How to Address Common Misconceptions About PEMF Therapy?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent years. The technology harnesses the power of electromagnetic fields to stimulate cellular repair and promote overall health. PEMF therapy's roots can be traced back to the mid-20th century, with significant advancements occurring in the past few decades.
The evolution of PEMF therapy has been marked by continuous refinement of equipment and protocols. Early devices were bulky and limited in their applications, while modern PEMF systems are more compact, versatile, and user-friendly. This progression has expanded the therapy's potential across various medical fields, including orthopedics, neurology, and pain management.
Despite its growing popularity, PEMF therapy faces numerous misconceptions that hinder its widespread acceptance. These misconceptions often stem from a lack of understanding about the therapy's mechanisms of action and its scientific basis. Addressing these misconceptions is crucial for the technology's future development and adoption.
The primary objective of this technical research report is to identify and address common misconceptions surrounding PEMF therapy. By doing so, we aim to provide a clearer understanding of the technology's capabilities, limitations, and potential applications. This clarification is essential for both healthcare professionals and potential patients to make informed decisions about the use of PEMF therapy.
Another key goal is to explore the current state of scientific evidence supporting PEMF therapy. This involves a comprehensive review of clinical studies, meta-analyses, and systematic reviews to establish a solid foundation for the therapy's efficacy and safety. By critically examining the available research, we can separate fact from fiction and provide a balanced perspective on PEMF therapy's potential benefits and risks.
Furthermore, this report seeks to outline the technological advancements driving the evolution of PEMF therapy. We will investigate cutting-edge developments in device design, treatment protocols, and targeted applications. Understanding these innovations is crucial for predicting future trends and identifying areas for further research and development.
Lastly, we aim to contextualize PEMF therapy within the broader landscape of alternative and complementary medicine. By comparing PEMF therapy to other non-invasive treatment modalities, we can better understand its unique advantages and potential synergies with existing therapies. This holistic approach will help position PEMF therapy accurately within the medical field and guide future research directions.
The evolution of PEMF therapy has been marked by continuous refinement of equipment and protocols. Early devices were bulky and limited in their applications, while modern PEMF systems are more compact, versatile, and user-friendly. This progression has expanded the therapy's potential across various medical fields, including orthopedics, neurology, and pain management.
Despite its growing popularity, PEMF therapy faces numerous misconceptions that hinder its widespread acceptance. These misconceptions often stem from a lack of understanding about the therapy's mechanisms of action and its scientific basis. Addressing these misconceptions is crucial for the technology's future development and adoption.
The primary objective of this technical research report is to identify and address common misconceptions surrounding PEMF therapy. By doing so, we aim to provide a clearer understanding of the technology's capabilities, limitations, and potential applications. This clarification is essential for both healthcare professionals and potential patients to make informed decisions about the use of PEMF therapy.
Another key goal is to explore the current state of scientific evidence supporting PEMF therapy. This involves a comprehensive review of clinical studies, meta-analyses, and systematic reviews to establish a solid foundation for the therapy's efficacy and safety. By critically examining the available research, we can separate fact from fiction and provide a balanced perspective on PEMF therapy's potential benefits and risks.
Furthermore, this report seeks to outline the technological advancements driving the evolution of PEMF therapy. We will investigate cutting-edge developments in device design, treatment protocols, and targeted applications. Understanding these innovations is crucial for predicting future trends and identifying areas for further research and development.
Lastly, we aim to contextualize PEMF therapy within the broader landscape of alternative and complementary medicine. By comparing PEMF therapy to other non-invasive treatment modalities, we can better understand its unique advantages and potential synergies with existing therapies. This holistic approach will help position PEMF therapy accurately within the medical field and guide future research directions.
Market Analysis for PEMF Devices
The PEMF (Pulsed Electromagnetic Field) therapy device market has shown significant growth in recent years, driven by increasing awareness of non-invasive treatment options and the rising prevalence of chronic pain conditions. The global PEMF therapy devices market was valued at approximately $500 million in 2020 and is projected to reach $1.2 billion by 2027, growing at a CAGR of around 12% during the forecast period.
The market for PEMF devices is segmented based on product type, application, end-user, and region. Product types include high-power devices used in clinical settings and low-power devices for home use. Applications span a wide range, including pain management, bone healing, neurological disorders, and wound healing. The end-user segments include hospitals, specialty clinics, and home healthcare settings.
North America currently dominates the PEMF device market, accounting for over 40% of the global market share. This is attributed to the high adoption rate of advanced medical technologies, well-established healthcare infrastructure, and increasing geriatric population. Europe follows as the second-largest market, with Germany, the UK, and France being key contributors. The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure and rising disposable incomes in countries like China and India.
