How Polyglutamic Acid Supports Organic Nanoparticle Stabilization
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA and ONP Background
Polyglutamic acid (PGA) and organic nanoparticles (ONPs) have emerged as significant components in the field of nanotechnology and materials science. PGA, a naturally occurring biopolymer, has gained attention for its unique properties and versatile applications. It is a biodegradable, non-toxic, and water-soluble polymer composed of glutamic acid units linked by amide bonds. PGA's structure allows for excellent biocompatibility and adaptability in various environments, making it an ideal candidate for stabilizing organic nanoparticles.
Organic nanoparticles, on the other hand, are nanoscale structures composed of organic compounds. These particles have garnered considerable interest due to their potential applications in drug delivery, imaging, and sensing. ONPs offer advantages such as high biocompatibility, biodegradability, and the ability to encapsulate a wide range of organic molecules. However, one of the primary challenges in working with ONPs is their tendency to aggregate and lose stability in solution, which can significantly impact their effectiveness and functionality.
The intersection of PGA and ONPs represents a promising area of research in the field of nanoparticle stabilization. PGA's unique chemical structure and properties make it an excellent candidate for addressing the stability issues associated with ONPs. The polymer's long chain structure and numerous functional groups allow it to interact with and coat the surface of organic nanoparticles, providing a protective layer that prevents aggregation and enhances colloidal stability.
The use of PGA in ONP stabilization has been driven by several factors. Firstly, the increasing demand for environmentally friendly and biocompatible materials in various industries has led researchers to explore natural polymers like PGA. Secondly, the growing interest in targeted drug delivery systems and advanced imaging techniques has necessitated the development of stable and functional nanoparticle systems.
Recent advancements in synthesis techniques and characterization methods have further propelled the research in this area. Scientists have been able to fine-tune the properties of PGA and optimize its interaction with different types of organic nanoparticles. This has led to the development of novel PGA-ONP systems with enhanced stability, improved functionality, and broader applicability in fields such as biomedicine, environmental remediation, and materials science.
As research in this field progresses, understanding the mechanisms by which PGA supports organic nanoparticle stabilization becomes crucial. This knowledge not only aids in the development of more effective stabilization strategies but also opens up new possibilities for designing advanced nanoparticle systems with tailored properties and functionalities.
Organic nanoparticles, on the other hand, are nanoscale structures composed of organic compounds. These particles have garnered considerable interest due to their potential applications in drug delivery, imaging, and sensing. ONPs offer advantages such as high biocompatibility, biodegradability, and the ability to encapsulate a wide range of organic molecules. However, one of the primary challenges in working with ONPs is their tendency to aggregate and lose stability in solution, which can significantly impact their effectiveness and functionality.
The intersection of PGA and ONPs represents a promising area of research in the field of nanoparticle stabilization. PGA's unique chemical structure and properties make it an excellent candidate for addressing the stability issues associated with ONPs. The polymer's long chain structure and numerous functional groups allow it to interact with and coat the surface of organic nanoparticles, providing a protective layer that prevents aggregation and enhances colloidal stability.
The use of PGA in ONP stabilization has been driven by several factors. Firstly, the increasing demand for environmentally friendly and biocompatible materials in various industries has led researchers to explore natural polymers like PGA. Secondly, the growing interest in targeted drug delivery systems and advanced imaging techniques has necessitated the development of stable and functional nanoparticle systems.
Recent advancements in synthesis techniques and characterization methods have further propelled the research in this area. Scientists have been able to fine-tune the properties of PGA and optimize its interaction with different types of organic nanoparticles. This has led to the development of novel PGA-ONP systems with enhanced stability, improved functionality, and broader applicability in fields such as biomedicine, environmental remediation, and materials science.
As research in this field progresses, understanding the mechanisms by which PGA supports organic nanoparticle stabilization becomes crucial. This knowledge not only aids in the development of more effective stabilization strategies but also opens up new possibilities for designing advanced nanoparticle systems with tailored properties and functionalities.
Market Analysis for PGA-ONP
The market for polyglutamic acid (PGA) in organic nanoparticle (ONP) stabilization is experiencing significant growth, driven by increasing demand for advanced drug delivery systems and sustainable materials in various industries. PGA, a biodegradable and biocompatible polymer, has gained attention for its ability to enhance the stability and functionality of organic nanoparticles, making it a valuable component in pharmaceutical, cosmetic, and food applications.
