How to Optimize Quantum Models for Effective Drug Targeting
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Computing in Drug Discovery: Background and Objectives
Quantum computing represents a paradigm shift in computational capabilities, leveraging quantum mechanical phenomena such as superposition and entanglement to process information in ways fundamentally different from classical computing. Since its theoretical conception in the early 1980s, quantum computing has evolved from abstract mathematical models to increasingly practical implementations, with significant milestones achieved in the last decade. The integration of quantum computing into drug discovery processes marks a natural progression in the field's application landscape.
The pharmaceutical industry faces mounting challenges in traditional drug discovery approaches, including escalating costs (estimated at $2.6 billion per new drug), extended development timelines (10-15 years average), and high failure rates (approximately 90%). These challenges create a compelling case for innovative computational methods that can accelerate and enhance the drug discovery pipeline.
Quantum computing offers transformative potential in addressing these challenges through its unique ability to model molecular interactions at the quantum level. Classical computational methods often struggle with accurately simulating complex biological systems due to the exponential scaling of computational resources required. Quantum algorithms, however, can potentially model these quantum mechanical interactions with greater fidelity and efficiency.
The primary technical objective in optimizing quantum models for drug targeting involves developing algorithms that can effectively simulate molecular interactions between potential drug compounds and biological targets. This includes accurate modeling of electronic structures, protein folding dynamics, and binding affinities—all critical aspects of drug efficacy and specificity.
Current quantum hardware remains limited by noise, decoherence, and qubit count constraints. Therefore, a key technical goal involves developing quantum algorithms that can operate effectively within these limitations while still providing meaningful advantages over classical methods. This includes the development of hybrid quantum-classical approaches that strategically leverage the strengths of both computational paradigms.
Looking forward, the field aims to achieve quantum advantage in specific drug discovery applications, where quantum computers can demonstrably outperform classical supercomputers in tasks relevant to pharmaceutical research. This includes more accurate prediction of molecular properties, faster virtual screening of compound libraries, and improved optimization of lead compounds.
The convergence of quantum computing with other emerging technologies—such as artificial intelligence, high-throughput screening, and advanced imaging techniques—presents opportunities for synergistic approaches to drug discovery. The ultimate technical vision involves creating an integrated computational platform where quantum models serve as a cornerstone for next-generation pharmaceutical research and development.
The pharmaceutical industry faces mounting challenges in traditional drug discovery approaches, including escalating costs (estimated at $2.6 billion per new drug), extended development timelines (10-15 years average), and high failure rates (approximately 90%). These challenges create a compelling case for innovative computational methods that can accelerate and enhance the drug discovery pipeline.
Quantum computing offers transformative potential in addressing these challenges through its unique ability to model molecular interactions at the quantum level. Classical computational methods often struggle with accurately simulating complex biological systems due to the exponential scaling of computational resources required. Quantum algorithms, however, can potentially model these quantum mechanical interactions with greater fidelity and efficiency.
The primary technical objective in optimizing quantum models for drug targeting involves developing algorithms that can effectively simulate molecular interactions between potential drug compounds and biological targets. This includes accurate modeling of electronic structures, protein folding dynamics, and binding affinities—all critical aspects of drug efficacy and specificity.
Current quantum hardware remains limited by noise, decoherence, and qubit count constraints. Therefore, a key technical goal involves developing quantum algorithms that can operate effectively within these limitations while still providing meaningful advantages over classical methods. This includes the development of hybrid quantum-classical approaches that strategically leverage the strengths of both computational paradigms.
Looking forward, the field aims to achieve quantum advantage in specific drug discovery applications, where quantum computers can demonstrably outperform classical supercomputers in tasks relevant to pharmaceutical research. This includes more accurate prediction of molecular properties, faster virtual screening of compound libraries, and improved optimization of lead compounds.
The convergence of quantum computing with other emerging technologies—such as artificial intelligence, high-throughput screening, and advanced imaging techniques—presents opportunities for synergistic approaches to drug discovery. The ultimate technical vision involves creating an integrated computational platform where quantum models serve as a cornerstone for next-generation pharmaceutical research and development.
