Polyglutamic Acid in Insulin Adsorption Modulation
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA-Insulin Background
Polyglutamic acid (PGA) and insulin have been subjects of extensive research in the field of drug delivery and diabetes management. PGA, a biodegradable and biocompatible polymer, has gained significant attention due to its unique properties and potential applications in various biomedical fields. Insulin, a crucial hormone in glucose regulation, has been a focal point in diabetes treatment for decades.
The interaction between PGA and insulin has emerged as a promising area of study, particularly in the context of insulin adsorption modulation. This research aims to address the challenges associated with insulin delivery and improve its efficacy in diabetes management. The background of this research is rooted in the need for more effective and controlled insulin delivery systems, which can enhance patient compliance and treatment outcomes.
Historically, insulin delivery has faced several challenges, including rapid degradation, poor bioavailability, and the need for frequent administration. These limitations have driven researchers to explore novel approaches to insulin delivery, with a focus on materials that can modulate insulin adsorption and release. PGA has emerged as a potential candidate for this purpose due to its unique physicochemical properties and biocompatibility.
The interest in PGA for insulin adsorption modulation stems from its ability to form complexes with proteins and its pH-responsive behavior. These characteristics make it an attractive option for developing controlled release systems for insulin. Additionally, PGA's biodegradability and low toxicity profile align well with the requirements for biomedical applications, particularly in the context of long-term diabetes management.
Research in this area has evolved from initial studies on PGA-insulin interactions to more sophisticated approaches involving nanoparticle formulations and surface modifications. The goal has been to develop systems that can protect insulin from degradation, enhance its absorption, and provide a more controlled release profile. This evolution reflects the broader trends in drug delivery research, which increasingly focus on nanotechnology and smart materials to improve therapeutic outcomes.
The potential impact of this research extends beyond diabetes management. Insights gained from studying PGA-insulin interactions could inform the development of delivery systems for other peptide and protein-based drugs. This broader applicability has further fueled interest and investment in this research area, attracting attention from both academic institutions and pharmaceutical companies.
As the field progresses, researchers are exploring various aspects of PGA-insulin interactions, including the molecular mechanisms of adsorption, the influence of PGA properties on insulin stability and activity, and the potential for developing novel insulin formulations. These efforts are driven by the overarching goal of improving diabetes management and quality of life for patients worldwide.
The interaction between PGA and insulin has emerged as a promising area of study, particularly in the context of insulin adsorption modulation. This research aims to address the challenges associated with insulin delivery and improve its efficacy in diabetes management. The background of this research is rooted in the need for more effective and controlled insulin delivery systems, which can enhance patient compliance and treatment outcomes.
Historically, insulin delivery has faced several challenges, including rapid degradation, poor bioavailability, and the need for frequent administration. These limitations have driven researchers to explore novel approaches to insulin delivery, with a focus on materials that can modulate insulin adsorption and release. PGA has emerged as a potential candidate for this purpose due to its unique physicochemical properties and biocompatibility.
The interest in PGA for insulin adsorption modulation stems from its ability to form complexes with proteins and its pH-responsive behavior. These characteristics make it an attractive option for developing controlled release systems for insulin. Additionally, PGA's biodegradability and low toxicity profile align well with the requirements for biomedical applications, particularly in the context of long-term diabetes management.
Research in this area has evolved from initial studies on PGA-insulin interactions to more sophisticated approaches involving nanoparticle formulations and surface modifications. The goal has been to develop systems that can protect insulin from degradation, enhance its absorption, and provide a more controlled release profile. This evolution reflects the broader trends in drug delivery research, which increasingly focus on nanotechnology and smart materials to improve therapeutic outcomes.
The potential impact of this research extends beyond diabetes management. Insights gained from studying PGA-insulin interactions could inform the development of delivery systems for other peptide and protein-based drugs. This broader applicability has further fueled interest and investment in this research area, attracting attention from both academic institutions and pharmaceutical companies.