Key market drivers include the growing prevalence of chronic pain conditions, increasing sports injuries, and the rising demand for non-invasive treatment options. The aging population in developed countries is also a significant factor, as older adults are more prone to conditions that can benefit from PEMF therapy. Additionally, technological advancements in PEMF devices, such as improved portability and user-friendly interfaces, are contributing to market growth.
However, the market faces challenges such as the lack of awareness about PEMF therapy among both patients and healthcare professionals, stringent regulatory requirements for medical devices, and the high cost of advanced PEMF systems. These factors may hinder market growth, particularly in developing regions.
The competitive landscape of the PEMF device market is characterized by the presence of both established medical device companies and specialized PEMF therapy manufacturers. Key players include OMI, Orthofix Medical Inc., Curatronic Ltd., and Swiss Bionic Solutions. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to gain a competitive edge.
Looking ahead, the PEMF device market is expected to continue its growth trajectory, driven by increasing clinical evidence supporting the efficacy of PEMF therapy, growing patient preference for non-pharmacological treatment options, and ongoing technological advancements in device design and functionality. The home healthcare segment, in particular, is anticipated to witness substantial growth as more patients seek convenient and cost-effective treatment options for chronic conditions.
The market for PEMF devices is segmented based on product type, application, end-user, and region. Product types include high-power devices used in clinical settings and low-power devices for home use. Applications span a wide range, including pain management, bone healing, neurological disorders, and wound healing. The end-user segments include hospitals, specialty clinics, and home healthcare settings.
North America currently dominates the PEMF device market, accounting for over 40% of the global market share. This is attributed to the high adoption rate of advanced medical technologies, well-established healthcare infrastructure, and increasing geriatric population. Europe follows as the second-largest market, with Germany, the UK, and France being key contributors. The Asia-Pacific region is expected to witness the fastest growth due to improving healthcare infrastructure and rising disposable incomes in countries like China and India.
Key market drivers include the growing prevalence of chronic pain conditions, increasing sports injuries, and the rising demand for non-invasive treatment options. The aging population in developed countries is also a significant factor, as older adults are more prone to conditions that can benefit from PEMF therapy. Additionally, technological advancements in PEMF devices, such as improved portability and user-friendly interfaces, are contributing to market growth.
However, the market faces challenges such as the lack of awareness about PEMF therapy among both patients and healthcare professionals, stringent regulatory requirements for medical devices, and the high cost of advanced PEMF systems. These factors may hinder market growth, particularly in developing regions.
The competitive landscape of the PEMF device market is characterized by the presence of both established medical device companies and specialized PEMF therapy manufacturers. Key players include OMI, Orthofix Medical Inc., Curatronic Ltd., and Swiss Bionic Solutions. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to gain a competitive edge.
Looking ahead, the PEMF device market is expected to continue its growth trajectory, driven by increasing clinical evidence supporting the efficacy of PEMF therapy, growing patient preference for non-pharmacological treatment options, and ongoing technological advancements in device design and functionality. The home healthcare segment, in particular, is anticipated to witness substantial growth as more patients seek convenient and cost-effective treatment options for chronic conditions.
Current Challenges in PEMF Therapy Perception
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a potential treatment for various health conditions. However, its widespread adoption faces several challenges due to common misconceptions and misunderstandings among both healthcare professionals and the general public.
One of the primary challenges is the lack of comprehensive understanding about the mechanism of action of PEMF therapy. Many people struggle to grasp how electromagnetic fields can influence biological processes, leading to skepticism about its efficacy. This knowledge gap often results in dismissal of PEMF therapy as pseudoscience or a placebo effect, hindering its acceptance in mainstream medicine.
Another significant challenge is the inconsistency in research methodologies and outcomes. While numerous studies have demonstrated positive effects of PEMF therapy, there is a lack of standardization in treatment protocols, frequencies, and durations used across different studies. This variability makes it difficult to draw definitive conclusions about the therapy's effectiveness for specific conditions, fueling doubts and misconceptions.
The perception of PEMF therapy as a "cure-all" treatment is another hurdle that needs to be addressed. Some proponents make exaggerated claims about its benefits, which can lead to unrealistic expectations and disappointment when results don't match the hype. This overpromising undermines the credibility of legitimate PEMF applications and reinforces skepticism among medical professionals.
Safety concerns also contribute to misconceptions about PEMF therapy. Despite its non-invasive nature, some individuals worry about potential long-term effects of exposure to electromagnetic fields. These concerns are often rooted in confusion between PEMF therapy and other forms of electromagnetic radiation, such as those emitted by cell phones or power lines.