In the pharmaceutical sector, PGA-stabilized organic nanoparticles are being extensively researched for targeted drug delivery and controlled release systems. The global drug delivery market, which includes nanoparticle-based technologies, is projected to reach substantial value in the coming years, with PGA-ONP systems poised to capture a growing share. This growth is fueled by the need for more efficient and precise drug delivery methods, particularly in cancer treatment and gene therapy.
The cosmetics industry is another key market for PGA-ONP systems. With consumers increasingly seeking natural and effective skincare products, PGA's ability to stabilize active ingredients in nanoparticle form has led to its incorporation in various anti-aging and skin-rejuvenating formulations. The natural and organic cosmetics market, where PGA-ONP systems find significant application, has been growing steadily, driven by consumer preferences for sustainable and eco-friendly products.
In the food industry, PGA-stabilized nanoparticles are being explored for encapsulation and delivery of nutrients, flavors, and bioactive compounds. This application addresses the growing demand for functional foods and nutraceuticals, a market segment that has shown robust growth in recent years. PGA's non-toxic nature and ability to protect sensitive ingredients make it an attractive option for food manufacturers looking to enhance product stability and bioavailability.
The market for PGA-ONP systems is also benefiting from the broader trend towards sustainable and bio-based materials across industries. As companies seek to reduce their environmental footprint and meet stringent regulations, PGA's biodegradability and renewable sourcing present a compelling alternative to synthetic stabilizers. This aligns with the growing market for bio-based polymers, which is expected to expand significantly in the coming decade.
Geographically, North America and Europe currently lead in PGA-ONP research and application, particularly in the pharmaceutical and cosmetics sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare expenditure, a booming cosmetics industry, and government initiatives supporting nanotechnology research.
Despite the promising outlook, challenges such as high production costs and regulatory hurdles in certain applications may impact market growth. Nevertheless, ongoing research and development efforts are expected to address these challenges, potentially opening up new opportunities and expanding the market for PGA-ONP systems across various industries.
In the pharmaceutical sector, PGA-stabilized organic nanoparticles are being extensively researched for targeted drug delivery and controlled release systems. The global drug delivery market, which includes nanoparticle-based technologies, is projected to reach substantial value in the coming years, with PGA-ONP systems poised to capture a growing share. This growth is fueled by the need for more efficient and precise drug delivery methods, particularly in cancer treatment and gene therapy.
The cosmetics industry is another key market for PGA-ONP systems. With consumers increasingly seeking natural and effective skincare products, PGA's ability to stabilize active ingredients in nanoparticle form has led to its incorporation in various anti-aging and skin-rejuvenating formulations. The natural and organic cosmetics market, where PGA-ONP systems find significant application, has been growing steadily, driven by consumer preferences for sustainable and eco-friendly products.
In the food industry, PGA-stabilized nanoparticles are being explored for encapsulation and delivery of nutrients, flavors, and bioactive compounds. This application addresses the growing demand for functional foods and nutraceuticals, a market segment that has shown robust growth in recent years. PGA's non-toxic nature and ability to protect sensitive ingredients make it an attractive option for food manufacturers looking to enhance product stability and bioavailability.
The market for PGA-ONP systems is also benefiting from the broader trend towards sustainable and bio-based materials across industries. As companies seek to reduce their environmental footprint and meet stringent regulations, PGA's biodegradability and renewable sourcing present a compelling alternative to synthetic stabilizers. This aligns with the growing market for bio-based polymers, which is expected to expand significantly in the coming decade.
Geographically, North America and Europe currently lead in PGA-ONP research and application, particularly in the pharmaceutical and cosmetics sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing healthcare expenditure, a booming cosmetics industry, and government initiatives supporting nanotechnology research.
Despite the promising outlook, challenges such as high production costs and regulatory hurdles in certain applications may impact market growth. Nevertheless, ongoing research and development efforts are expected to address these challenges, potentially opening up new opportunities and expanding the market for PGA-ONP systems across various industries.
