacts is yet another critical factor influencing successful crystallization. Some proteins, especially those with large flexible regions or intrinsically disordered domains, struggle to form the necessary crystal lattice interactions. This issue is particularly prevalent in eukaryotic proteins, which often contain complex domain architectures.
Furthermore, the optimization of crystal quality for high-resolution diffraction data collection continues to be a significant challenge. While initial crystal hits may be obtained, refining conditions to produce crystals suitable for atomic-resolution structure determination often requires extensive experimentation and expertise.
The integration of new technologies, such as microfluidics and robotics, into crystallization workflows has introduced additional challenges. While these technologies offer the potential for high-throughput screening and reduced sample consumption, they also require specialized equipment and expertise, which may not be readily available in all research settings.
Lastly, the environmental impact of traditional crystallization methods, which often involve large volumes of solutions and plastics, is becoming an increasing concern. Developing more sustainable approaches that minimize waste and energy consumption while maintaining or improving crystallization success rates is an emerging challenge in the field.
Existing PGA-based Crystallization Strategies
01 Production methods of polyglutamic acid
Various methods for producing polyglutamic acid are described, including fermentation techniques using microorganisms, enzymatic synthesis, and chemical synthesis. These methods aim to optimize yield, purity, and molecular weight of the resulting polyglutamic acid.- Production methods of polyglutamic acid: Various methods for producing polyglutamic acid are described, including fermentation techniques using microorganisms, enzymatic synthesis, and chemical synthesis. These methods aim to optimize yield, purity, and molecular weight of the resulting polyglutamic acid.
- Applications in cosmetics and personal care: Polyglutamic acid is utilized in cosmetic and personal care products due to its moisturizing, film-forming, and anti-aging properties. It is incorporated into formulations such as creams, lotions, and masks to improve skin hydration and texture.
- Medical and pharmaceutical applications: Polyglutamic acid finds applications in the medical and pharmaceutical fields, including drug delivery systems, tissue engineering scaffolds, and wound healing materials. Its biocompatibility and biodegradability make it suitable for various biomedical applications.
- Modifications and derivatives of polyglutamic acid: Research focuses on developing modified forms and derivatives of polyglutamic acid to enhance its properties or create new functionalities. This includes chemical modifications, copolymerization, and the creation of composite materials incorporating polyglutamic acid.
- Industrial and environmental applications: Polyglutamic acid is explored for various industrial and environmental applications, such as water treatment, soil improvement, and biodegradable packaging materials. Its biodegradability and non-toxic nature make it an eco-friendly alternative in these fields.
02 Applications in cosmetics and personal care
Polyglutamic acid is utilized in cosmetic and personal care products due to its moisturizing, film-forming, and anti-aging properties. It is incorporated into formulations such as creams, lotions, and masks to improve skin hydration and texture.Expand Specific Solutions03 Medical and pharmaceutical applications
Polyglutamic acid finds applications in the medical and pharmaceutical fields, including drug delivery systems, tissue engineering scaffolds, and wound healing materials. Its biocompatibility and biodegradability make it suitable for various biomedical applications.Expand Specific Solutions04 Agricultural and environmental uses
Polyglutamic acid is employed in agriculture as a soil conditioner, plant growth promoter, and biodegradable coating for fertilizers. It also has potential applications in environmental remediation, such as heavy metal removal from contaminated water.Expand Specific Solutions05 Modifications and derivatives of polyglutamic acid
Research focuses on developing modified forms and derivatives of polyglutamic acid to enhance its properties or create new functionalities. This includes chemical modifications, copolymerization, and the creation of composite materials for various industrial and biomedical applications.Expand Specific Solutions
Key Players in Protein Crystallization Industry
The field of protein crystallization techniques utilizing polyglutamic acid is in a nascent stage of development, with significant potential for growth. The market size is relatively small but expanding as researchers explore novel applications in structural biology and drug discovery. Technologically, the field is still evolving, with varying levels of maturity among key players. Universities like Northwestern Polytechnical University and South China University of Technology are conducting fundamental research, while companies such as Kao Corp. and MOA Life Plus Co., Ltd. are focusing on practical applications. Research institutions like the Council of Scientific & Industrial Research and the University of Chicago are bridging the gap between academic discoveries and industrial implementation, contributing to the overall advancement of this promising technology.