The regulatory landscape surrounding PEMF devices adds another layer of complexity to public perception. The lack of clear guidelines and standards for PEMF devices in some regions has led to a proliferation of products with varying quality and effectiveness. This situation makes it challenging for consumers and healthcare providers to distinguish between legitimate therapeutic devices and ineffective or potentially harmful ones.
Lastly, the integration of PEMF therapy into conventional medical practice faces resistance due to the traditional divide between alternative and mainstream medicine. Many healthcare professionals are hesitant to recommend or use PEMF therapy due to limited exposure during their medical training and a preference for more established treatment modalities.
One of the primary challenges is the lack of comprehensive understanding about the mechanism of action of PEMF therapy. Many people struggle to grasp how electromagnetic fields can influence biological processes, leading to skepticism about its efficacy. This knowledge gap often results in dismissal of PEMF therapy as pseudoscience or a placebo effect, hindering its acceptance in mainstream medicine.
Another significant challenge is the inconsistency in research methodologies and outcomes. While numerous studies have demonstrated positive effects of PEMF therapy, there is a lack of standardization in treatment protocols, frequencies, and durations used across different studies. This variability makes it difficult to draw definitive conclusions about the therapy's effectiveness for specific conditions, fueling doubts and misconceptions.
The perception of PEMF therapy as a "cure-all" treatment is another hurdle that needs to be addressed. Some proponents make exaggerated claims about its benefits, which can lead to unrealistic expectations and disappointment when results don't match the hype. This overpromising undermines the credibility of legitimate PEMF applications and reinforces skepticism among medical professionals.
Safety concerns also contribute to misconceptions about PEMF therapy. Despite its non-invasive nature, some individuals worry about potential long-term effects of exposure to electromagnetic fields. These concerns are often rooted in confusion between PEMF therapy and other forms of electromagnetic radiation, such as those emitted by cell phones or power lines.
The regulatory landscape surrounding PEMF devices adds another layer of complexity to public perception. The lack of clear guidelines and standards for PEMF devices in some regions has led to a proliferation of products with varying quality and effectiveness. This situation makes it challenging for consumers and healthcare providers to distinguish between legitimate therapeutic devices and ineffective or potentially harmful ones.
Lastly, the integration of PEMF therapy into conventional medical practice faces resistance due to the traditional divide between alternative and mainstream medicine. Many healthcare professionals are hesitant to recommend or use PEMF therapy due to limited exposure during their medical training and a preference for more established treatment modalities.
Existing Strategies for PEMF Education
01 Misconception about PEMF therapy effectiveness
There's a common misconception that PEMF therapy is a cure-all treatment. While it has shown promise in various applications, its effectiveness can vary depending on the condition being treated and individual factors. PEMF therapy is generally considered a complementary treatment and should not replace conventional medical care. Understanding its limitations and appropriate uses is crucial for managing expectations.- Misconception about PEMF effectiveness: There's a common misconception that PEMF therapy is ineffective or pseudoscientific. However, numerous studies and clinical trials have demonstrated its potential benefits in various medical applications, including pain management, bone healing, and tissue regeneration. The therapy works by stimulating cellular activity and promoting natural healing processes in the body.
- Misunderstanding of PEMF safety: Some people believe that PEMF therapy is unsafe or harmful due to its use of electromagnetic fields. In reality, when used properly and under professional guidance, PEMF therapy is generally considered safe with minimal side effects. The electromagnetic fields used in PEMF are typically low-intensity and pulsed, which differentiates them from potentially harmful continuous electromagnetic fields.
- Confusion about PEMF application methods: There's often confusion about how PEMF therapy should be applied. Some believe it requires direct skin contact or invasive procedures. In fact, PEMF can be applied non-invasively through various devices, including mats, pads, and localized applicators. The therapy can penetrate through clothing and even casts, making it versatile for different treatment scenarios.
- Overestimation of immediate results: A common misconception is that PEMF therapy provides instant or miraculous results. While some patients may experience immediate relief, especially in pain management cases, the full benefits of PEMF therapy often require consistent application over time. The therapy works by gradually improving cellular function and promoting the body's natural healing processes, which can take weeks or months to show significant results.
- Misunderstanding of PEMF's scope of application: Some people mistakenly believe that PEMF therapy is limited to a narrow range of conditions, typically musculoskeletal issues. In reality, research has shown potential applications in a wide variety of health concerns, including mental health, cardiovascular health, and even certain neurological conditions. The therapy's ability to influence cellular activity gives it a broad spectrum of potential uses in healthcare.