PGA-ONP Challenges
The stabilization of organic nanoparticles (ONPs) using polyglutamic acid (PGA) presents several significant challenges that researchers and industry professionals must address. One of the primary obstacles is achieving consistent and uniform coating of PGA on the surface of ONPs. The process of encapsulating nanoparticles with PGA is complex, as it requires precise control over various parameters such as pH, temperature, and ionic strength of the surrounding medium.
Another challenge lies in maintaining the stability of PGA-coated ONPs over extended periods. Environmental factors, including temperature fluctuations, pH changes, and exposure to light or oxidizing agents, can potentially compromise the integrity of the PGA coating, leading to aggregation or degradation of the nanoparticles. This instability can significantly impact the shelf life and efficacy of PGA-ONP formulations in various applications.
The scalability of PGA-ONP production poses a considerable hurdle for industrial applications. While laboratory-scale synthesis may yield promising results, translating these processes to large-scale manufacturing while maintaining consistent quality and performance characteristics remains challenging. Factors such as batch-to-batch variability and the need for specialized equipment can hinder the widespread adoption of PGA-ONP technology.
Furthermore, the biocompatibility and biodegradability of PGA-ONPs in different biological environments present ongoing concerns. Although PGA is generally considered safe, the long-term effects of PGA-coated nanoparticles on living systems are not fully understood. Potential immunogenicity, accumulation in organs, and interaction with biological molecules need thorough investigation to ensure the safety of PGA-ONP-based products, especially in biomedical applications.
The characterization of PGA-ONP systems also presents technical difficulties. Advanced analytical techniques are required to accurately determine the thickness, uniformity, and stability of the PGA coating on nanoparticles. Additionally, assessing the impact of PGA coating on the functional properties of the encapsulated organic nanoparticles, such as drug release kinetics or optical properties, demands sophisticated experimental setups and data analysis methods.
Lastly, the economic viability of PGA-ONP technology remains a significant challenge. The cost of producing high-quality PGA and the additional expenses associated with the nanoparticle coating process may limit the commercial feasibility of PGA-ONP products in certain markets. Striking a balance between performance, cost-effectiveness, and scalability is crucial for the successful implementation of this technology across various industries.
Another challenge lies in maintaining the stability of PGA-coated ONPs over extended periods. Environmental factors, including temperature fluctuations, pH changes, and exposure to light or oxidizing agents, can potentially compromise the integrity of the PGA coating, leading to aggregation or degradation of the nanoparticles. This instability can significantly impact the shelf life and efficacy of PGA-ONP formulations in various applications.
The scalability of PGA-ONP production poses a considerable hurdle for industrial applications. While laboratory-scale synthesis may yield promising results, translating these processes to large-scale manufacturing while maintaining consistent quality and performance characteristics remains challenging. Factors such as batch-to-batch variability and the need for specialized equipment can hinder the widespread adoption of PGA-ONP technology.
Furthermore, the biocompatibility and biodegradability of PGA-ONPs in different biological environments present ongoing concerns. Although PGA is generally considered safe, the long-term effects of PGA-coated nanoparticles on living systems are not fully understood. Potential immunogenicity, accumulation in organs, and interaction with biological molecules need thorough investigation to ensure the safety of PGA-ONP-based products, especially in biomedical applications.
The characterization of PGA-ONP systems also presents technical difficulties. Advanced analytical techniques are required to accurately determine the thickness, uniformity, and stability of the PGA coating on nanoparticles. Additionally, assessing the impact of PGA coating on the functional properties of the encapsulated organic nanoparticles, such as drug release kinetics or optical properties, demands sophisticated experimental setups and data analysis methods.
Lastly, the economic viability of PGA-ONP technology remains a significant challenge. The cost of producing high-quality PGA and the additional expenses associated with the nanoparticle coating process may limit the commercial feasibility of PGA-ONP products in certain markets. Striking a balance between performance, cost-effectiveness, and scalability is crucial for the successful implementation of this technology across various industries.