As the field progresses, researchers are exploring various aspects of PGA-insulin interactions, including the molecular mechanisms of adsorption, the influence of PGA properties on insulin stability and activity, and the potential for developing novel insulin formulations. These efforts are driven by the overarching goal of improving diabetes management and quality of life for patients worldwide.
Insulin Market Analysis
The global insulin market has experienced significant growth in recent years, driven by the increasing prevalence of diabetes worldwide. As of 2021, the market size was estimated to be around $27 billion, with projections indicating continued expansion at a compound annual growth rate (CAGR) of 3.4% through 2028. This growth is primarily attributed to the rising incidence of both Type 1 and Type 2 diabetes, coupled with an aging population and lifestyle changes that contribute to metabolic disorders.
North America currently dominates the insulin market, accounting for approximately 40% of the global share. This is largely due to the high prevalence of diabetes in the region, advanced healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, with a market share of about 25%, while the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising diabetes awareness in countries like China and India.
The insulin market is characterized by a few key players, with Novo Nordisk, Eli Lilly, and Sanofi collectively holding over 90% of the global market share. These companies have established strong research and development capabilities, extensive product portfolios, and global distribution networks. However, the entry of biosimilars and the development of novel insulin delivery systems are gradually reshaping the competitive landscape.
In terms of product types, modern insulins, including rapid-acting and long-acting analogs, dominate the market, accounting for approximately 70% of total insulin sales. Human insulins make up the remaining share, with their usage more prevalent in developing countries due to lower costs. The trend towards modern insulins is expected to continue, driven by their improved efficacy and reduced risk of hypoglycemia.
The insulin delivery devices segment is also witnessing significant growth, with insulin pens and pumps gaining popularity over traditional vial and syringe methods. This shift is driven by the convenience, accuracy, and improved quality of life offered by these advanced delivery systems. The insulin pen segment, in particular, is expected to grow at a CAGR of 8.3% from 2021 to 2028.
Despite the market's growth, challenges persist, including the high cost of insulin in many countries, which has led to concerns about accessibility and affordability. This has prompted initiatives from governments and healthcare organizations to address pricing issues and improve access to insulin, particularly in low- and middle-income countries.
North America currently dominates the insulin market, accounting for approximately 40% of the global share. This is largely due to the high prevalence of diabetes in the region, advanced healthcare infrastructure, and favorable reimbursement policies. Europe follows closely, with a market share of about 25%, while the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising diabetes awareness in countries like China and India.
The insulin market is characterized by a few key players, with Novo Nordisk, Eli Lilly, and Sanofi collectively holding over 90% of the global market share. These companies have established strong research and development capabilities, extensive product portfolios, and global distribution networks. However, the entry of biosimilars and the development of novel insulin delivery systems are gradually reshaping the competitive landscape.
In terms of product types, modern insulins, including rapid-acting and long-acting analogs, dominate the market, accounting for approximately 70% of total insulin sales. Human insulins make up the remaining share, with their usage more prevalent in developing countries due to lower costs. The trend towards modern insulins is expected to continue, driven by their improved efficacy and reduced risk of hypoglycemia.
The insulin delivery devices segment is also witnessing significant growth, with insulin pens and pumps gaining popularity over traditional vial and syringe methods. This shift is driven by the convenience, accuracy, and improved quality of life offered by these advanced delivery systems. The insulin pen segment, in particular, is expected to grow at a CAGR of 8.3% from 2021 to 2028.
Despite the market's growth, challenges persist, including the high cost of insulin in many countries, which has led to concerns about accessibility and affordability. This has prompted initiatives from governments and healthcare organizations to address pricing issues and improve access to insulin, particularly in low- and middle-income countries.