Market Analysis for Quantum-Enabled Pharmaceutical Solutions
The quantum computing pharmaceutical market is experiencing unprecedented growth, driven by the increasing complexity of drug discovery processes and the limitations of classical computational methods. Current market valuations indicate that quantum-enabled pharmaceutical solutions could reach a market size of $15 billion by 2030, with a compound annual growth rate of approximately 24% from 2023 to 2030. This growth trajectory is supported by significant investments from both pharmaceutical giants and technology companies seeking competitive advantages in drug development.
Market demand for quantum-enabled drug targeting solutions stems primarily from three key factors. First, the escalating costs of traditional drug development, which currently averages $2.6 billion per approved drug with a timeline of 10-15 years. Quantum computing promises to reduce these costs by up to 30% through more efficient molecular modeling and binding affinity predictions. Second, the increasing complexity of target diseases, particularly in oncology and neurodegenerative disorders, requires computational power beyond classical capabilities. Third, the growing emphasis on personalized medicine necessitates more sophisticated modeling of drug-patient interactions at the molecular level.
Geographically, North America currently dominates the quantum pharmaceutical market with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the fastest growth rate of 28% annually, driven by substantial government investments in quantum technologies in China, Japan, and Singapore.
The market segmentation reveals distinct application areas with varying levels of maturity. Molecular structure analysis represents the largest current segment (40% of applications), followed by drug-target interaction modeling (30%), pharmacokinetic simulations (20%), and quantum machine learning for biomarker discovery (10%). The fastest-growing segment is quantum machine learning applications, projected to expand at 32% annually as algorithms mature and quantum hardware capabilities improve.
Customer segments include large pharmaceutical companies (65% of current market), biotechnology firms (20%), academic research institutions (10%), and government research agencies (5%). Large pharmaceutical companies are primarily motivated by reducing time-to-market and development costs, while biotechnology firms seek competitive advantages through novel therapeutic approaches enabled by quantum computing.
Key market barriers include the high cost of quantum computing infrastructure, limited quantum talent pool, regulatory uncertainties regarding computational drug discovery validation, and the current technical limitations of quantum hardware. Despite these challenges, market adoption is accelerating as hybrid classical-quantum approaches demonstrate tangible benefits in early-stage drug discovery processes.
Market demand for quantum-enabled drug targeting solutions stems primarily from three key factors. First, the escalating costs of traditional drug development, which currently averages $2.6 billion per approved drug with a timeline of 10-15 years. Quantum computing promises to reduce these costs by up to 30% through more efficient molecular modeling and binding affinity predictions. Second, the increasing complexity of target diseases, particularly in oncology and neurodegenerative disorders, requires computational power beyond classical capabilities. Third, the growing emphasis on personalized medicine necessitates more sophisticated modeling of drug-patient interactions at the molecular level.
Geographically, North America currently dominates the quantum pharmaceutical market with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the fastest growth rate of 28% annually, driven by substantial government investments in quantum technologies in China, Japan, and Singapore.
The market segmentation reveals distinct application areas with varying levels of maturity. Molecular structure analysis represents the largest current segment (40% of applications), followed by drug-target interaction modeling (30%), pharmacokinetic simulations (20%), and quantum machine learning for biomarker discovery (10%). The fastest-growing segment is quantum machine learning applications, projected to expand at 32% annually as algorithms mature and quantum hardware capabilities improve.
Customer segments include large pharmaceutical companies (65% of current market), biotechnology firms (20%), academic research institutions (10%), and government research agencies (5%). Large pharmaceutical companies are primarily motivated by reducing time-to-market and development costs, while biotechnology firms seek competitive advantages through novel therapeutic approaches enabled by quantum computing.
Key market barriers include the high cost of quantum computing infrastructure, limited quantum talent pool, regulatory uncertainties regarding computational drug discovery validation, and the current technical limitations of quantum hardware. Despite these challenges, market adoption is accelerating as hybrid classical-quantum approaches demonstrate tangible benefits in early-stage drug discovery processes.
Current Quantum Models and Technical Limitations
Quantum computing has emerged as a promising frontier for drug discovery, offering computational capabilities that classical computers cannot match. Current quantum models for drug targeting primarily fall into three categories: quantum molecular simulations, quantum machine learning algorithms, and quantum-classical hybrid approaches. Quantum molecular simulations leverage quantum mechanics principles to accurately model molecular interactions at the atomic level, providing insights into drug-target binding affinities with unprecedented precision.