02 Misunderstanding of PEMF therapy mechanisms
Some people misunderstand how PEMF therapy works at a cellular level. PEMF therapy uses electromagnetic fields to stimulate cellular activity and promote healing. However, it's not a direct energy transfer or a form of radiation therapy. The misconception about its mechanism can lead to unrealistic expectations or unfounded fears about its safety and efficacy.Expand Specific Solutions03 Confusion about PEMF therapy safety
There's often confusion about the safety of PEMF therapy, with some believing it might be harmful due to its use of electromagnetic fields. In reality, when used as directed, PEMF therapy is generally considered safe for most people. However, it's important to note that certain individuals, such as those with electronic implants or pregnant women, should consult with a healthcare provider before using PEMF devices.Expand Specific Solutions04 Overestimation of PEMF therapy's immediate effects
A common misconception is that PEMF therapy provides immediate, dramatic results. While some users may experience quick relief, especially for pain management, the full benefits of PEMF therapy often require consistent, long-term use. Understanding that results may be gradual and cumulative is important for setting realistic expectations and maintaining a proper treatment regimen.Expand Specific Solutions05 Misunderstanding of PEMF device specifications
There's often confusion about the importance of various PEMF device specifications, such as frequency, intensity, and waveform. Some believe that higher intensity or frequency always equates to better results. In reality, different conditions may respond better to specific parameters, and more isn't always better. Understanding the appropriate settings for specific applications is crucial for optimal results and safety.Expand Specific Solutions
Key PEMF Device Manufacturers and Researchers
The competitive landscape for addressing misconceptions about PEMF therapy is evolving, with the market currently in a growth phase. As the global PEMF therapy market expands, estimated to reach $1.8 billion by 2025, companies are focusing on educating consumers and healthcare professionals. The technology's maturity varies across applications, with established players like Regenesis Biomedical and Venus Concept leading in medical device development. Emerging companies such as SofPulse and Galvanize Therapeutics are innovating in specific therapeutic areas, while research institutions like the National University of Singapore and Swiss Federal Institute of Technology contribute to advancing the scientific understanding of PEMF therapy. The industry is seeing increased collaboration between academia and commercial entities to address misconceptions and validate therapeutic claims.
Regenesis Biomedical, Inc.
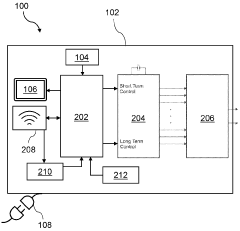
Technical Solution: Regenesis Biomedical addresses PEMF therapy misconceptions through their Provant Therapy System. This FDA-cleared device utilizes pulsed radiofrequency energy to promote healing and reduce pain. The company emphasizes education on the therapy's mechanism of action, which involves stimulating cellular repair and reducing inflammation. They provide clinical evidence demonstrating the efficacy of PEMF in various conditions, including post-operative pain and edema reduction[1]. Regenesis also focuses on explaining the non-invasive nature of PEMF and its lack of significant side effects, addressing safety concerns often associated with electromagnetic therapies[2].
Strengths: FDA-cleared device, strong clinical evidence base. Weaknesses: Limited to specific frequency range, may not address all PEMF applications.
Venus Concept Ltd.
Technical Solution: Venus Concept tackles PEMF misconceptions through their Venus Pulse technology. Their approach integrates PEMF with other modalities like radiofrequency and magnetic pulse therapy for aesthetic and medical applications. The company focuses on educating practitioners and patients about the synergistic effects of combined therapies, demonstrating how PEMF enhances cellular metabolism and blood circulation[3]. Venus Concept's devices are designed to deliver precise, targeted PEMF treatments, addressing concerns about the therapy's specificity. They provide extensive training materials and clinical studies to support the efficacy of their PEMF-based treatments in areas such as pain management and skin rejuvenation[4].
Strengths: Multi-modal approach, extensive practitioner training. Weaknesses: Primarily focused on aesthetic applications, may not address all medical PEMF uses.