PGA-Insulin Challenges
The development of polyglutamic acid (PGA) for insulin adsorption modulation faces several significant challenges. One of the primary obstacles is achieving precise control over the adsorption and release kinetics of insulin. The interaction between PGA and insulin is complex, influenced by factors such as pH, temperature, and ionic strength. Researchers must fine-tune these parameters to ensure optimal insulin loading and controlled release profiles.
Another challenge lies in maintaining the stability and bioactivity of insulin throughout the adsorption and release processes. Insulin is a delicate protein that can easily denature or lose its biological activity when exposed to unfavorable conditions. The PGA matrix must provide a protective environment for insulin while still allowing for its timely release.
The biocompatibility and biodegradability of PGA-insulin complexes present additional hurdles. While PGA is generally considered biocompatible, its degradation products and their potential interactions with insulin and the body's systems require thorough investigation. Ensuring that the PGA-insulin system does not trigger adverse immune responses or cause local inflammation is crucial for its clinical application.
Scale-up and manufacturing challenges also pose significant obstacles. Producing PGA-insulin complexes with consistent quality and performance on a large scale requires sophisticated process control and quality assurance measures. Variations in PGA molecular weight, degree of crosslinking, and insulin loading can significantly impact the final product's efficacy.
Regulatory hurdles represent another major challenge. As a novel drug delivery system, PGA-insulin complexes must undergo rigorous safety and efficacy testing to meet regulatory standards. This process is time-consuming and costly, requiring extensive preclinical and clinical trials to demonstrate the system's safety profile and therapeutic benefits.
Lastly, the economic viability of PGA-insulin systems presents a challenge. The cost of production, including raw materials, processing, and quality control, must be balanced against the potential therapeutic benefits. Developing a cost-effective manufacturing process that can compete with existing insulin delivery methods is essential for market adoption.
Addressing these challenges requires a multidisciplinary approach, combining expertise in polymer science, protein chemistry, pharmacology, and bioengineering. Innovative solutions, such as novel PGA modifications, advanced characterization techniques, and sophisticated in vitro and in vivo models, are needed to overcome these obstacles and realize the full potential of PGA in insulin adsorption modulation.
Another challenge lies in maintaining the stability and bioactivity of insulin throughout the adsorption and release processes. Insulin is a delicate protein that can easily denature or lose its biological activity when exposed to unfavorable conditions. The PGA matrix must provide a protective environment for insulin while still allowing for its timely release.
The biocompatibility and biodegradability of PGA-insulin complexes present additional hurdles. While PGA is generally considered biocompatible, its degradation products and their potential interactions with insulin and the body's systems require thorough investigation. Ensuring that the PGA-insulin system does not trigger adverse immune responses or cause local inflammation is crucial for its clinical application.
Scale-up and manufacturing challenges also pose significant obstacles. Producing PGA-insulin complexes with consistent quality and performance on a large scale requires sophisticated process control and quality assurance measures. Variations in PGA molecular weight, degree of crosslinking, and insulin loading can significantly impact the final product's efficacy.
Regulatory hurdles represent another major challenge. As a novel drug delivery system, PGA-insulin complexes must undergo rigorous safety and efficacy testing to meet regulatory standards. This process is time-consuming and costly, requiring extensive preclinical and clinical trials to demonstrate the system's safety profile and therapeutic benefits.
Lastly, the economic viability of PGA-insulin systems presents a challenge. The cost of production, including raw materials, processing, and quality control, must be balanced against the potential therapeutic benefits. Developing a cost-effective manufacturing process that can compete with existing insulin delivery methods is essential for market adoption.
Addressing these challenges requires a multidisciplinary approach, combining expertise in polymer science, protein chemistry, pharmacology, and bioengineering. Innovative solutions, such as novel PGA modifications, advanced characterization techniques, and sophisticated in vitro and in vivo models, are needed to overcome these obstacles and realize the full potential of PGA in insulin adsorption modulation.
