How to Enhance Montmorillonite's Surface Area for Catalysis
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Montmorillonite Catalysis Background and Objectives
Montmorillonite, a naturally occurring clay mineral belonging to the smectite group, has emerged as a significant material in the field of heterogeneous catalysis over the past several decades. The evolution of montmorillonite as a catalyst support and catalyst has been marked by continuous innovation, driven by its unique layered structure, ion exchange capacity, and surface properties. Initially utilized in simple acid-catalyzed reactions, montmorillonite has progressively found applications in more complex catalytic processes including organic transformations, polymerization reactions, and environmental remediation.
The technological trajectory of montmorillonite in catalysis has been characterized by a shift from using natural, minimally processed clay to highly engineered forms with enhanced surface properties. This evolution reflects broader trends in materials science and catalysis, where increasing control over material structure at the nanoscale has enabled significant performance improvements. Recent advances in characterization techniques have further accelerated our understanding of montmorillonite's surface chemistry and catalytic mechanisms.
Current research focuses on maximizing the effective surface area of montmorillonite to enhance its catalytic performance. This objective is particularly crucial as surface area directly correlates with the number of accessible active sites, thereby influencing reaction rates, selectivity, and catalyst lifetime. Traditional montmorillonite typically exhibits surface areas ranging from 30-80 m²/g, which is modest compared to other catalytic materials such as zeolites or metal-organic frameworks.
The primary technical goal of this research is to develop systematic approaches to significantly increase montmorillonite's surface area beyond 200 m²/g while maintaining its structural integrity and beneficial properties. This enhancement would position montmorillonite as a more competitive option in industrial catalysis applications where high surface area materials are preferred. Secondary objectives include improving the thermal and mechanical stability of modified montmorillonite and developing scalable, environmentally friendly modification processes.
Achieving these objectives would address several limitations currently facing montmorillonite catalysts, including diffusion constraints, limited active site accessibility, and relatively low catalytic activity per unit mass. The enhanced surface area would particularly benefit reactions involving bulky molecules or those requiring high catalyst loading, potentially opening new application domains for montmorillonite-based catalysts in fine chemical synthesis, pharmaceutical manufacturing, and green chemistry processes.
The technological advancement in this field aligns with broader industry trends toward more efficient, sustainable catalytic processes that minimize waste and energy consumption. By improving the fundamental properties of an abundant, naturally occurring material like montmorillonite, this research aims to contribute to the development of next-generation catalysts that balance performance, cost, and environmental considerations.
The technological trajectory of montmorillonite in catalysis has been characterized by a shift from using natural, minimally processed clay to highly engineered forms with enhanced surface properties. This evolution reflects broader trends in materials science and catalysis, where increasing control over material structure at the nanoscale has enabled significant performance improvements. Recent advances in characterization techniques have further accelerated our understanding of montmorillonite's surface chemistry and catalytic mechanisms.
Current research focuses on maximizing the effective surface area of montmorillonite to enhance its catalytic performance. This objective is particularly crucial as surface area directly correlates with the number of accessible active sites, thereby influencing reaction rates, selectivity, and catalyst lifetime. Traditional montmorillonite typically exhibits surface areas ranging from 30-80 m²/g, which is modest compared to other catalytic materials such as zeolites or metal-organic frameworks.
The primary technical goal of this research is to develop systematic approaches to significantly increase montmorillonite's surface area beyond 200 m²/g while maintaining its structural integrity and beneficial properties. This enhancement would position montmorillonite as a more competitive option in industrial catalysis applications where high surface area materials are preferred. Secondary objectives include improving the thermal and mechanical stability of modified montmorillonite and developing scalable, environmentally friendly modification processes.
Achieving these objectives would address several limitations currently facing montmorillonite catalysts, including diffusion constraints, limited active site accessibility, and relatively low catalytic activity per unit mass. The enhanced surface area would particularly benefit reactions involving bulky molecules or those requiring high catalyst loading, potentially opening new application domains for montmorillonite-based catalysts in fine chemical synthesis, pharmaceutical manufacturing, and green chemistry processes.