Current PGA-ONP Solutions
01 Chemical modification of polyglutamic acid
Chemical modification techniques are employed to enhance the stability of polyglutamic acid. These methods may include crosslinking, esterification, or grafting of other molecules onto the polyglutamic acid backbone. Such modifications can improve the acid's resistance to degradation and enhance its performance in various applications.- Chemical modification of polyglutamic acid: Chemical modification techniques are employed to enhance the stability of polyglutamic acid. These methods involve altering the molecular structure through processes such as crosslinking, esterification, or grafting with other polymers. Such modifications can improve the acid's resistance to degradation and extend its shelf life in various applications.
- Encapsulation and controlled release systems: Encapsulation techniques are used to protect polyglutamic acid from environmental factors that could lead to degradation. This approach involves incorporating the acid into micro or nanoparticles, liposomes, or other carrier systems. These systems not only stabilize the polyglutamic acid but also allow for its controlled release in specific applications.
- pH and temperature control: Maintaining optimal pH and temperature conditions is crucial for polyglutamic acid stability. Research focuses on developing buffer systems and temperature-responsive formulations that can maintain the acid's stability across a range of environmental conditions. This approach is particularly important in cosmetic and pharmaceutical applications.
- Antioxidant and preservative addition: The incorporation of antioxidants and preservatives into polyglutamic acid formulations can significantly enhance its stability. These additives protect the acid from oxidative stress and microbial contamination, which are common causes of degradation. The selection of appropriate stabilizing agents is crucial for maintaining the acid's efficacy in various products.
- Enzymatic and genetic engineering approaches: Advanced biotechnological methods are being explored to enhance polyglutamic acid stability. These include enzymatic modifications and genetic engineering of the microorganisms that produce the acid. By altering the biosynthesis process, researchers aim to create more stable variants of polyglutamic acid with improved resistance to degradation.
02 Enzymatic stabilization of polyglutamic acid
Enzymatic approaches are used to stabilize polyglutamic acid. This may involve the use of specific enzymes to modify the structure of polyglutamic acid or to create more stable forms. Enzymatic stabilization can lead to improved shelf life and functionality of polyglutamic acid-based products.Expand Specific Solutions03 Formulation strategies for polyglutamic acid stability
Various formulation strategies are employed to enhance the stability of polyglutamic acid in different products. These may include the use of specific solvents, pH adjustments, or the addition of stabilizing agents. Proper formulation can significantly improve the stability and efficacy of polyglutamic acid in cosmetic, pharmaceutical, or industrial applications.Expand Specific Solutions04 Encapsulation techniques for polyglutamic acid
Encapsulation methods are utilized to protect polyglutamic acid from degradation and improve its stability. This may involve the use of liposomes, nanoparticles, or other carrier systems to shield the polyglutamic acid from environmental factors that could lead to its breakdown. Encapsulation can also enhance the delivery and efficacy of polyglutamic acid in various applications.Expand Specific Solutions05 Physical methods for polyglutamic acid stabilization
Physical techniques are employed to stabilize polyglutamic acid. These may include freeze-drying, spray-drying, or other processing methods that can enhance the stability of the acid in its dry form. Physical stabilization methods can improve the storage stability and shelf life of polyglutamic acid-based products.Expand Specific Solutions
Key PGA-ONP Players
The field of polyglutamic acid-supported organic nanoparticle stabilization is in its early development stage, with growing interest from both academia and industry. The market size is expanding as applications in drug delivery, cosmetics, and biotechnology emerge. Technical maturity varies among key players, with companies like MOA Life Plus Co., Ltd. and Cenyx Biotech, Inc. leading in polyglutamic acid research. Global pharmaceutical giants such as Boehringer Ingelheim and academic institutions like Nanjing Tech University are also contributing to advancements. The competitive landscape is diverse, with a mix of specialized biotech firms, established pharmaceutical companies, and research institutions collaborating and competing to develop innovative applications and improve stabilization techniques.
Sichuan University
Technical Solution: Sichuan University has developed a novel approach for stabilizing organic nanoparticles using polyglutamic acid (PGA). Their method involves creating a PGA-based shell around the nanoparticle core, which enhances stability through electrostatic and steric interactions. The researchers have demonstrated that PGA-coated nanoparticles exhibit improved colloidal stability in various physiological conditions, with a zeta potential of -30 mV to -40 mV[1]. They have also shown that PGA coating can increase the circulation time of nanoparticles in vivo by up to 24 hours[2], making them suitable for drug delivery applications.