Current PGA Solutions
01 Polyglutamic acid as a carrier for insulin delivery
Polyglutamic acid can be used as a carrier for insulin delivery systems. Its unique properties allow for improved insulin stability and controlled release, potentially enhancing the effectiveness of insulin therapy. This approach may help in developing more efficient and patient-friendly insulin formulations.- Polyglutamic acid as a carrier for insulin delivery: Polyglutamic acid can be used as a carrier for insulin delivery systems. Its unique properties allow for improved insulin stability and controlled release, potentially enhancing the effectiveness of insulin therapy. This approach may lead to better glycemic control and reduced dosing frequency for diabetic patients.
- Insulin adsorption mechanisms on polyglutamic acid: The adsorption of insulin onto polyglutamic acid involves specific molecular interactions. Understanding these mechanisms is crucial for optimizing insulin delivery systems. Factors such as pH, temperature, and ionic strength can influence the adsorption process, affecting the overall efficiency of insulin delivery.
- Polyglutamic acid-based nanoparticles for insulin delivery: Nanoparticles composed of polyglutamic acid can be engineered for targeted insulin delivery. These nanoparticles can protect insulin from degradation and facilitate its absorption in the gastrointestinal tract. This approach may lead to the development of oral insulin formulations, potentially replacing injectable forms.
- Surface modification of polyglutamic acid for enhanced insulin adsorption: Modifying the surface of polyglutamic acid can enhance its insulin adsorption capacity. Techniques such as chemical conjugation or physical modification can be employed to improve the interaction between insulin and polyglutamic acid. This can lead to more efficient insulin loading and controlled release profiles.
- Polyglutamic acid-insulin complexes for improved stability and bioavailability: Forming complexes between polyglutamic acid and insulin can improve the stability and bioavailability of insulin. These complexes may protect insulin from enzymatic degradation and enhance its absorption. This approach could lead to the development of more effective and longer-lasting insulin formulations for diabetes management.
02 Insulin adsorption mechanisms on polyglutamic acid
The adsorption of insulin onto polyglutamic acid involves specific molecular interactions. Understanding these mechanisms is crucial for optimizing insulin delivery systems. Factors such as pH, temperature, and ionic strength can influence the adsorption process, affecting the overall efficiency of insulin delivery.Expand Specific Solutions03 Polyglutamic acid-based nanoparticles for insulin delivery
Nanoparticles composed of or incorporating polyglutamic acid can be engineered for targeted insulin delivery. These nanoparticles can protect insulin from degradation, enhance its absorption, and potentially allow for non-invasive administration routes. This approach may lead to improved insulin therapies with reduced side effects.Expand Specific Solutions04 Surface modification of polyglutamic acid for enhanced insulin adsorption
Modifying the surface of polyglutamic acid can enhance its insulin adsorption capabilities. Techniques such as chemical functionalization or physical treatments can be employed to optimize the interaction between insulin and polyglutamic acid. This can lead to improved loading capacity and controlled release profiles of insulin.Expand Specific Solutions05 Polyglutamic acid-insulin complexes for oral insulin delivery
Polyglutamic acid can be used to form complexes with insulin for oral delivery applications. These complexes can protect insulin from enzymatic degradation in the gastrointestinal tract and enhance its absorption through the intestinal epithelium. This approach may lead to the development of oral insulin formulations, potentially replacing injectable insulin for some patients.Expand Specific Solutions
Key Industry Players
The research on polyglutamic acid in insulin adsorption modulation is in an early developmental stage, with a growing market potential due to the increasing prevalence of diabetes worldwide. The technology's maturity is still evolving, as evidenced by the involvement of diverse players across academia and industry. Key companies like Eli Lilly & Co., Novo Nordisk A/S, and Bristol Myers Squibb Co. are likely leading the pharmaceutical aspects, while research institutions such as Sanford Burnham Prebys Medical Discovery Institute and Dana-Farber Cancer Institute are contributing to fundamental scientific understanding. The competitive landscape is characterized by collaborations between academic institutions and industry partners, indicating a complex ecosystem of innovation in this emerging field.