The technological advancement in this field aligns with broader industry trends toward more efficient, sustainable catalytic processes that minimize waste and energy consumption. By improving the fundamental properties of an abundant, naturally occurring material like montmorillonite, this research aims to contribute to the development of next-generation catalysts that balance performance, cost, and environmental considerations.
Market Analysis for High Surface Area Clay Catalysts
The global market for high surface area clay catalysts, particularly those based on montmorillonite, has experienced significant growth driven by increasing demand across multiple industrial sectors. The catalyst market was valued at approximately $33.9 billion in 2022 and is projected to reach $47.2 billion by 2030, growing at a CAGR of 4.2%. Within this broader market, clay-based catalysts represent a specialized segment with distinctive growth characteristics.
The petroleum refining industry remains the largest consumer of enhanced montmorillonite catalysts, accounting for roughly 40% of market demand. These catalysts are crucial in fluid catalytic cracking (FCC) processes, hydrocracking, and isomerization reactions. The push toward processing heavier crude oils and meeting stricter environmental regulations has intensified the need for more efficient clay catalysts with optimized surface areas.
Environmental applications constitute the fastest-growing segment, expanding at approximately 6.8% annually. This growth is primarily driven by increasing regulatory pressure to reduce industrial emissions and treat wastewater effectively. High surface area montmorillonite catalysts have demonstrated exceptional performance in removing heavy metals, organic pollutants, and pharmaceutical residues from water systems.
The fine chemicals and pharmaceutical sectors collectively represent about 25% of the market share. These industries value montmorillonite-based catalysts for their selectivity in organic synthesis reactions, particularly in asymmetric catalysis and green chemistry applications. The ability to fine-tune surface properties makes these materials increasingly attractive for high-value chemical production.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for 38% of global consumption, followed by North America (27%) and Europe (24%). China and India are experiencing the most rapid growth rates due to expanding industrial bases and increasing environmental regulations. The Middle East is emerging as a significant market due to its petrochemical industry's continued development.
Key market drivers include the global shift toward sustainable manufacturing processes, increasing research in nanocatalysis, and growing demand for specialty chemicals. The trend toward catalyst recovery and recycling is also influencing market dynamics, with enhanced montmorillonite catalysts offering advantages in regeneration capabilities.
Market challenges include competition from synthetic zeolites and metal-organic frameworks (MOFs), which can offer more precise pore size distribution. Additionally, the variability in natural clay compositions presents standardization challenges for industrial applications requiring consistent performance metrics.
The petroleum refining industry remains the largest consumer of enhanced montmorillonite catalysts, accounting for roughly 40% of market demand. These catalysts are crucial in fluid catalytic cracking (FCC) processes, hydrocracking, and isomerization reactions. The push toward processing heavier crude oils and meeting stricter environmental regulations has intensified the need for more efficient clay catalysts with optimized surface areas.
Environmental applications constitute the fastest-growing segment, expanding at approximately 6.8% annually. This growth is primarily driven by increasing regulatory pressure to reduce industrial emissions and treat wastewater effectively. High surface area montmorillonite catalysts have demonstrated exceptional performance in removing heavy metals, organic pollutants, and pharmaceutical residues from water systems.
The fine chemicals and pharmaceutical sectors collectively represent about 25% of the market share. These industries value montmorillonite-based catalysts for their selectivity in organic synthesis reactions, particularly in asymmetric catalysis and green chemistry applications. The ability to fine-tune surface properties makes these materials increasingly attractive for high-value chemical production.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for 38% of global consumption, followed by North America (27%) and Europe (24%). China and India are experiencing the most rapid growth rates due to expanding industrial bases and increasing environmental regulations. The Middle East is emerging as a significant market due to its petrochemical industry's continued development.
Key market drivers include the global shift toward sustainable manufacturing processes, increasing research in nanocatalysis, and growing demand for specialty chemicals. The trend toward catalyst recovery and recycling is also influencing market dynamics, with enhanced montmorillonite catalysts offering advantages in regeneration capabilities.
Market challenges include competition from synthetic zeolites and metal-organic frameworks (MOFs), which can offer more precise pore size distribution. Additionally, the variability in natural clay compositions presents standardization challenges for industrial applications requiring consistent performance metrics.