Scientific Evidence Supporting PEMF Efficacy
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
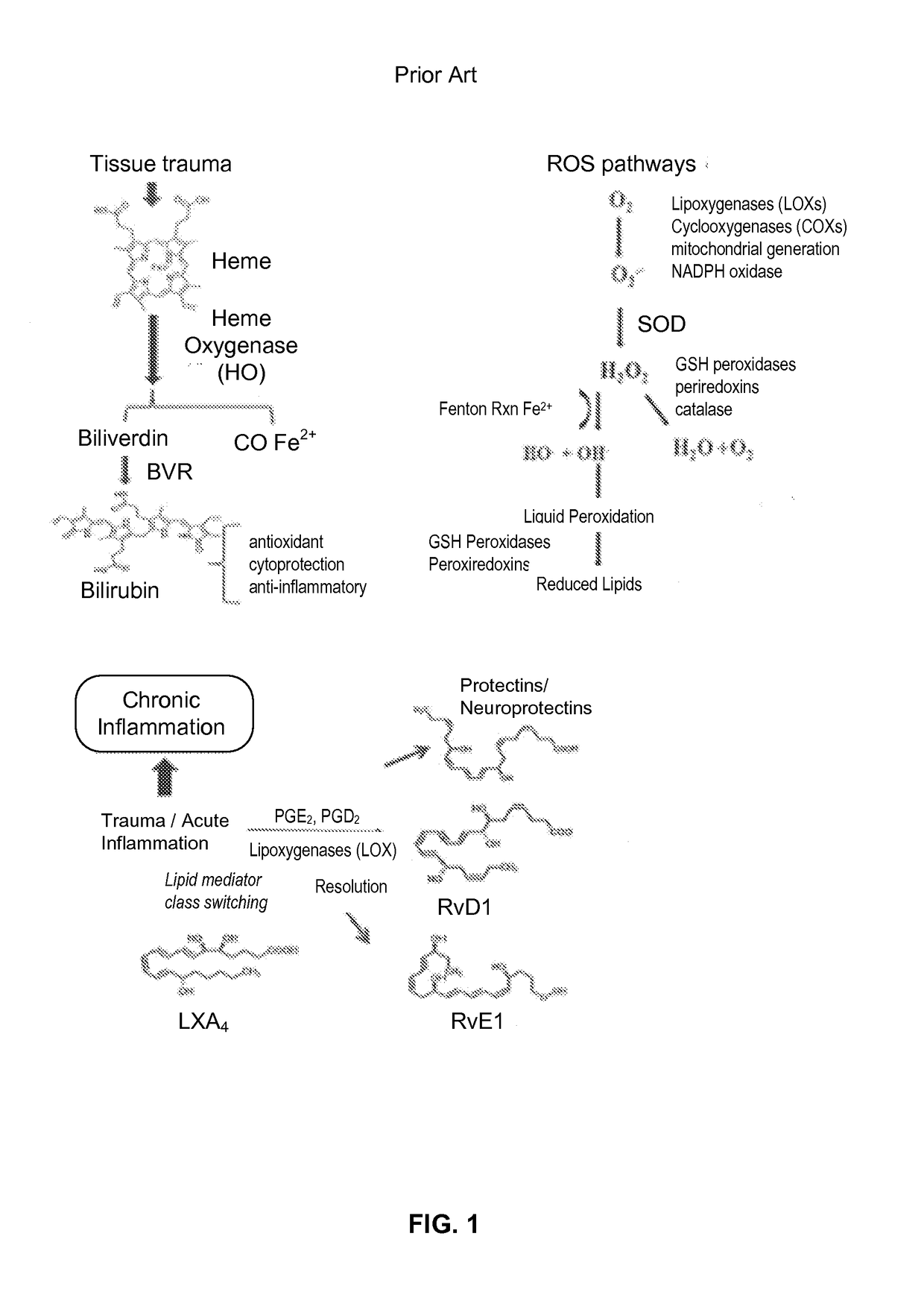
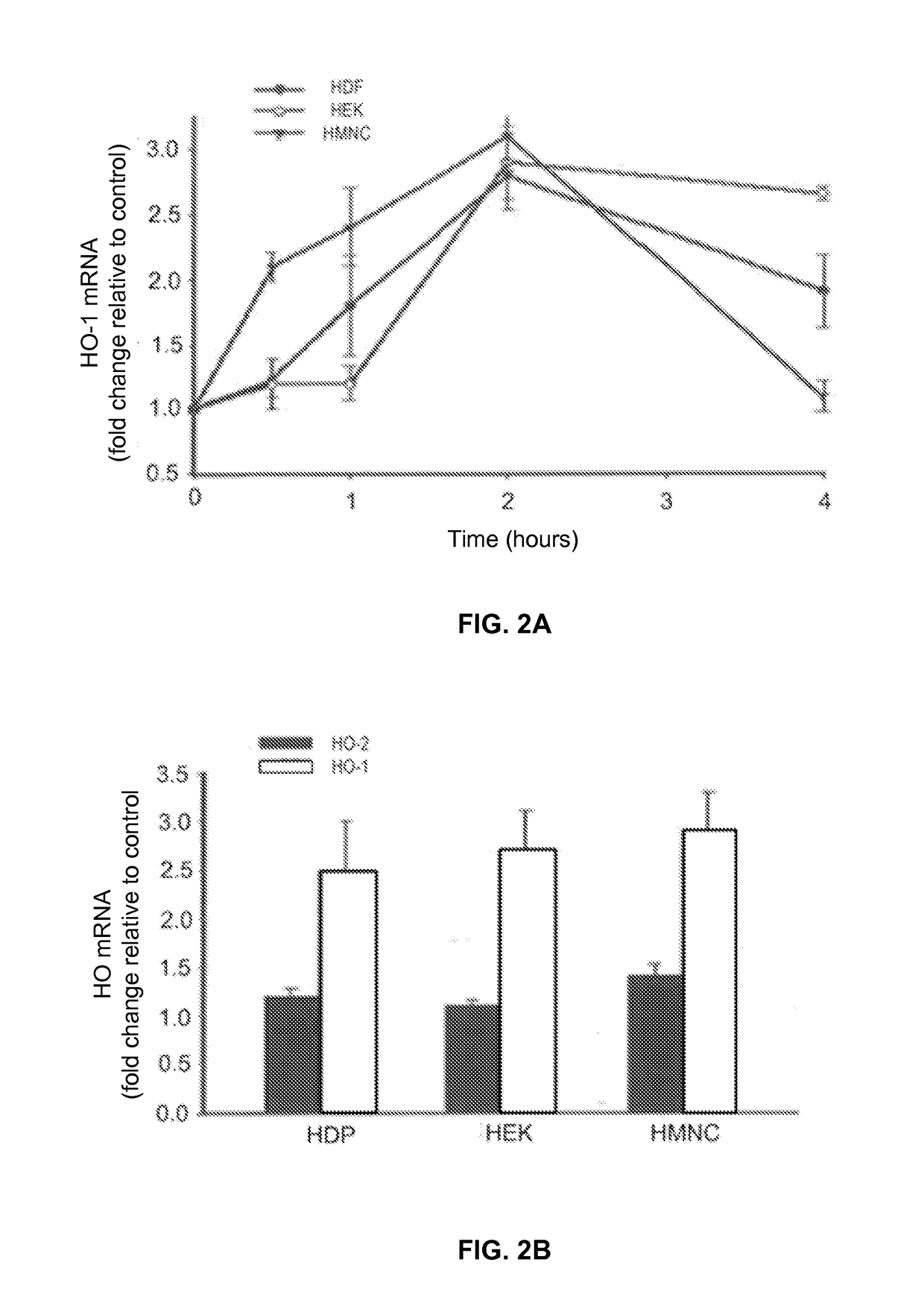
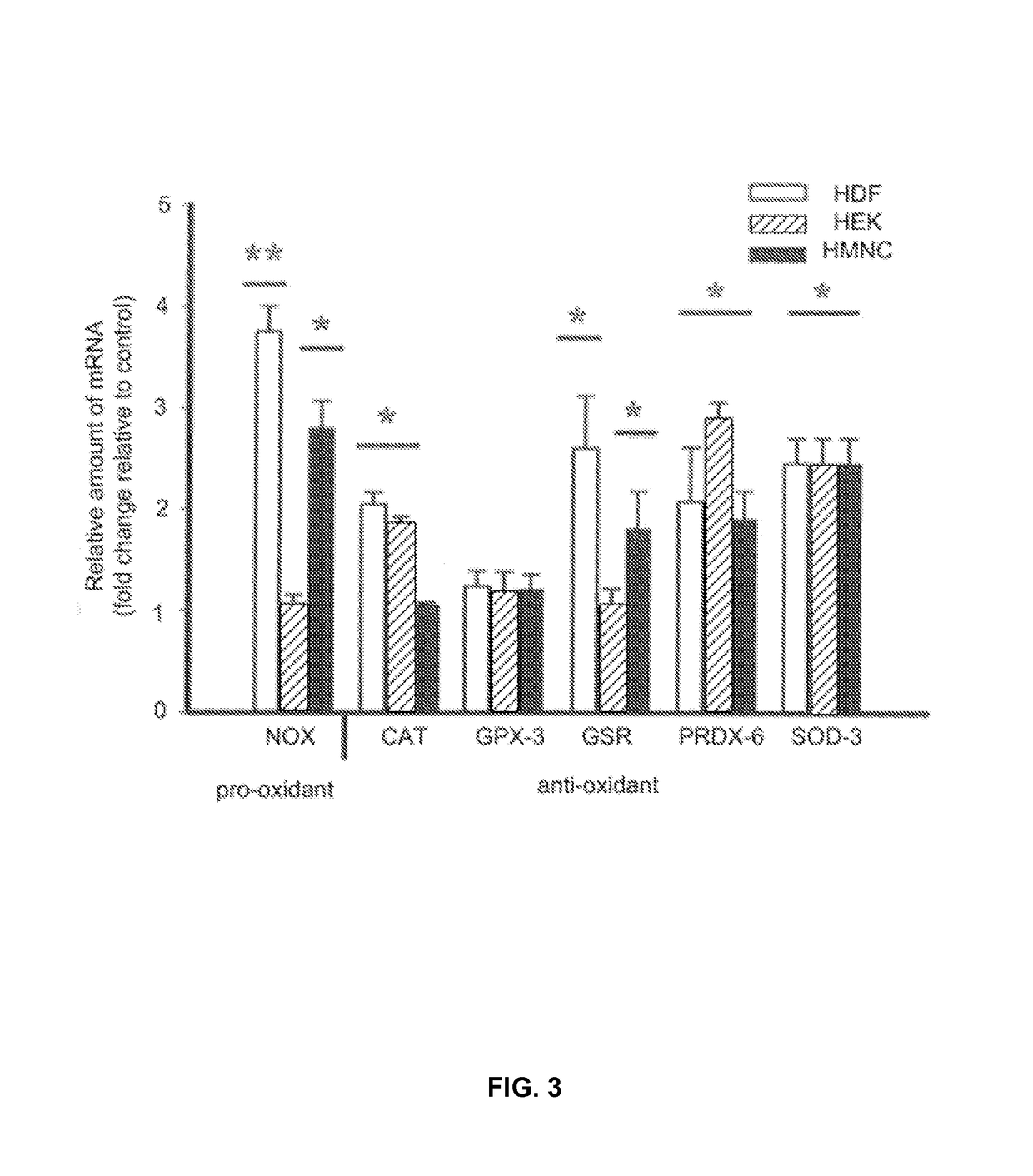
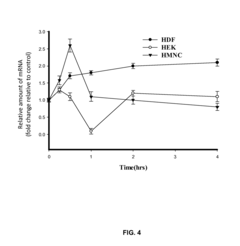
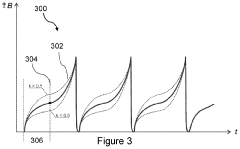
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
A pulsed electromagnetic field apparatus and method for generating frequencies
PatentWO2024127242A1
Innovation
- A PEMF apparatus with a pulse generator and electromagnetic field generation means that uses modified sawtooth waveforms with pre-stress and relaxation periods, and quasi-sine signals with pulse width modulation, along with a feedback circuit for frequency stability and precision, and a bifilar antenna for scalar wave generation.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices plays a crucial role in addressing common misconceptions about PEMF therapy. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device.
The FDA's regulatory process ensures that PEMF devices meet specific safety and effectiveness standards before they can be marketed to consumers. This helps to dispel misconceptions about the safety of PEMF therapy, as devices that have undergone FDA clearance have been evaluated for potential risks and benefits.