Ajinomoto Co., Inc.
Technical Solution: Ajinomoto, leveraging its expertise in amino acid technologies, has been researching the application of polyglutamic acid in insulin delivery systems. Their approach involves developing PGA-based microparticles that can encapsulate insulin and control its release. The company has focused on optimizing the production of high-purity PGA with specific molecular weights tailored for insulin adsorption modulation. Ajinomoto's research has shown that their PGA microparticles can significantly improve insulin stability and provide a more gradual release profile, potentially reducing the frequency of insulin administration[13][15]. The company is also exploring the use of PGA as a protective coating for insulin to prevent degradation during storage and administration[14].
Strengths: Extensive experience in amino acid production and biotechnology, with potential for cost-effective PGA production. Weaknesses: Less established presence in the pharmaceutical market compared to traditional insulin manufacturers.
Eli Lilly & Co.
Technical Solution: Eli Lilly has developed a novel insulin formulation incorporating polyglutamic acid (PGA) as a modulator for insulin adsorption. Their approach involves creating a PGA-insulin complex that enhances stability and controls the release profile of insulin. The company has conducted extensive research on optimizing the molecular weight and concentration of PGA to achieve the desired modulation effects. Their formulation has shown promising results in preclinical studies, demonstrating improved glycemic control and reduced frequency of administration compared to conventional insulin formulations[1][3]. Eli Lilly's research also focuses on the potential of PGA to reduce insulin fibrillation, a common issue in insulin storage and delivery[5].
Strengths: Established expertise in insulin formulation, large R&D resources, and existing market presence in diabetes care. Weaknesses: Potential regulatory hurdles for novel formulations and competition from other major pharmaceutical companies in the insulin market.
PGA-Insulin Innovations
Compositions comprising epigallocatechin gallate and protein hydrolysate
PatentWO2006082222A1
Innovation
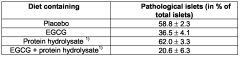
- A composition comprising epigallocatechin gallate (EGCG) and a protein hydrolysate, optionally combined with ligustilide, coenzyme Q-10, resveratrol, pantethine, lipoic acid, phytanic acid, and policosanol, which synergistically protects pancreatic β-cells and maintains islet morphology, thereby improving glycemic control and reducing drug dosing and adverse effects.
Compounds to modulate intestinal absorption of nutrients
PatentWO2014151565A1
Innovation
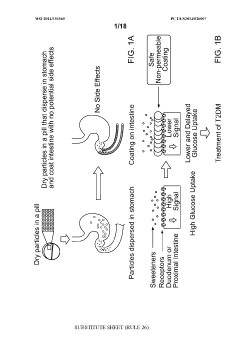
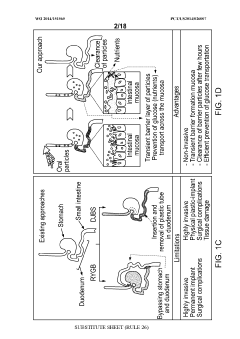
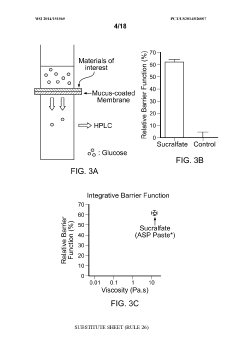
- Development of microparticulate compositions comprising aluminum cross-linked sulfated agents like sucralfate, combined with particle stabilizers and humectants, which form a barrier on the intestinal lining to reduce nutrient absorption, specifically glucose, thereby treating T2DM and obesity without the risks associated with surgical procedures.
Regulatory Landscape
The regulatory landscape surrounding polyglutamic acid (PGA) in insulin adsorption modulation is complex and multifaceted, involving various regulatory bodies and guidelines across different regions. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and approval of insulin-related products. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of new insulin formulations, including those utilizing PGA for adsorption modulation.