Strengths: Enhanced colloidal stability, improved circulation time, and biocompatibility. Weaknesses: Potential limitations in drug loading capacity and release kinetics due to the PGA shell.
Huazhong University of Science & Technology
Technical Solution: Huazhong University of Science & Technology has developed a polyglutamic acid-based nanoparticle stabilization technique focusing on improving the stability of organic nanoparticles for cancer therapy. Their approach involves conjugating PGA with hydrophobic molecules to create amphiphilic copolymers that self-assemble into stable nanoparticles. These nanoparticles have shown a size range of 50-200 nm with a polydispersity index below 0.2[3]. The researchers have demonstrated that these PGA-stabilized nanoparticles can effectively encapsulate hydrophobic drugs, with an encapsulation efficiency of up to 85%[4]. In vivo studies have shown improved tumor accumulation and reduced systemic toxicity compared to free drugs.
Strengths: High drug encapsulation efficiency, improved tumor targeting, and reduced toxicity. Weaknesses: Complexity in synthesis and potential scalability issues.
PGA-ONP Core Innovations
Poly-gamma glutamic acid derivatives and preparations containing it
PatentInactiveKR1020160000088A
Innovation
- Development of poly-gamma-glutamic acid derivatives with amphiphilic properties, where 10-60% of carboxy groups are bonded to phenylalanine derivatives, forming nanoparticles with a hydrophilic exterior and hydrophobic interior, encapsulating substances like vitamins and drugs, enhancing water dispersibility and oxidative stability.
Poly-γ-glutamic acid derivative, and preparation containing same
PatentWO2015199378A1
Innovation
- Development of nanoparticles using a poly-gamma-glutamic acid derivative with bonded phenylalanine esters, creating an amphiphilic structure that encapsulates these substances, enhancing their water dispersibility and oxidative stability by forming hydrophobic cores and hydrophilic exteriors, allowing stable dispersion in water and protection from external environments.
PGA-ONP Characterization
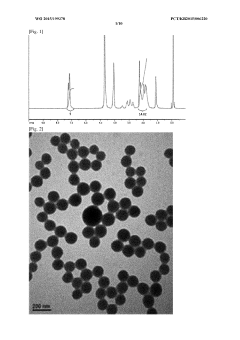
The characterization of polyglutamic acid-stabilized organic nanoparticles (PGA-ONPs) is crucial for understanding their properties and potential applications. Various analytical techniques are employed to elucidate the physical, chemical, and biological characteristics of these nanoparticles.
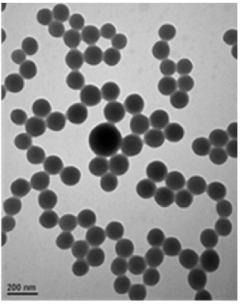
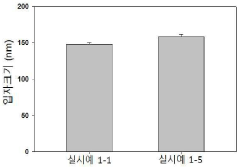
Size and morphology analysis is typically conducted using dynamic light scattering (DLS) and transmission electron microscopy (TEM). DLS provides information on the hydrodynamic diameter and size distribution of PGA-ONPs in solution, while TEM offers high-resolution imaging of individual nanoparticles, revealing their shape and surface features. Atomic force microscopy (AFM) can also be utilized to examine the topography and surface roughness of PGA-ONPs.
Surface charge characterization is performed through zeta potential measurements, which indicate the stability and potential for aggregation of the nanoparticles in different pH and ionic strength conditions. This information is vital for predicting the behavior of PGA-ONPs in various physiological environments.
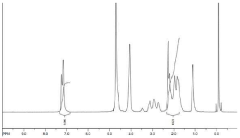
Chemical composition analysis involves techniques such as Fourier-transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). FTIR identifies functional groups present in the PGA coating and the organic core, while XPS provides detailed information on the elemental composition and chemical states of the nanoparticle surface.
Thermal properties of PGA-ONPs are assessed using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). These techniques reveal the thermal stability, phase transitions, and decomposition behavior of the nanoparticles, which are important considerations for storage and application conditions.