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements on manufacturers, including enhanced clinical evaluation and post-market surveillance. This regulatory framework helps to address misconceptions about the efficacy of PEMF therapy by requiring manufacturers to provide robust clinical evidence supporting their claims.
Health Canada regulates PEMF devices as Class II medical devices under the Medical Devices Regulations. Similar to the FDA, Health Canada requires manufacturers to obtain a Medical Device License before marketing their products. This regulatory oversight helps to ensure that PEMF devices sold in Canada meet established safety and performance standards.
The regulatory frameworks in these jurisdictions also mandate proper labeling and instructions for use, which can help address misconceptions about the appropriate applications and limitations of PEMF therapy. By requiring clear and accurate product information, regulators aim to prevent misunderstandings and promote informed decision-making among consumers and healthcare professionals.
It's important to note that regulatory requirements may vary depending on the specific claims made about a PEMF device. Devices marketed for general wellness purposes may be subject to different regulations compared to those claiming to treat specific medical conditions. This distinction helps to clarify the intended use of PEMF devices and prevent misconceptions about their therapeutic scope.
Regulatory bodies also monitor advertising and marketing claims related to PEMF devices. By enforcing regulations against false or misleading claims, these agencies help to combat misinformation and address common misconceptions about the capabilities of PEMF therapy. This oversight extends to both traditional advertising channels and digital platforms, including social media and e-commerce websites.
The FDA's regulatory process ensures that PEMF devices meet specific safety and effectiveness standards before they can be marketed to consumers. This helps to dispel misconceptions about the safety of PEMF therapy, as devices that have undergone FDA clearance have been evaluated for potential risks and benefits.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements on manufacturers, including enhanced clinical evaluation and post-market surveillance. This regulatory framework helps to address misconceptions about the efficacy of PEMF therapy by requiring manufacturers to provide robust clinical evidence supporting their claims.
Health Canada regulates PEMF devices as Class II medical devices under the Medical Devices Regulations. Similar to the FDA, Health Canada requires manufacturers to obtain a Medical Device License before marketing their products. This regulatory oversight helps to ensure that PEMF devices sold in Canada meet established safety and performance standards.