Current Limitations in Montmorillonite Surface Modification
Despite montmorillonite's promising catalytic properties, several significant limitations hinder its effective surface modification for enhanced catalysis applications. The primary challenge lies in its layered structure, where the interlayer spaces are often occupied by exchangeable cations and water molecules, restricting access to potential reactive sites. This structural constraint limits the effective surface area available for catalytic reactions, particularly when dealing with larger substrate molecules.
Traditional modification methods such as acid activation and ion exchange often provide only modest improvements in surface area, typically increasing from 30-80 m²/g to 150-300 m²/g. These approaches frequently result in partial collapse of the clay structure, leading to inconsistent catalytic performance and reduced stability during repeated catalytic cycles.
Another significant limitation is the hydrophilic nature of natural montmorillonite, which causes preferential adsorption of water molecules over organic reactants. This characteristic severely impedes catalytic efficiency in non-aqueous reaction systems, which constitute a substantial portion of industrial catalytic processes. Current hydrophobization techniques using quaternary ammonium compounds often block catalytic sites while attempting to modify surface properties.
The heterogeneity of natural montmorillonite deposits presents additional challenges, as variations in composition and impurities lead to inconsistent modification results. Commercial montmorillonite samples can contain 5-15% impurities including quartz, feldspar, and carbonates, which interfere with uniform surface modification and catalytic performance.
Temperature stability represents another critical limitation. While montmorillonite maintains structural integrity up to approximately 500°C, many surface modification agents decompose at much lower temperatures (200-300°C), restricting applications in high-temperature catalytic processes. This thermal constraint significantly narrows the range of potential industrial applications.
Current pillaring techniques using metal oxide clusters, while effective at increasing interlayer spacing, often result in reduced accessibility to acid sites and create diffusion limitations for reactant molecules. Research indicates that only 40-60% of theoretical acid sites remain accessible after conventional pillaring procedures.
Finally, scaling up laboratory modification protocols to industrial levels presents substantial challenges in maintaining consistent surface properties and catalytic performance. Batch-to-batch variations in modified montmorillonite catalysts can exceed 15-20% in surface area and 25-30% in catalytic activity, making quality control and process standardization particularly difficult for commercial applications.
Traditional modification methods such as acid activation and ion exchange often provide only modest improvements in surface area, typically increasing from 30-80 m²/g to 150-300 m²/g. These approaches frequently result in partial collapse of the clay structure, leading to inconsistent catalytic performance and reduced stability during repeated catalytic cycles.
Another significant limitation is the hydrophilic nature of natural montmorillonite, which causes preferential adsorption of water molecules over organic reactants. This characteristic severely impedes catalytic efficiency in non-aqueous reaction systems, which constitute a substantial portion of industrial catalytic processes. Current hydrophobization techniques using quaternary ammonium compounds often block catalytic sites while attempting to modify surface properties.
The heterogeneity of natural montmorillonite deposits presents additional challenges, as variations in composition and impurities lead to inconsistent modification results. Commercial montmorillonite samples can contain 5-15% impurities including quartz, feldspar, and carbonates, which interfere with uniform surface modification and catalytic performance.
Temperature stability represents another critical limitation. While montmorillonite maintains structural integrity up to approximately 500°C, many surface modification agents decompose at much lower temperatures (200-300°C), restricting applications in high-temperature catalytic processes. This thermal constraint significantly narrows the range of potential industrial applications.
Current pillaring techniques using metal oxide clusters, while effective at increasing interlayer spacing, often result in reduced accessibility to acid sites and create diffusion limitations for reactant molecules. Research indicates that only 40-60% of theoretical acid sites remain accessible after conventional pillaring procedures.
Finally, scaling up laboratory modification protocols to industrial levels presents substantial challenges in maintaining consistent surface properties and catalytic performance. Batch-to-batch variations in modified montmorillonite catalysts can exceed 15-20% in surface area and 25-30% in catalytic activity, making quality control and process standardization particularly difficult for commercial applications.