Quantum machine learning models, particularly quantum neural networks and quantum support vector machines, have demonstrated potential for analyzing complex biological datasets and identifying patterns that classical algorithms might miss. These models excel at processing high-dimensional pharmaceutical data and can potentially accelerate the identification of promising drug candidates.
Hybrid quantum-classical approaches represent the most practical implementation in today's technological landscape. These models strategically delegate computationally intensive tasks to quantum processors while handling other operations on classical hardware, creating a balanced workflow that maximizes current quantum capabilities while mitigating their limitations.
Despite these advancements, significant technical limitations persist. Quantum coherence remains a fundamental challenge, as quantum states are extremely fragile and susceptible to environmental noise. Current quantum processors typically maintain coherence for only microseconds to milliseconds, severely restricting the complexity and duration of calculations possible for drug modeling applications.
Qubit scalability presents another major hurdle. While effective drug targeting models require thousands to millions of qubits for realistic molecular simulations, today's most advanced quantum computers offer only around 100-1000 qubits with limited connectivity and high error rates, constraining the size and complexity of molecular systems that can be accurately modeled.
Error correction mechanisms, though theoretically promising, remain practically challenging to implement at scale. The overhead required for quantum error correction can multiply the required physical qubits by factors of 10-1000, pushing truly fault-tolerant quantum computing for comprehensive drug discovery further into the future.
Algorithm development also faces significant challenges. Quantum algorithms for chemistry simulations, such as Variational Quantum Eigensolver (VQE) and Quantum Approximate Optimization Algorithm (QAOA), still struggle with convergence issues and require extensive classical pre-processing and post-processing, limiting their standalone effectiveness for drug targeting applications.
Quantum machine learning models, particularly quantum neural networks and quantum support vector machines, have demonstrated potential for analyzing complex biological datasets and identifying patterns that classical algorithms might miss. These models excel at processing high-dimensional pharmaceutical data and can potentially accelerate the identification of promising drug candidates.
Hybrid quantum-classical approaches represent the most practical implementation in today's technological landscape. These models strategically delegate computationally intensive tasks to quantum processors while handling other operations on classical hardware, creating a balanced workflow that maximizes current quantum capabilities while mitigating their limitations.
Despite these advancements, significant technical limitations persist. Quantum coherence remains a fundamental challenge, as quantum states are extremely fragile and susceptible to environmental noise. Current quantum processors typically maintain coherence for only microseconds to milliseconds, severely restricting the complexity and duration of calculations possible for drug modeling applications.
Qubit scalability presents another major hurdle. While effective drug targeting models require thousands to millions of qubits for realistic molecular simulations, today's most advanced quantum computers offer only around 100-1000 qubits with limited connectivity and high error rates, constraining the size and complexity of molecular systems that can be accurately modeled.
Error correction mechanisms, though theoretically promising, remain practically challenging to implement at scale. The overhead required for quantum error correction can multiply the required physical qubits by factors of 10-1000, pushing truly fault-tolerant quantum computing for comprehensive drug discovery further into the future.
Algorithm development also faces significant challenges. Quantum algorithms for chemistry simulations, such as Variational Quantum Eigensolver (VQE) and Quantum Approximate Optimization Algorithm (QAOA), still struggle with convergence issues and require extensive classical pre-processing and post-processing, limiting their standalone effectiveness for drug targeting applications.
Existing Quantum Approaches for Molecular Targeting
01 Quantum Computing Algorithms for Optimization
Quantum computing algorithms are being developed to solve complex optimization problems more efficiently than classical methods. These algorithms leverage quantum properties such as superposition and entanglement to explore multiple solution paths simultaneously. They can be applied to various optimization challenges including resource allocation, scheduling, and logistics, potentially providing exponential speedups for certain problem classes.- Quantum Computing Optimization Algorithms: Various quantum algorithms have been developed to optimize complex computational problems. These algorithms leverage quantum mechanical properties such as superposition and entanglement to achieve computational advantages over classical methods. They are particularly effective for optimization problems in fields like logistics, finance, and machine learning, where they can potentially find optimal or near-optimal solutions more efficiently than traditional approaches.