The regulatory frameworks in these jurisdictions also mandate proper labeling and instructions for use, which can help address misconceptions about the appropriate applications and limitations of PEMF therapy. By requiring clear and accurate product information, regulators aim to prevent misunderstandings and promote informed decision-making among consumers and healthcare professionals.
It's important to note that regulatory requirements may vary depending on the specific claims made about a PEMF device. Devices marketed for general wellness purposes may be subject to different regulations compared to those claiming to treat specific medical conditions. This distinction helps to clarify the intended use of PEMF devices and prevent misconceptions about their therapeutic scope.
Regulatory bodies also monitor advertising and marketing claims related to PEMF devices. By enforcing regulations against false or misleading claims, these agencies help to combat misinformation and address common misconceptions about the capabilities of PEMF therapy. This oversight extends to both traditional advertising channels and digital platforms, including social media and e-commerce websites.
PEMF Therapy Safety Profile
PEMF (Pulsed Electromagnetic Field) therapy has gained significant attention in recent years as a non-invasive treatment option for various health conditions. However, concerns about its safety profile have led to misconceptions among potential users and healthcare professionals. To address these concerns, it is crucial to examine the extensive body of research and clinical studies that have been conducted on PEMF therapy.
Numerous studies have demonstrated that PEMF therapy, when used as directed, has a favorable safety profile. The therapy utilizes low-frequency electromagnetic fields, typically in the range of 1-100 Hz, which are well below the levels known to cause harmful biological effects. These fields are similar in strength to the Earth's natural magnetic field, making them generally well-tolerated by the human body.
One of the key safety aspects of PEMF therapy is its non-invasive nature. Unlike many pharmaceutical interventions or surgical procedures, PEMF therapy does not require breaking the skin or introducing foreign substances into the body. This significantly reduces the risk of complications such as infections or adverse drug reactions.
Clinical trials have consistently shown that PEMF therapy has minimal side effects when used appropriately. The most commonly reported side effects are mild and transient, including temporary discomfort or tingling sensations during treatment. These effects typically subside quickly after the therapy session ends and are not associated with any long-term negative consequences.
It is important to note that PEMF therapy devices are regulated by health authorities in many countries. For example, in the United States, the Food and Drug Administration (FDA) has cleared several PEMF devices for various medical applications, including bone healing and pain management. This regulatory oversight helps ensure that commercially available PEMF devices meet specific safety and efficacy standards.
While PEMF therapy is generally considered safe for most individuals, there are certain populations for whom caution is advised. Pregnant women, individuals with implanted electronic devices such as pacemakers, and those with active cancer are typically advised to consult with their healthcare providers before undergoing PEMF therapy. These precautions are primarily based on theoretical concerns rather than documented adverse effects.
To further address safety concerns, it is essential to emphasize the importance of using PEMF devices as directed by manufacturers and healthcare professionals. Proper usage, including adherence to recommended treatment durations and frequencies, helps ensure that users receive the intended benefits while minimizing any potential risks.
Numerous studies have demonstrated that PEMF therapy, when used as directed, has a favorable safety profile. The therapy utilizes low-frequency electromagnetic fields, typically in the range of 1-100 Hz, which are well below the levels known to cause harmful biological effects. These fields are similar in strength to the Earth's natural magnetic field, making them generally well-tolerated by the human body.
One of the key safety aspects of PEMF therapy is its non-invasive nature. Unlike many pharmaceutical interventions or surgical procedures, PEMF therapy does not require breaking the skin or introducing foreign substances into the body. This significantly reduces the risk of complications such as infections or adverse drug reactions.
Clinical trials have consistently shown that PEMF therapy has minimal side effects when used appropriately. The most commonly reported side effects are mild and transient, including temporary discomfort or tingling sensations during treatment. These effects typically subside quickly after the therapy session ends and are not associated with any long-term negative consequences.
It is important to note that PEMF therapy devices are regulated by health authorities in many countries. For example, in the United States, the Food and Drug Administration (FDA) has cleared several PEMF devices for various medical applications, including bone healing and pain management. This regulatory oversight helps ensure that commercially available PEMF devices meet specific safety and efficacy standards.
While PEMF therapy is generally considered safe for most individuals, there are certain populations for whom caution is advised. Pregnant women, individuals with implanted electronic devices such as pacemakers, and those with active cancer are typically advised to consult with their healthcare providers before undergoing PEMF therapy. These precautions are primarily based on theoretical concerns rather than documented adverse effects.
To further address safety concerns, it is essential to emphasize the importance of using PEMF devices as directed by manufacturers and healthcare professionals. Proper usage, including adherence to recommended treatment durations and frequencies, helps ensure that users receive the intended benefits while minimizing any potential risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!