Current Methods for Montmorillonite Surface Area Enhancement
01 Measurement and characterization of montmorillonite surface area
Various techniques are used to measure and characterize the surface area of montmorillonite clay, including BET (Brunauer-Emmett-Teller) method, nitrogen adsorption, and other gas adsorption techniques. These methods help determine the specific surface area, which is typically in the range of 600-800 m²/g for natural montmorillonite. The high surface area is attributed to the layered structure of montmorillonite, which provides both external and internal surfaces for adsorption.- Measurement and characterization of montmorillonite surface area: Various techniques are used to measure and characterize the surface area of montmorillonite clay, including BET (Brunauer-Emmett-Teller) method, nitrogen adsorption, and other analytical approaches. The specific surface area of montmorillonite typically ranges from 600-800 m²/g, which contributes to its high adsorption capacity and ion exchange properties. These measurements are crucial for understanding the clay's performance in various applications.
- Surface modification of montmorillonite to enhance properties: Surface modification techniques are applied to montmorillonite to alter its surface area and enhance specific properties. These modifications include organic treatment with surfactants, acid activation, pillaring with metal oxides, and thermal treatments. Modified montmorillonite often exhibits increased basal spacing, improved dispersion in polymers, and enhanced adsorption capabilities, making it more effective in applications such as nanocomposites, wastewater treatment, and catalysis.
- Montmorillonite in polymer nanocomposites: Montmorillonite's high surface area makes it an excellent nanofiller in polymer composites. When properly exfoliated, the clay platelets with their large aspect ratio and surface area create extensive interfacial regions with the polymer matrix, resulting in improved mechanical properties, thermal stability, barrier properties, and flame retardancy. The surface area characteristics of montmorillonite directly influence the degree of exfoliation and the resulting performance enhancements in the nanocomposite materials.
- Montmorillonite as adsorbent for environmental applications: The high specific surface area of montmorillonite makes it an effective adsorbent for environmental remediation applications. It can adsorb heavy metals, organic pollutants, dyes, and other contaminants from water and soil. The adsorption capacity is directly related to the available surface area, which can be further enhanced through various modification techniques. Montmorillonite-based adsorbents are used in wastewater treatment, soil remediation, and as barriers in landfills to prevent contaminant migration.
- Montmorillonite in pharmaceutical and cosmetic applications: The large surface area of montmorillonite is beneficial in pharmaceutical and cosmetic formulations. It serves as an effective carrier for drug delivery systems, providing controlled release of active ingredients. In cosmetics, montmorillonite acts as a rheological modifier, stabilizer, and absorbent for oils and impurities. The surface area characteristics influence its ability to interact with active ingredients, improve product stability, and enhance the efficacy of formulations in both pharmaceutical and cosmetic applications.
02 Modification techniques to enhance montmorillonite surface area
Various modification techniques can be employed to enhance the surface area of montmorillonite, including acid activation, pillaring with metal oxides, organic modification, and thermal treatment. These modifications can increase the basal spacing between the clay layers, create mesopores, and expose more active sites, resulting in significantly increased surface area. Modified montmorillonites with enhanced surface areas show improved performance in various applications such as adsorption, catalysis, and as polymer fillers.Expand Specific Solutions03 Applications utilizing montmorillonite's high surface area
The high surface area of montmorillonite makes it valuable for numerous applications including environmental remediation (adsorption of pollutants and heavy metals), catalysis, drug delivery systems, and as reinforcing fillers in polymer nanocomposites. The large surface area provides abundant active sites for adsorption, ion exchange, and chemical reactions, making montmorillonite an effective material for removing contaminants from water and gases, supporting catalytic reactions, and improving mechanical properties of composite materials.Expand Specific Solutions04 Relationship between surface area and adsorption capacity
The surface area of montmorillonite directly influences its adsorption capacity for various substances including organic compounds, heavy metals, and gases. Higher surface area generally correlates with increased adsorption capacity due to more available binding sites. Factors affecting this relationship include the nature of the adsorbate, pH conditions, temperature, and the presence of competing ions. Understanding this relationship is crucial for optimizing montmorillonite-based adsorbents for specific applications in water treatment, gas purification, and other separation processes.Expand Specific Solutions05 Nanocomposites utilizing montmorillonite's surface properties
Montmorillonite's high surface area makes it an excellent nanofiller for polymer nanocomposites. When properly exfoliated or intercalated within polymer matrices, montmorillonite nanolayers with high aspect ratios and surface areas can significantly enhance mechanical, thermal, barrier, and flame-retardant properties of the resulting materials. The interface between the clay surface and polymer matrix plays a crucial role in determining the final properties of these nanocomposites, which find applications in packaging, automotive parts, construction materials, and flame-retardant coatings.Expand Specific Solutions
Leading Research Groups and Companies in Clay Catalysis
The montmorillonite surface area enhancement for catalysis market is currently in a growth phase, with increasing applications across petrochemical, environmental, and fine chemical sectors. The global market size is expanding due to rising demand for efficient catalysts in industrial processes. Technologically, this field is moderately mature but experiencing continuous innovation. Leading players include BASF Corp. and W.R. Grace & Co., who leverage their extensive chemical expertise; Toyota Motor Corp. and Cataler Corp., focusing on automotive catalyst applications; while research institutions like China University of Geosciences and Council of Scientific & Industrial Research drive fundamental advancements. Chinese companies such as PetroChina and Sinopec are increasingly investing in this technology, particularly for petroleum refining applications.