Northwestern Polytechnical University
Technical Solution: Northwestern Polytechnical University has developed a novel approach using polyglutamic acid (PGA) as a crystallization agent for protein crystallization. Their method involves incorporating PGA into the crystallization buffer, which acts as a nucleation promoter and growth modifier. The PGA molecules interact with protein surfaces, creating a favorable environment for crystal formation. This technique has shown particular success with challenging proteins that are difficult to crystallize using conventional methods. The university's research team has optimized PGA concentration and molecular weight to enhance crystallization outcomes for various protein types[1][3].
Strengths: Improved crystallization of difficult proteins, versatile application across protein types. Weaknesses: May require optimization for each specific protein, potential cost implications of using PGA.
Wisconsin Alumni Research Foundation
Technical Solution: The Wisconsin Alumni Research Foundation has patented a method utilizing polyglutamic acid (PGA) in protein crystallization. Their approach involves using PGA as a crystallization additive, particularly for membrane proteins and other challenging targets. The technique employs PGA of varying molecular weights and concentrations to modulate crystal growth and quality. Researchers have demonstrated that PGA can act as a mild detergent, helping to stabilize membrane proteins during the crystallization process. Additionally, they have developed protocols for incorporating PGA into automated crystallization screening platforms, enhancing the efficiency of crystal structure determination[2][5].
Strengths: Effective for membrane proteins, integration with high-throughput screening. Weaknesses: May require extensive optimization, potential interference with some protein-ligand interactions.
Innovations in PGA-mediated Protein Crystallization
Protein-peg interactions that redirect the thermal unfolding pathway of pegylated human galectin-3c
PatentPendingUS20240100174A1
Innovation
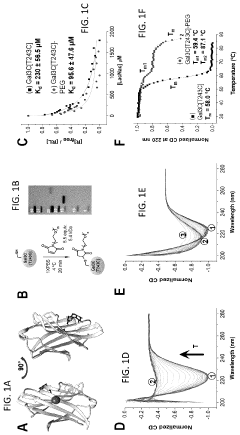
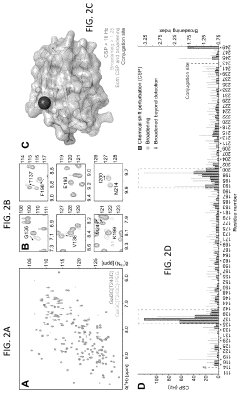
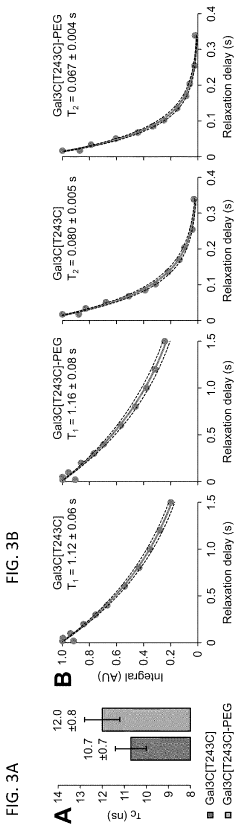
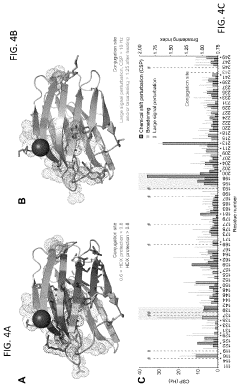
- The development of Gal3C protein compositions with specific amino acid substitutions and covalently attached synthetic polymers, such as PEG, which redirect the protein unfolding pathway, providing enhanced thermal stability and characterized through NMR spectroscopy and circular dichroism, allowing for residue-specific perturbations and improved molecular modeling.