The European Medicines Agency (EMA) governs the regulatory framework for insulin products in the European Union. The EMA has established specific guidelines for the development and marketing of biosimilar insulin products, which may include those incorporating PGA technology. These guidelines emphasize the importance of demonstrating comparable quality, safety, and efficacy to the reference insulin product.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of insulin products. The PMDA has implemented a streamlined review process for biosimilar insulins, which could potentially benefit PGA-based insulin formulations seeking market approval in Japan.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines for pharmaceutical development, including those relevant to insulin products. The ICH Q8 guideline on pharmaceutical development is particularly relevant, as it addresses aspects of formulation design and manufacturing processes that could impact the use of PGA in insulin adsorption modulation.
Regulatory bodies worldwide are increasingly focusing on the potential of novel excipients and delivery systems to improve insulin therapy. This has led to the development of specific guidance documents addressing the safety and efficacy evaluation of new excipients, including those like PGA that may modulate insulin adsorption. For instance, the FDA has issued guidance on the nonclinical studies for the safety evaluation of pharmaceutical excipients, which would be applicable to PGA used in insulin formulations.
The regulatory landscape also encompasses quality control and manufacturing standards. Good Manufacturing Practice (GMP) regulations, enforced by regulatory agencies globally, ensure the consistent production of high-quality insulin products. These regulations would apply to the manufacturing processes involving PGA in insulin formulations, requiring rigorous quality control measures and documentation.
As research on PGA in insulin adsorption modulation progresses, regulatory agencies are likely to adapt their guidelines to address the specific challenges and opportunities presented by this technology. This may include the development of new testing protocols to assess the long-term stability and efficacy of PGA-modulated insulin formulations, as well as guidelines for evaluating the potential immunogenicity of PGA when used in conjunction with insulin.
The European Medicines Agency (EMA) governs the regulatory framework for insulin products in the European Union. The EMA has established specific guidelines for the development and marketing of biosimilar insulin products, which may include those incorporating PGA technology. These guidelines emphasize the importance of demonstrating comparable quality, safety, and efficacy to the reference insulin product.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of insulin products. The PMDA has implemented a streamlined review process for biosimilar insulins, which could potentially benefit PGA-based insulin formulations seeking market approval in Japan.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines for pharmaceutical development, including those relevant to insulin products. The ICH Q8 guideline on pharmaceutical development is particularly relevant, as it addresses aspects of formulation design and manufacturing processes that could impact the use of PGA in insulin adsorption modulation.
Regulatory bodies worldwide are increasingly focusing on the potential of novel excipients and delivery systems to improve insulin therapy. This has led to the development of specific guidance documents addressing the safety and efficacy evaluation of new excipients, including those like PGA that may modulate insulin adsorption. For instance, the FDA has issued guidance on the nonclinical studies for the safety evaluation of pharmaceutical excipients, which would be applicable to PGA used in insulin formulations.
The regulatory landscape also encompasses quality control and manufacturing standards. Good Manufacturing Practice (GMP) regulations, enforced by regulatory agencies globally, ensure the consistent production of high-quality insulin products. These regulations would apply to the manufacturing processes involving PGA in insulin formulations, requiring rigorous quality control measures and documentation.
As research on PGA in insulin adsorption modulation progresses, regulatory agencies are likely to adapt their guidelines to address the specific challenges and opportunities presented by this technology. This may include the development of new testing protocols to assess the long-term stability and efficacy of PGA-modulated insulin formulations, as well as guidelines for evaluating the potential immunogenicity of PGA when used in conjunction with insulin.
Biocompatibility Studies
Biocompatibility studies are crucial in assessing the safety and efficacy of polyglutamic acid (PGA) for insulin adsorption modulation. These studies evaluate the interaction between PGA and biological systems, ensuring that the material does not elicit adverse reactions when in contact with living tissues or bodily fluids.