BASF Corp.
Technical Solution: BASF has developed a proprietary acid activation process for montmorillonite enhancement that significantly increases surface area from typical 30-70 m²/g to over 300 m²/g. Their approach involves controlled acid treatment with H2SO4 or HCl at precise concentrations (0.5-2.0M) and temperatures (70-95°C), followed by washing and calcination steps. This process selectively dissolves octahedral sheets while preserving the tetrahedral structure, creating mesopores (2-50nm) that enhance accessibility to active sites. BASF has further innovated by incorporating pillaring techniques using polyoxocations of Al, Zr, or Ti between clay layers, which prevents layer collapse during thermal treatments and creates permanent microporous networks with controlled pore dimensions. Their catalysts demonstrate exceptional thermal stability up to 500°C and controlled acidity profiles suitable for various petrochemical applications.
Strengths: Superior control over pore size distribution and acidity, excellent thermal stability, and scalable industrial production capabilities. Their acid-activated montmorillonites show 3-5 times higher catalytic activity in alkylation and isomerization reactions compared to conventional catalysts. Weakness: Higher production costs due to multi-step processing and acid handling requirements, potential environmental concerns from acid waste streams.
China University of Geosciences
Technical Solution: China University of Geosciences has pioneered an innovative "exfoliation-reassembly" approach to dramatically enhance montmorillonite's surface area for catalytic applications. Their method involves complete delamination of the clay structure into individual nanosheets followed by controlled reassembly into a house-of-cards structure with significantly enhanced surface area (350-450 m²/g). The process begins with intercalation using large organic cations (typically quaternary ammonium compounds with long alkyl chains), followed by rapid thermal shock treatment that causes explosive delamination. These dispersed nanosheets are then reassembled under carefully controlled pH and ionic strength conditions to create a three-dimensional network with abundant mesopores (10-30 nm) and exposed edge sites. The researchers have further enhanced this approach by incorporating functional nanoparticles (TiO2, CeO2, ZnO) during the reassembly process, creating multifunctional catalytic systems. Their materials show exceptional performance in photocatalytic applications, including water purification and selective organic transformations, with reaction rates 3-5 times higher than conventional supported catalysts due to the dramatically increased accessibility of active sites.
Strengths: Extraordinarily high surface area compared to conventional acid-activated or pillared clays; highly accessible active sites without diffusion limitations; versatile platform for incorporating multiple functional components. Weaknesses: Complex synthesis procedure with critical dependence on reassembly conditions; potential for structural collapse under high-temperature catalytic conditions; scalability challenges for industrial production.
Key Patents and Innovations in Clay Mineral Modification
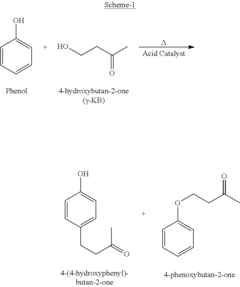
Process for the preparation of 4-(4-hydroxyphenyl)but any- one using solid acid clay catalyst
PatentWO2009118755A2
Innovation
- The development of a process using acid-activated Montmorillonite clay as a solid acid catalyst for the Friedel-Crafts alkylation of phenol with 4-hydroxybutan-2-one, which achieves high selectivity and conversion by optimizing catalyst preparation and reaction conditions.