- Quantum Machine Learning Models: Quantum machine learning combines quantum computing principles with machine learning techniques to create more powerful predictive models. These hybrid approaches can process complex datasets more efficiently, potentially offering advantages in pattern recognition, classification, and prediction tasks. Quantum neural networks and other quantum-enhanced learning algorithms are being developed to handle increasingly complex data structures and optimization challenges.
- Quantum Communication Network Optimization: Optimization of quantum communication networks involves developing efficient protocols for quantum key distribution, quantum teleportation, and secure information transfer. These networks require specialized optimization techniques to maintain quantum coherence over distance, minimize noise, and maximize entanglement fidelity. Advanced models are being developed to optimize the routing, scheduling, and resource allocation in quantum networks.
- Quantum Error Correction and Mitigation: Quantum error correction and mitigation techniques are essential for optimizing the performance of quantum models in the presence of noise and decoherence. These approaches involve developing mathematical frameworks and algorithms that can detect and correct errors in quantum systems, improving the reliability and accuracy of quantum computations. Optimization of error correction codes and fault-tolerant protocols is crucial for scaling quantum systems.
- Quantum-Classical Hybrid Optimization: Hybrid quantum-classical optimization approaches combine the strengths of both quantum and classical computing paradigms. These methods typically use quantum processors for specific computationally intensive subtasks while classical computers handle coordination and other parts of the algorithm. Variational quantum algorithms, quantum approximate optimization algorithms, and quantum-enhanced sampling techniques fall into this category, offering practical approaches to optimization problems on near-term quantum hardware.
02 Quantum Machine Learning Models
Quantum machine learning combines quantum computing principles with machine learning techniques to create more powerful predictive models. These hybrid approaches can process complex datasets more efficiently, recognize patterns that classical algorithms might miss, and optimize model parameters through quantum techniques. Applications include data classification, clustering, and feature selection with potential advantages in computational speed and accuracy.Expand Specific Solutions03 Quantum-Inspired Optimization for Classical Systems
Quantum-inspired optimization techniques adapt quantum principles for implementation on classical computing systems. These methods simulate quantum behaviors like tunneling and interference to escape local optima in complex optimization landscapes. By incorporating concepts from quantum mechanics into classical algorithms, these approaches can improve optimization performance without requiring actual quantum hardware, making them more accessible for current applications.Expand Specific Solutions04 Quantum Annealing for Combinatorial Optimization
Quantum annealing represents a specialized approach to solving combinatorial optimization problems by leveraging quantum fluctuations to find low-energy states corresponding to optimal solutions. This technique is particularly effective for problems that can be formulated as Ising models or quadratic unconstrained binary optimization. Quantum annealing hardware can navigate complex energy landscapes more efficiently than classical simulated annealing methods for certain problem structures.Expand Specific Solutions05 Hybrid Quantum-Classical Optimization Frameworks
Hybrid quantum-classical optimization frameworks combine the strengths of both computing paradigms to address complex optimization challenges. These approaches typically use quantum processors for computationally intensive subroutines while classical computers handle coordination, pre-processing, and post-processing tasks. The variational quantum eigensolver and quantum approximate optimization algorithm are examples of such hybrid methods that iteratively refine solutions through quantum-classical feedback loops.Expand Specific Solutions
Leading Organizations in Quantum Drug Discovery
The quantum modeling for drug targeting landscape is currently in an early growth phase, characterized by a blend of academic research and commercial applications. The market size is expanding rapidly, with projections suggesting significant growth as quantum computing becomes more accessible. Technologically, we're seeing varying levels of maturity across key players. IBM and Bayer are leveraging established quantum computing expertise to develop pharmaceutical applications, while specialized companies like Kuano Ltd. are pioneering quantum-AI hybrid approaches specifically for drug discovery. Academic institutions (National University of Singapore, École Normale Supérieure) are advancing fundamental research, while pharmaceutical companies (Takeda, Sanofi) are increasingly investing in quantum capabilities to enhance their drug development pipelines. The field is witnessing convergence between quantum computing experts and pharmaceutical researchers, creating a dynamic competitive environment.
Kuano Ltd.