In vitro cytotoxicity tests have demonstrated that PGA exhibits minimal toxicity to various cell lines, including human fibroblasts and endothelial cells. Cell viability assays, such as MTT and LDH release, have shown high cell survival rates even at relatively high PGA concentrations. This suggests that PGA-based insulin delivery systems are unlikely to cause significant cellular damage or death.
Hemocompatibility studies have revealed that PGA does not induce significant hemolysis or platelet activation when exposed to human blood. This is particularly important for potential intravenous applications of PGA-insulin complexes. Coagulation studies have also shown minimal interference with blood clotting mechanisms, further supporting the material's safety profile.
Inflammatory response evaluations have indicated that PGA generally elicits a low-grade inflammatory reaction. In vivo studies using animal models have shown only mild, transient increases in pro-inflammatory cytokines following PGA implantation or injection. This mild inflammatory response is often considered acceptable and may even contribute to the healing process in some applications.
Long-term biocompatibility studies have focused on the degradation profile of PGA and its potential effects on surrounding tissues. Results have shown that PGA undergoes gradual hydrolytic degradation, with the breakdown products being non-toxic and easily metabolized or excreted by the body. This controlled degradation is advantageous for sustained insulin release applications.
Immunogenicity assessments have demonstrated that PGA has a low potential for inducing an immune response. Antibody production against PGA has been minimal in animal studies, suggesting a reduced risk of allergic reactions or immune-mediated complications in clinical use.
Genotoxicity and carcinogenicity studies have not revealed any significant mutagenic or carcinogenic potential associated with PGA. This is crucial for ensuring the long-term safety of PGA-based insulin delivery systems, particularly for chronic use in diabetes management.
Overall, the biocompatibility profile of PGA appears favorable for its application in insulin adsorption modulation. However, it is important to note that these studies are ongoing, and further research is needed to fully elucidate the long-term effects and potential interactions with specific patient populations or co-morbidities.
In vitro cytotoxicity tests have demonstrated that PGA exhibits minimal toxicity to various cell lines, including human fibroblasts and endothelial cells. Cell viability assays, such as MTT and LDH release, have shown high cell survival rates even at relatively high PGA concentrations. This suggests that PGA-based insulin delivery systems are unlikely to cause significant cellular damage or death.
Hemocompatibility studies have revealed that PGA does not induce significant hemolysis or platelet activation when exposed to human blood. This is particularly important for potential intravenous applications of PGA-insulin complexes. Coagulation studies have also shown minimal interference with blood clotting mechanisms, further supporting the material's safety profile.
Inflammatory response evaluations have indicated that PGA generally elicits a low-grade inflammatory reaction. In vivo studies using animal models have shown only mild, transient increases in pro-inflammatory cytokines following PGA implantation or injection. This mild inflammatory response is often considered acceptable and may even contribute to the healing process in some applications.
Long-term biocompatibility studies have focused on the degradation profile of PGA and its potential effects on surrounding tissues. Results have shown that PGA undergoes gradual hydrolytic degradation, with the breakdown products being non-toxic and easily metabolized or excreted by the body. This controlled degradation is advantageous for sustained insulin release applications.
Immunogenicity assessments have demonstrated that PGA has a low potential for inducing an immune response. Antibody production against PGA has been minimal in animal studies, suggesting a reduced risk of allergic reactions or immune-mediated complications in clinical use.
Genotoxicity and carcinogenicity studies have not revealed any significant mutagenic or carcinogenic potential associated with PGA. This is crucial for ensuring the long-term safety of PGA-based insulin delivery systems, particularly for chronic use in diabetes management.
Overall, the biocompatibility profile of PGA appears favorable for its application in insulin adsorption modulation. However, it is important to note that these studies are ongoing, and further research is needed to fully elucidate the long-term effects and potential interactions with specific patient populations or co-morbidities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!