Process for the Preparation of 4-(4-hydroxyphenyl)butan-2-one Using Solid Acid Clay Catalyst
PatentInactiveUS20110257439A1
Innovation
- The development of an acid-activated Montmorillonite clay catalyst with specific structural and porosity characteristics is used for the Friedel-Crafts alkylation of phenol with 4-hydroxybutan-2-one, enhancing selectivity and conversion efficiency while employing an eco-friendly and recyclable catalyst.
Environmental Impact and Sustainability Considerations
The enhancement of montmorillonite's surface area for catalysis applications must be evaluated not only for its technical efficacy but also for its environmental implications. The extraction and processing of montmorillonite clay involves mining operations that can lead to habitat disruption, soil erosion, and landscape alteration. These environmental impacts necessitate responsible mining practices, including site rehabilitation and minimization of ecological footprints.
Water usage represents another significant environmental concern in montmorillonite processing. Traditional modification methods often require substantial amounts of water for washing and purification steps. Implementing closed-loop water systems and developing water-efficient modification techniques can substantially reduce the environmental burden associated with montmorillonite enhancement processes.
Chemical treatments employed to increase montmorillonite's surface area frequently involve acids, bases, or organic compounds that may pose environmental risks if improperly managed. The development of greener modification approaches using bio-based reagents, ionic liquids, or mechanochemical methods offers promising alternatives that reduce reliance on hazardous chemicals while maintaining or improving catalytic performance.
Energy consumption during thermal activation processes represents another environmental challenge. Conventional calcination methods require high temperatures, resulting in significant carbon emissions. Microwave-assisted or sonochemical modification techniques present more energy-efficient alternatives that can reduce the carbon footprint of montmorillonite enhancement while potentially improving surface characteristics.
The sustainability profile of enhanced montmorillonite catalysts extends beyond production to include their operational lifetime and end-of-life considerations. Developing montmorillonite catalysts with improved regeneration capabilities and resistance to deactivation can extend their useful life, thereby reducing waste generation and resource consumption. Additionally, exploring pathways for recycling spent montmorillonite catalysts or repurposing them for secondary applications contributes to circular economy principles.
Life cycle assessment (LCA) methodologies should be applied to comprehensively evaluate the environmental impacts of different montmorillonite enhancement strategies. Such analyses enable informed decision-making by quantifying environmental trade-offs between increased catalytic performance and potential environmental burdens across the entire value chain, from raw material extraction to catalyst disposal or recycling.
Water usage represents another significant environmental concern in montmorillonite processing. Traditional modification methods often require substantial amounts of water for washing and purification steps. Implementing closed-loop water systems and developing water-efficient modification techniques can substantially reduce the environmental burden associated with montmorillonite enhancement processes.
Chemical treatments employed to increase montmorillonite's surface area frequently involve acids, bases, or organic compounds that may pose environmental risks if improperly managed. The development of greener modification approaches using bio-based reagents, ionic liquids, or mechanochemical methods offers promising alternatives that reduce reliance on hazardous chemicals while maintaining or improving catalytic performance.
Energy consumption during thermal activation processes represents another environmental challenge. Conventional calcination methods require high temperatures, resulting in significant carbon emissions. Microwave-assisted or sonochemical modification techniques present more energy-efficient alternatives that can reduce the carbon footprint of montmorillonite enhancement while potentially improving surface characteristics.
The sustainability profile of enhanced montmorillonite catalysts extends beyond production to include their operational lifetime and end-of-life considerations. Developing montmorillonite catalysts with improved regeneration capabilities and resistance to deactivation can extend their useful life, thereby reducing waste generation and resource consumption. Additionally, exploring pathways for recycling spent montmorillonite catalysts or repurposing them for secondary applications contributes to circular economy principles.