Technical Solution: Kuano has developed a specialized quantum computing platform specifically designed for drug discovery applications. Their approach focuses on using quantum algorithms to model enzyme active sites and predict drug-target interactions with greater accuracy than classical methods. Kuano's quantum models incorporate both the electronic structure calculations needed for accurate molecular modeling and machine learning techniques to identify patterns in biological data. They've implemented variational quantum algorithms that can efficiently simulate molecular properties relevant to drug binding, including quantum effects that classical computers struggle to model accurately. Kuano's platform includes quantum-enhanced virtual screening capabilities that can evaluate potential drug candidates against specific targets more efficiently than traditional high-throughput screening methods[5]. Their quantum models are particularly optimized for modeling transition states in enzymatic reactions, which are critical for designing effective enzyme inhibitors as potential drugs. Kuano has developed proprietary methods for reducing quantum circuit complexity while maintaining simulation accuracy, allowing their algorithms to run effectively on current noisy intermediate-scale quantum (NISQ) devices. Their approach combines quantum computing with AI techniques to extract maximum value from limited quantum resources.
Strengths: Kuano's specialized focus on quantum computing for drug discovery has allowed them to develop highly optimized algorithms for this specific application. Their deep expertise in both quantum computing and computational chemistry enables practical applications despite current hardware limitations. Weaknesses: As a smaller company, Kuano has more limited resources compared to larger competitors, potentially constraining their ability to develop and deploy quantum solutions at scale. Their specialized approach may also be less adaptable to other computational chemistry problems outside their core focus areas.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda has developed an innovative quantum computing approach for drug targeting that integrates quantum algorithms with their extensive pharmaceutical expertise. Their platform utilizes quantum machine learning techniques to analyze complex biological data and identify novel drug targets with higher accuracy than conventional methods. Takeda's quantum models are specifically designed to simulate protein-ligand interactions at the quantum mechanical level, accounting for effects that classical models typically approximate. They've implemented quantum algorithms that can efficiently search chemical spaces containing billions of potential compounds to identify those with optimal binding properties for specific targets. Takeda's approach includes quantum-enhanced molecular dynamics simulations that can more accurately predict drug behavior in biological systems, including absorption, distribution, metabolism, and excretion properties[6]. Their quantum models incorporate error mitigation techniques specifically designed for pharmaceutical applications, ensuring reliable results despite the limitations of current quantum hardware. Takeda has also developed hybrid quantum-classical workflows that integrate quantum computing into existing drug discovery pipelines, allowing for practical applications that leverage both computing paradigms.
Strengths: Takeda's extensive pharmaceutical expertise and resources allow them to effectively translate quantum computing advantages into practical drug discovery applications. Their global research network facilitates testing and validation of quantum-derived insights across multiple therapeutic areas. Weaknesses: The approach requires significant investment in both quantum computing expertise and infrastructure, creating high barriers to full implementation. The quantum advantage remains theoretical for many applications until more powerful and reliable quantum hardware becomes available.
Key Quantum Techniques for Drug-Target Interactions
Quantum computing algorithms for accelerated drug discovery
PatentPendingIN202411014372A
Innovation
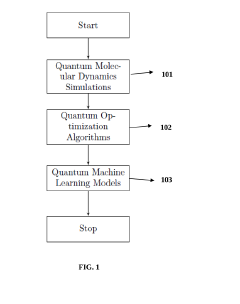
- The integration of quantum computing algorithms, including quantum molecular dynamics simulations, optimization algorithms, and machine learning models, utilizes qubits to simulate molecule interactions, search chemical spaces, and predict pharmacokinetic and pharmacodynamic properties, enabling faster and more accurate identification of drug candidates with desired characteristics.
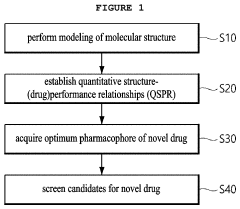
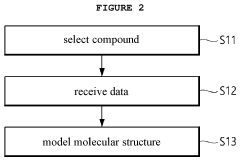
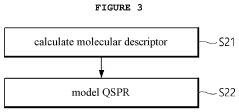
Method for screening of target-based drugs through numerical inversion of quantitative structure-(DRUG)performance relationships and molecular dynamics simulation
PatentActiveUS20200342960A1
Innovation
- A drug screening method utilizing numerical inversions of a quantitative structure-(drug)performance relationships (QSPR) model and molecular dynamics simulation to identify optimal pharmacophores and select candidate compounds for novel target-based drugs, reducing the need for costly clinical tests and false compound synthesis.