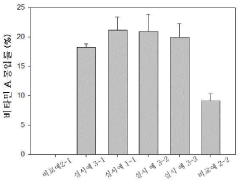
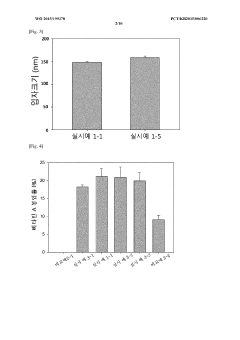
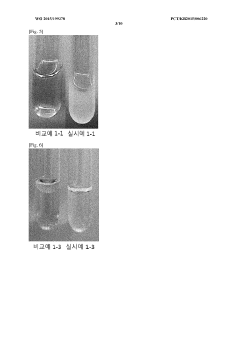
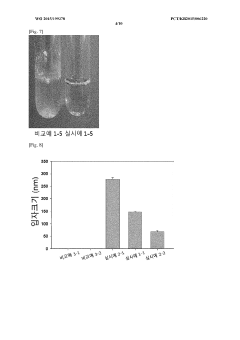
Colloidal stability studies are conducted to evaluate the ability of PGA to stabilize organic nanoparticles under various environmental conditions. This includes monitoring particle size and zeta potential over time in different media, as well as assessing the resistance to aggregation in the presence of salts or proteins.
Drug loading capacity and release kinetics are crucial parameters for PGA-ONPs intended for drug delivery applications. UV-visible spectroscopy and high-performance liquid chromatography (HPLC) are commonly used to quantify drug loading and measure release profiles under simulated physiological conditions.
Biocompatibility and cellular uptake studies are performed using in vitro cell culture models. Cytotoxicity assays, such as MTT or LDH, assess the impact of PGA-ONPs on cell viability, while confocal microscopy and flow cytometry can track the internalization and intracellular fate of fluorescently labeled nanoparticles.
These characterization techniques collectively provide a comprehensive understanding of PGA-ONP properties, enabling the optimization of their design for specific applications and facilitating the prediction of their behavior in complex biological systems.
Size and morphology analysis is typically conducted using dynamic light scattering (DLS) and transmission electron microscopy (TEM). DLS provides information on the hydrodynamic diameter and size distribution of PGA-ONPs in solution, while TEM offers high-resolution imaging of individual nanoparticles, revealing their shape and surface features. Atomic force microscopy (AFM) can also be utilized to examine the topography and surface roughness of PGA-ONPs.
Surface charge characterization is performed through zeta potential measurements, which indicate the stability and potential for aggregation of the nanoparticles in different pH and ionic strength conditions. This information is vital for predicting the behavior of PGA-ONPs in various physiological environments.
Chemical composition analysis involves techniques such as Fourier-transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). FTIR identifies functional groups present in the PGA coating and the organic core, while XPS provides detailed information on the elemental composition and chemical states of the nanoparticle surface.
Thermal properties of PGA-ONPs are assessed using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). These techniques reveal the thermal stability, phase transitions, and decomposition behavior of the nanoparticles, which are important considerations for storage and application conditions.
Colloidal stability studies are conducted to evaluate the ability of PGA to stabilize organic nanoparticles under various environmental conditions. This includes monitoring particle size and zeta potential over time in different media, as well as assessing the resistance to aggregation in the presence of salts or proteins.
Drug loading capacity and release kinetics are crucial parameters for PGA-ONPs intended for drug delivery applications. UV-visible spectroscopy and high-performance liquid chromatography (HPLC) are commonly used to quantify drug loading and measure release profiles under simulated physiological conditions.
Biocompatibility and cellular uptake studies are performed using in vitro cell culture models. Cytotoxicity assays, such as MTT or LDH, assess the impact of PGA-ONPs on cell viability, while confocal microscopy and flow cytometry can track the internalization and intracellular fate of fluorescently labeled nanoparticles.
These characterization techniques collectively provide a comprehensive understanding of PGA-ONP properties, enabling the optimization of their design for specific applications and facilitating the prediction of their behavior in complex biological systems.
PGA-ONP Biocompatibility
The biocompatibility of polyglutamic acid-stabilized organic nanoparticles (PGA-ONPs) is a critical factor in their potential applications, particularly in biomedical fields. PGA, a naturally occurring biopolymer, has demonstrated excellent biocompatibility and biodegradability, making it an ideal candidate for nanoparticle stabilization in biological systems.