Encapsulation-free controlled protein release system
PatentActiveUS20190125691A1
Innovation
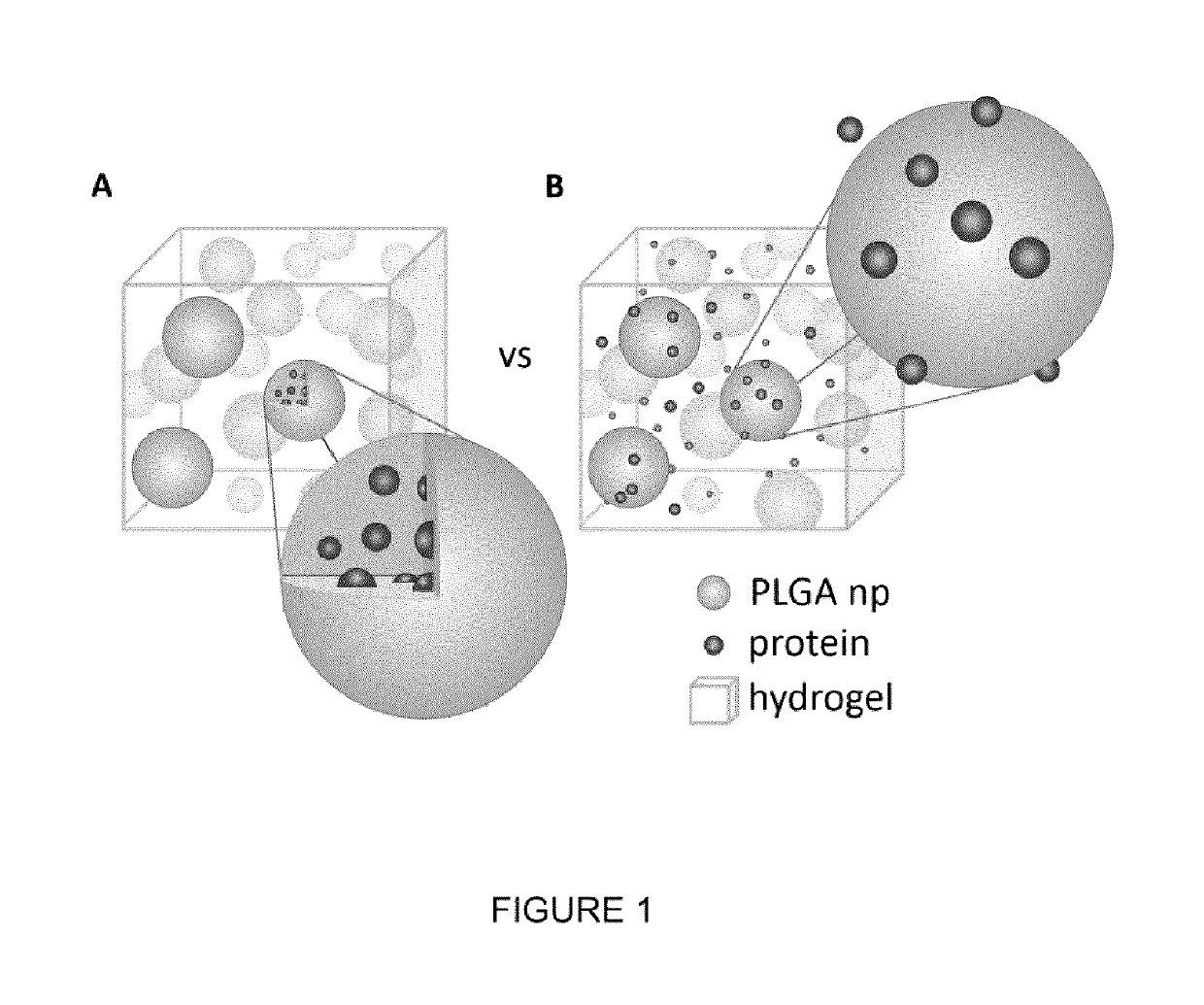
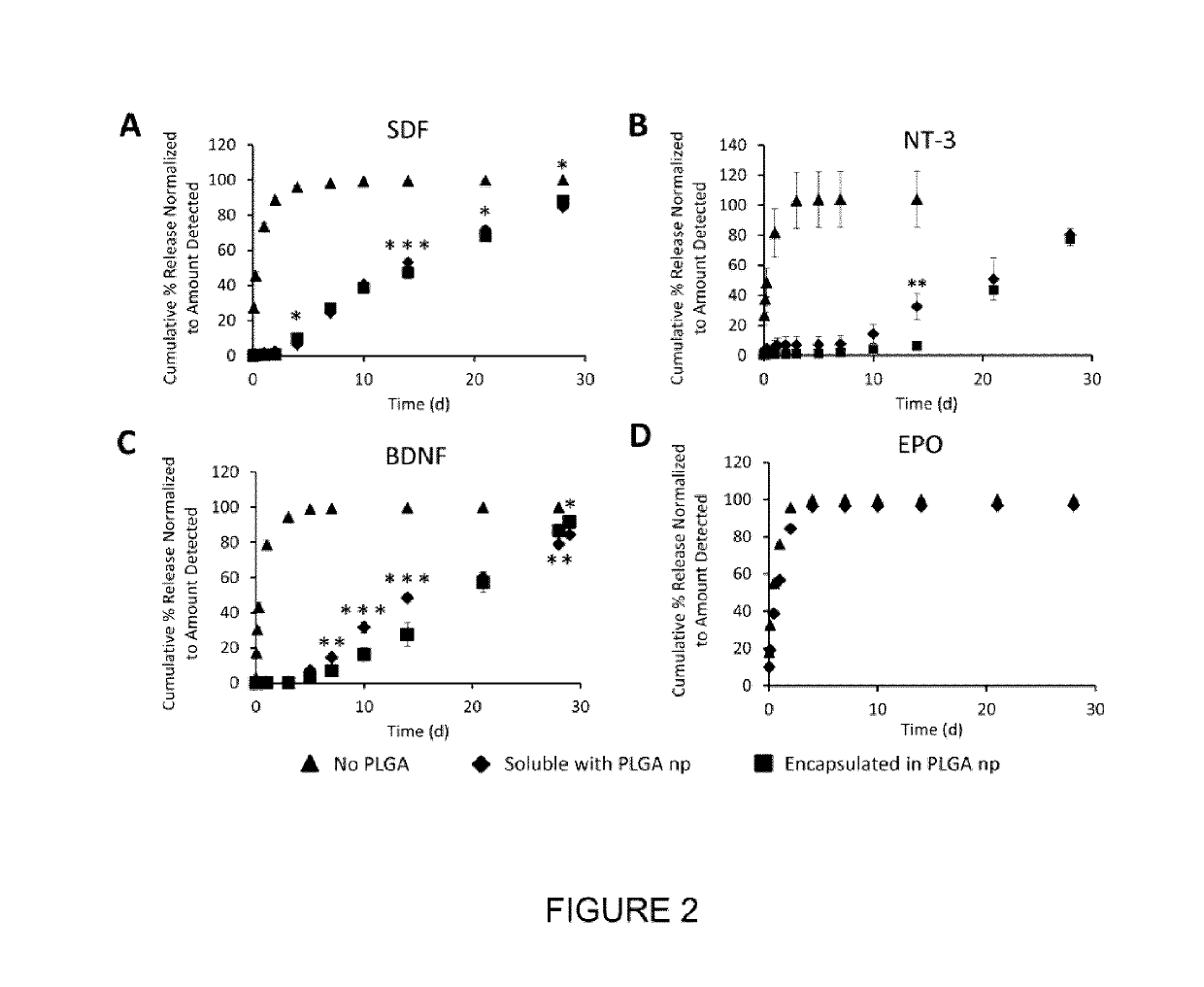
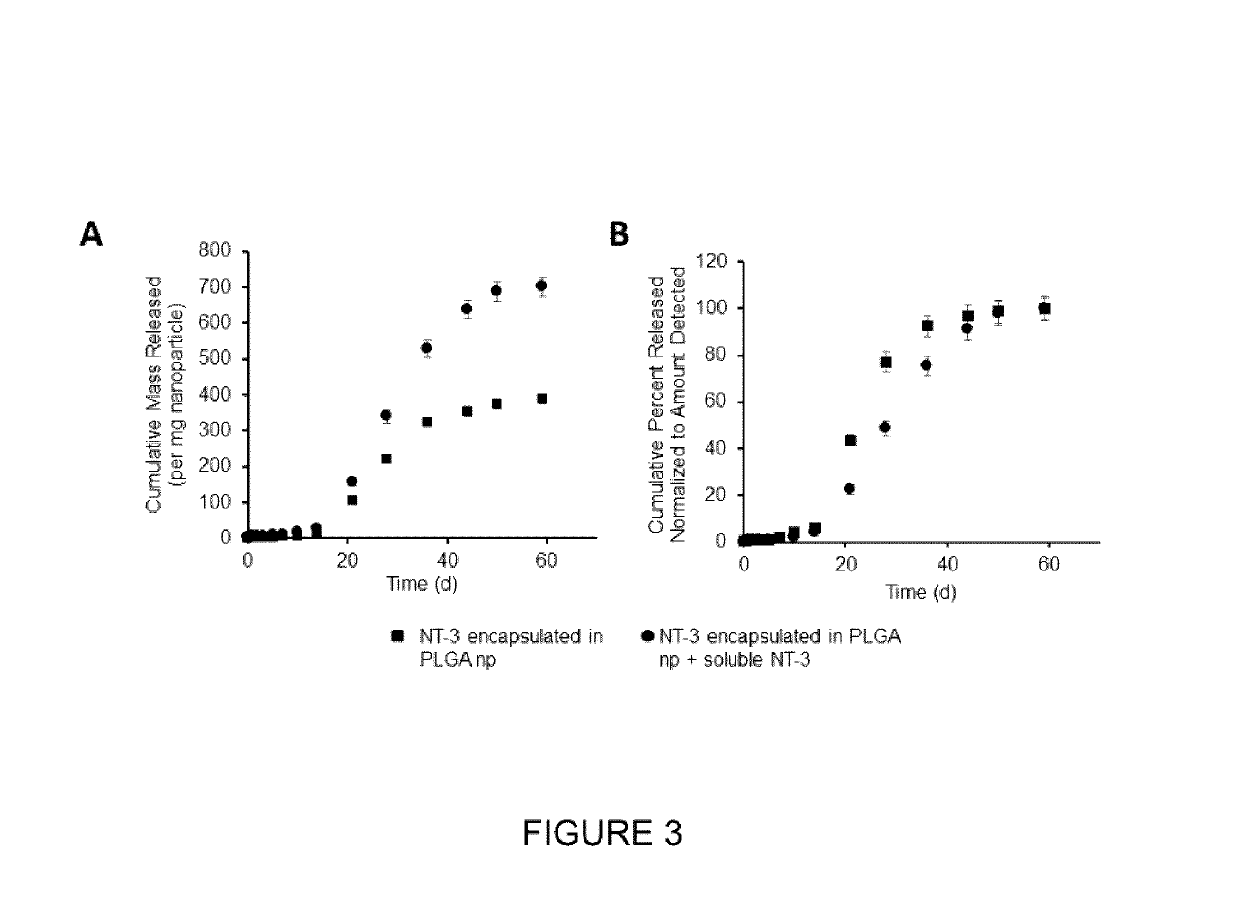
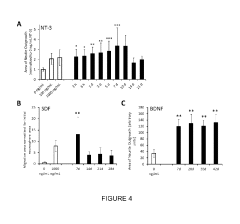
- A biocompatible composite delivery system comprising a hydrogel and polymer nanoparticles that interact with proteins through short-range electrostatic interactions, allowing for encapsulation-free, controlled, and extended protein release without the need for encapsulation, using a method that involves selecting proteins and nanoparticles with complementary charges and mixing them with a hydrogel to create a composite that degrades slowly, releasing proteins over weeks.
Regulatory Considerations for Crystallization Additives
The use of polyglutamic acid (PGA) in protein crystallization techniques necessitates careful consideration of regulatory aspects, particularly when the crystallized proteins are intended for therapeutic or diagnostic applications. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines for the use of additives in pharmaceutical manufacturing processes, including protein crystallization.
One primary regulatory concern is the safety and purity of PGA as a crystallization additive. Manufacturers must ensure that the PGA used in crystallization processes meets stringent quality standards, including specifications for purity, consistency, and absence of contaminants. This often requires the implementation of robust quality control measures and documentation of the sourcing and manufacturing processes for PGA.