Quantum-Classical Hybrid Systems for Drug Optimization
Quantum-Classical Hybrid Systems for Drug Optimization represent a significant advancement in computational approaches to drug discovery. These systems leverage the complementary strengths of quantum computing and classical computing architectures to address the complex challenges of molecular modeling and drug-target interactions. The integration of these two computing paradigms creates a powerful framework that can overcome the limitations inherent in purely classical or purely quantum approaches.
The fundamental architecture of hybrid systems typically involves quantum processors handling computationally intensive tasks such as quantum chemistry calculations and molecular simulations, while classical computers manage data pre-processing, post-processing, and coordination of the overall workflow. This division of labor optimizes computational efficiency and enables researchers to tackle previously intractable problems in drug discovery.
Several implementation models have emerged in recent years, including variational quantum-classical algorithms like VQE (Variational Quantum Eigensolver) and QAOA (Quantum Approximate Optimization Algorithm), which have shown particular promise for drug optimization applications. These algorithms utilize quantum processors to explore vast chemical spaces while classical optimization routines refine the results, creating an iterative feedback loop that progressively improves targeting accuracy.
The advantage of hybrid systems lies in their ability to handle the multi-scale nature of drug-target interactions. Quantum components excel at modeling electron-level quantum mechanical effects crucial for understanding binding affinities and molecular properties, while classical components efficiently manage larger-scale molecular dynamics and data analysis tasks. This multi-scale capability is essential for developing drugs with optimal binding profiles and minimal off-target effects.
Recent advancements in hybrid system frameworks include improved error mitigation techniques that enhance the reliability of quantum computations despite current hardware limitations. Noise-aware algorithms and error-correction protocols have significantly increased the practical utility of quantum-classical approaches for real-world drug discovery applications, even on NISQ (Noisy Intermediate-Scale Quantum) devices currently available.
Industry adoption of quantum-classical hybrid systems has accelerated, with pharmaceutical companies establishing dedicated quantum computing research divisions. Collaborative efforts between technology providers and pharmaceutical researchers have resulted in specialized software platforms that streamline the implementation of hybrid approaches for specific drug discovery workflows, making these advanced techniques more accessible to medicinal chemists and computational biologists.
The fundamental architecture of hybrid systems typically involves quantum processors handling computationally intensive tasks such as quantum chemistry calculations and molecular simulations, while classical computers manage data pre-processing, post-processing, and coordination of the overall workflow. This division of labor optimizes computational efficiency and enables researchers to tackle previously intractable problems in drug discovery.
Several implementation models have emerged in recent years, including variational quantum-classical algorithms like VQE (Variational Quantum Eigensolver) and QAOA (Quantum Approximate Optimization Algorithm), which have shown particular promise for drug optimization applications. These algorithms utilize quantum processors to explore vast chemical spaces while classical optimization routines refine the results, creating an iterative feedback loop that progressively improves targeting accuracy.
The advantage of hybrid systems lies in their ability to handle the multi-scale nature of drug-target interactions. Quantum components excel at modeling electron-level quantum mechanical effects crucial for understanding binding affinities and molecular properties, while classical components efficiently manage larger-scale molecular dynamics and data analysis tasks. This multi-scale capability is essential for developing drugs with optimal binding profiles and minimal off-target effects.
Recent advancements in hybrid system frameworks include improved error mitigation techniques that enhance the reliability of quantum computations despite current hardware limitations. Noise-aware algorithms and error-correction protocols have significantly increased the practical utility of quantum-classical approaches for real-world drug discovery applications, even on NISQ (Noisy Intermediate-Scale Quantum) devices currently available.
Industry adoption of quantum-classical hybrid systems has accelerated, with pharmaceutical companies establishing dedicated quantum computing research divisions. Collaborative efforts between technology providers and pharmaceutical researchers have resulted in specialized software platforms that streamline the implementation of hybrid approaches for specific drug discovery workflows, making these advanced techniques more accessible to medicinal chemists and computational biologists.