Life cycle assessment (LCA) methodologies should be applied to comprehensively evaluate the environmental impacts of different montmorillonite enhancement strategies. Such analyses enable informed decision-making by quantifying environmental trade-offs between increased catalytic performance and potential environmental burdens across the entire value chain, from raw material extraction to catalyst disposal or recycling.
Scale-up Challenges and Industrial Implementation
Scaling up montmorillonite surface area enhancement processes from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. The transition requires careful consideration of equipment design, process parameters, and economic factors. Laboratory methods that successfully enhance surface area often utilize small quantities under highly controlled conditions, which cannot be directly replicated at industrial scale.
One primary challenge is maintaining uniform treatment conditions across large batches. Acid activation, thermal treatment, and pillaring processes all require precise temperature control, mixing efficiency, and reaction time management. As batch sizes increase, heat and mass transfer limitations can lead to inconsistent surface area enhancement and variable catalytic performance across the processed material.
Equipment design represents another critical hurdle. Industrial-scale acid treatment vessels must withstand corrosive conditions while providing adequate mixing. Thermal treatment furnaces need to ensure even temperature distribution throughout large clay volumes. Pillaring processes require specialized equipment for intercalation and subsequent processing steps. The capital investment for such specialized equipment can be substantial, necessitating thorough economic analysis.
Process reproducibility and quality control become increasingly complex at industrial scale. Montmorillonite's natural variability compounds this challenge, as feedstock from different sources or even different mining locations within the same deposit can exhibit varying chemical compositions and physical properties. Developing robust quality control protocols and feedstock pre-screening methods is essential for consistent catalytic performance.
Waste management presents additional complications, particularly for acid activation processes. The large volumes of acidic waste require neutralization and proper disposal, adding operational costs and environmental considerations. Water consumption for washing steps can also be substantial, necessitating water recycling systems for sustainable operation.
Energy efficiency represents a significant economic factor in scale-up efforts. Thermal treatments and drying processes consume considerable energy, directly impacting production costs. Implementing heat recovery systems and optimizing thermal processes can improve economic viability while reducing environmental impact.
Despite these challenges, several companies have successfully implemented industrial-scale montmorillonite modification processes, demonstrating commercial viability. These implementations typically involve continuous or semi-continuous processing rather than batch operations, with specialized monitoring systems to ensure consistent quality. Successful scale-up often requires iterative process development with pilot-scale testing serving as a critical intermediate step between laboratory research and full industrial implementation.
One primary challenge is maintaining uniform treatment conditions across large batches. Acid activation, thermal treatment, and pillaring processes all require precise temperature control, mixing efficiency, and reaction time management. As batch sizes increase, heat and mass transfer limitations can lead to inconsistent surface area enhancement and variable catalytic performance across the processed material.
Equipment design represents another critical hurdle. Industrial-scale acid treatment vessels must withstand corrosive conditions while providing adequate mixing. Thermal treatment furnaces need to ensure even temperature distribution throughout large clay volumes. Pillaring processes require specialized equipment for intercalation and subsequent processing steps. The capital investment for such specialized equipment can be substantial, necessitating thorough economic analysis.
Process reproducibility and quality control become increasingly complex at industrial scale. Montmorillonite's natural variability compounds this challenge, as feedstock from different sources or even different mining locations within the same deposit can exhibit varying chemical compositions and physical properties. Developing robust quality control protocols and feedstock pre-screening methods is essential for consistent catalytic performance.
Waste management presents additional complications, particularly for acid activation processes. The large volumes of acidic waste require neutralization and proper disposal, adding operational costs and environmental considerations. Water consumption for washing steps can also be substantial, necessitating water recycling systems for sustainable operation.
Energy efficiency represents a significant economic factor in scale-up efforts. Thermal treatments and drying processes consume considerable energy, directly impacting production costs. Implementing heat recovery systems and optimizing thermal processes can improve economic viability while reducing environmental impact.
Despite these challenges, several companies have successfully implemented industrial-scale montmorillonite modification processes, demonstrating commercial viability. These implementations typically involve continuous or semi-continuous processing rather than batch operations, with specialized monitoring systems to ensure consistent quality. Successful scale-up often requires iterative process development with pilot-scale testing serving as a critical intermediate step between laboratory research and full industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!