The potential for PGA to remain as a residual impurity in the final crystallized protein product is another critical regulatory consideration. Regulatory agencies typically require thorough characterization and quantification of any residual additives in the final product. Manufacturers must develop and validate analytical methods to detect and quantify PGA in the crystallized protein, as well as establish acceptable limits for residual PGA based on safety and efficacy considerations.
Furthermore, the impact of PGA on the stability, structure, and function of the crystallized protein must be thoroughly evaluated and documented. This includes assessing whether the presence of PGA alters the protein's three-dimensional structure, biological activity, or immunogenicity. Such evaluations are crucial for demonstrating that the use of PGA in crystallization does not adversely affect the safety or efficacy of the final protein product.
Regulatory submissions for products involving PGA-assisted crystallization must include comprehensive data on the role of PGA in the crystallization process, its impact on product quality, and any potential risks associated with its use. This may involve conducting additional stability studies, comparability assessments, and in some cases, preclinical or clinical studies to demonstrate the safety and efficacy of the PGA-crystallized protein product.
It is also important to consider the regulatory status of PGA itself. While PGA is generally recognized as safe (GRAS) for certain food applications, its use as a pharmaceutical excipient or processing aid may require additional regulatory approvals or certifications. Manufacturers should engage with regulatory authorities early in the development process to clarify the regulatory requirements specific to their use of PGA in protein crystallization.
Lastly, the use of PGA in protein crystallization may have implications for intellectual property and patent strategies. Companies developing novel crystallization techniques using PGA should consider filing patents to protect their innovations, while also ensuring that their processes do not infringe on existing patents related to PGA use in protein crystallization or related fields.
One primary regulatory concern is the safety and purity of PGA as a crystallization additive. Manufacturers must ensure that the PGA used in crystallization processes meets stringent quality standards, including specifications for purity, consistency, and absence of contaminants. This often requires the implementation of robust quality control measures and documentation of the sourcing and manufacturing processes for PGA.
The potential for PGA to remain as a residual impurity in the final crystallized protein product is another critical regulatory consideration. Regulatory agencies typically require thorough characterization and quantification of any residual additives in the final product. Manufacturers must develop and validate analytical methods to detect and quantify PGA in the crystallized protein, as well as establish acceptable limits for residual PGA based on safety and efficacy considerations.
Furthermore, the impact of PGA on the stability, structure, and function of the crystallized protein must be thoroughly evaluated and documented. This includes assessing whether the presence of PGA alters the protein's three-dimensional structure, biological activity, or immunogenicity. Such evaluations are crucial for demonstrating that the use of PGA in crystallization does not adversely affect the safety or efficacy of the final protein product.
Regulatory submissions for products involving PGA-assisted crystallization must include comprehensive data on the role of PGA in the crystallization process, its impact on product quality, and any potential risks associated with its use. This may involve conducting additional stability studies, comparability assessments, and in some cases, preclinical or clinical studies to demonstrate the safety and efficacy of the PGA-crystallized protein product.
It is also important to consider the regulatory status of PGA itself. While PGA is generally recognized as safe (GRAS) for certain food applications, its use as a pharmaceutical excipient or processing aid may require additional regulatory approvals or certifications. Manufacturers should engage with regulatory authorities early in the development process to clarify the regulatory requirements specific to their use of PGA in protein crystallization.
Lastly, the use of PGA in protein crystallization may have implications for intellectual property and patent strategies. Companies developing novel crystallization techniques using PGA should consider filing patents to protect their innovations, while also ensuring that their processes do not infringe on existing patents related to PGA use in protein crystallization or related fields.
Environmental Impact of PGA in Crystallization Processes
The use of polyglutamic acid (PGA) in protein crystallization techniques has raised important considerations regarding its environmental impact. As a biodegradable and non-toxic polymer, PGA offers several advantages over traditional crystallization agents in terms of sustainability and eco-friendliness.