Regulatory Considerations for Quantum-Designed Therapeutics
The regulatory landscape for quantum-designed therapeutics represents a complex and evolving framework that pharmaceutical companies must navigate carefully. Current regulatory bodies, including the FDA in the United States and the EMA in Europe, have not yet established specific guidelines for quantum-computed drug candidates, creating a regulatory gray area that requires proactive engagement with authorities.
Quantum-designed drugs present unique regulatory challenges due to their computational origin and potentially novel mechanisms of action. The validation of in silico models used in quantum computing approaches requires substantial documentation to demonstrate reliability and reproducibility. Regulatory agencies typically require evidence that computational models accurately predict biological interactions, necessitating comprehensive validation studies comparing quantum predictions with experimental results.
Data integrity and algorithmic transparency emerge as critical regulatory considerations. Pharmaceutical developers must maintain detailed records of quantum computational methods, including algorithm specifications, error correction techniques, and simulation parameters. This documentation becomes essential for regulatory submissions and may require new standards for quantum computational reproducibility that exceed traditional computational chemistry requirements.
Safety assessment frameworks may need adaptation for quantum-designed therapeutics, particularly when these technologies identify novel binding sites or unconventional molecular interactions. Regulatory bodies will likely require enhanced pharmacovigilance protocols and potentially longer clinical trial observation periods for first-in-class quantum-designed drugs to monitor for unforeseen biological effects.
Intellectual property protection presents another regulatory dimension, as patent offices worldwide are still developing frameworks for quantum-derived innovations. Companies must carefully structure patent applications to protect both the quantum methods employed and the resulting therapeutic compounds, potentially requiring new approaches to pharmaceutical IP strategy.
International harmonization of regulatory approaches remains limited, with significant variations in how different jurisdictions may evaluate quantum-designed therapeutics. Companies pursuing global markets should engage with multiple regulatory authorities early in development to align expectations and requirements, potentially through programs like FDA-EMA parallel scientific advice.
Establishing regulatory precedent will be crucial for the field's advancement. Early adopters who successfully navigate regulatory pathways with quantum-designed drugs will likely shape future regulatory frameworks, suggesting that pharmaceutical companies should consider regulatory strategy as a competitive advantage rather than merely a compliance exercise.
Quantum-designed drugs present unique regulatory challenges due to their computational origin and potentially novel mechanisms of action. The validation of in silico models used in quantum computing approaches requires substantial documentation to demonstrate reliability and reproducibility. Regulatory agencies typically require evidence that computational models accurately predict biological interactions, necessitating comprehensive validation studies comparing quantum predictions with experimental results.
Data integrity and algorithmic transparency emerge as critical regulatory considerations. Pharmaceutical developers must maintain detailed records of quantum computational methods, including algorithm specifications, error correction techniques, and simulation parameters. This documentation becomes essential for regulatory submissions and may require new standards for quantum computational reproducibility that exceed traditional computational chemistry requirements.
Safety assessment frameworks may need adaptation for quantum-designed therapeutics, particularly when these technologies identify novel binding sites or unconventional molecular interactions. Regulatory bodies will likely require enhanced pharmacovigilance protocols and potentially longer clinical trial observation periods for first-in-class quantum-designed drugs to monitor for unforeseen biological effects.
Intellectual property protection presents another regulatory dimension, as patent offices worldwide are still developing frameworks for quantum-derived innovations. Companies must carefully structure patent applications to protect both the quantum methods employed and the resulting therapeutic compounds, potentially requiring new approaches to pharmaceutical IP strategy.
International harmonization of regulatory approaches remains limited, with significant variations in how different jurisdictions may evaluate quantum-designed therapeutics. Companies pursuing global markets should engage with multiple regulatory authorities early in development to align expectations and requirements, potentially through programs like FDA-EMA parallel scientific advice.
Establishing regulatory precedent will be crucial for the field's advancement. Early adopters who successfully navigate regulatory pathways with quantum-designed drugs will likely shape future regulatory frameworks, suggesting that pharmaceutical companies should consider regulatory strategy as a competitive advantage rather than merely a compliance exercise.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!