PGA-ONPs have shown promising results in various in vitro and in vivo studies, exhibiting low cytotoxicity and minimal immune response. The biocompatibility of these nanoparticles is largely attributed to the properties of PGA itself, which is non-toxic, non-immunogenic, and readily metabolized by the body.
In cellular studies, PGA-ONPs have demonstrated high cell viability rates across multiple cell lines, including both normal and cancer cells. The nanoparticles do not significantly alter cell morphology or proliferation rates at therapeutic concentrations, indicating their potential for safe use in targeted drug delivery and imaging applications.
Furthermore, in vivo studies have shown that PGA-ONPs are well-tolerated when administered systemically. They exhibit prolonged circulation times in the bloodstream without triggering significant immune responses or causing adverse effects on major organs. This characteristic is particularly advantageous for applications requiring extended exposure, such as sustained drug release or long-term imaging.
The biodegradability of PGA-ONPs is another crucial aspect of their biocompatibility. As the PGA coating breaks down over time, it is metabolized into non-toxic byproducts that can be easily eliminated from the body. This property reduces the risk of long-term accumulation and potential toxicity associated with non-degradable nanoparticles.
Additionally, PGA-ONPs have demonstrated the ability to cross biological barriers, including the blood-brain barrier, without causing significant damage or disruption to these protective structures. This feature opens up possibilities for targeted delivery of therapeutics to previously challenging areas, such as the central nervous system.
The surface properties of PGA-ONPs also contribute to their biocompatibility. The hydrophilic nature of PGA helps to create a stable aqueous dispersion of the nanoparticles, reducing aggregation and improving their interaction with biological fluids and cellular membranes. This characteristic enhances the overall stability and efficacy of the nanoparticle system in physiological environments.
In conclusion, the biocompatibility of PGA-ONPs represents a significant advantage in the development of advanced drug delivery systems and diagnostic tools. Their low toxicity, biodegradability, and ability to interact safely with biological systems make them promising candidates for a wide range of biomedical applications, from cancer therapy to neurological treatments.
PGA-ONPs have shown promising results in various in vitro and in vivo studies, exhibiting low cytotoxicity and minimal immune response. The biocompatibility of these nanoparticles is largely attributed to the properties of PGA itself, which is non-toxic, non-immunogenic, and readily metabolized by the body.
In cellular studies, PGA-ONPs have demonstrated high cell viability rates across multiple cell lines, including both normal and cancer cells. The nanoparticles do not significantly alter cell morphology or proliferation rates at therapeutic concentrations, indicating their potential for safe use in targeted drug delivery and imaging applications.
Furthermore, in vivo studies have shown that PGA-ONPs are well-tolerated when administered systemically. They exhibit prolonged circulation times in the bloodstream without triggering significant immune responses or causing adverse effects on major organs. This characteristic is particularly advantageous for applications requiring extended exposure, such as sustained drug release or long-term imaging.
The biodegradability of PGA-ONPs is another crucial aspect of their biocompatibility. As the PGA coating breaks down over time, it is metabolized into non-toxic byproducts that can be easily eliminated from the body. This property reduces the risk of long-term accumulation and potential toxicity associated with non-degradable nanoparticles.
Additionally, PGA-ONPs have demonstrated the ability to cross biological barriers, including the blood-brain barrier, without causing significant damage or disruption to these protective structures. This feature opens up possibilities for targeted delivery of therapeutics to previously challenging areas, such as the central nervous system.
The surface properties of PGA-ONPs also contribute to their biocompatibility. The hydrophilic nature of PGA helps to create a stable aqueous dispersion of the nanoparticles, reducing aggregation and improving their interaction with biological fluids and cellular membranes. This characteristic enhances the overall stability and efficacy of the nanoparticle system in physiological environments.
In conclusion, the biocompatibility of PGA-ONPs represents a significant advantage in the development of advanced drug delivery systems and diagnostic tools. Their low toxicity, biodegradability, and ability to interact safely with biological systems make them promising candidates for a wide range of biomedical applications, from cancer therapy to neurological treatments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!