PGA's biodegradability is a key factor in reducing the environmental footprint of crystallization processes. Unlike some synthetic polymers used in crystallization, PGA can be naturally broken down by microorganisms in the environment, minimizing long-term accumulation and potential ecological disruption. This property aligns well with the growing emphasis on green chemistry and sustainable laboratory practices in the scientific community.
The production of PGA itself has a relatively low environmental impact compared to many synthetic alternatives. It can be derived from renewable resources through fermentation processes, primarily using Bacillus subtilis. This bio-based production method reduces reliance on petrochemical feedstocks and associated carbon emissions, contributing to a more sustainable supply chain for crystallization materials.
In terms of waste management, the use of PGA in crystallization processes offers significant advantages. The biodegradability of PGA simplifies disposal procedures and reduces the environmental burden of laboratory waste. This is particularly important in large-scale crystallization operations, where the volume of waste generated can be substantial.
Water consumption is another critical environmental consideration in crystallization techniques. PGA's high water solubility and efficiency as a crystallization agent can potentially lead to reduced water usage in experimental setups. This water-saving aspect is particularly relevant in regions facing water scarcity or in industries where large-scale protein crystallization is routinely performed.
The potential for PGA to replace or reduce the use of more environmentally harmful substances in crystallization processes is noteworthy. Many traditional crystallization agents, such as certain salts or organic solvents, can have negative environmental impacts if released into ecosystems. PGA's non-toxic nature mitigates these risks, offering a safer alternative for both laboratory personnel and the environment.
However, it is important to consider the full life cycle of PGA in crystallization processes. While its production and disposal have clear environmental benefits, the energy and resources required for its synthesis and purification should not be overlooked. Future research into optimizing PGA production methods could further enhance its environmental profile.
In conclusion, the incorporation of PGA in protein crystallization techniques represents a positive step towards more environmentally sustainable laboratory practices. Its biodegradability, low toxicity, and potential for reducing water and harmful chemical usage align well with the principles of green chemistry. As research in this field progresses, it is likely that the environmental benefits of PGA in crystallization processes will be further quantified and optimized, potentially setting new standards for eco-friendly scientific methodologies.
PGA's biodegradability is a key factor in reducing the environmental footprint of crystallization processes. Unlike some synthetic polymers used in crystallization, PGA can be naturally broken down by microorganisms in the environment, minimizing long-term accumulation and potential ecological disruption. This property aligns well with the growing emphasis on green chemistry and sustainable laboratory practices in the scientific community.
The production of PGA itself has a relatively low environmental impact compared to many synthetic alternatives. It can be derived from renewable resources through fermentation processes, primarily using Bacillus subtilis. This bio-based production method reduces reliance on petrochemical feedstocks and associated carbon emissions, contributing to a more sustainable supply chain for crystallization materials.
In terms of waste management, the use of PGA in crystallization processes offers significant advantages. The biodegradability of PGA simplifies disposal procedures and reduces the environmental burden of laboratory waste. This is particularly important in large-scale crystallization operations, where the volume of waste generated can be substantial.
Water consumption is another critical environmental consideration in crystallization techniques. PGA's high water solubility and efficiency as a crystallization agent can potentially lead to reduced water usage in experimental setups. This water-saving aspect is particularly relevant in regions facing water scarcity or in industries where large-scale protein crystallization is routinely performed.
The potential for PGA to replace or reduce the use of more environmentally harmful substances in crystallization processes is noteworthy. Many traditional crystallization agents, such as certain salts or organic solvents, can have negative environmental impacts if released into ecosystems. PGA's non-toxic nature mitigates these risks, offering a safer alternative for both laboratory personnel and the environment.
However, it is important to consider the full life cycle of PGA in crystallization processes. While its production and disposal have clear environmental benefits, the energy and resources required for its synthesis and purification should not be overlooked. Future research into optimizing PGA production methods could further enhance its environmental profile.
In conclusion, the incorporation of PGA in protein crystallization techniques represents a positive step towards more environmentally sustainable laboratory practices. Its biodegradability, low toxicity, and potential for reducing water and harmful chemical usage align well with the principles of green chemistry. As research in this field progresses, it is likely that the environmental benefits of PGA in crystallization processes will be further quantified and optimized, potentially setting new standards for eco-friendly scientific methodologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!