How to Test Kaolinite's Response to Temperature Fluctuations
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kaolinite Thermal Testing Background and Objectives
Kaolinite, a common clay mineral with the chemical formula Al₂Si₂O₅(OH)₄, has been extensively utilized across various industries including ceramics, paper manufacturing, cosmetics, and increasingly in advanced materials science. The thermal behavior of kaolinite represents a critical area of study due to its significant implications for industrial applications and geological processes. Understanding how kaolinite responds to temperature fluctuations is essential for optimizing manufacturing processes, ensuring product quality, and developing innovative applications.
The historical context of kaolinite thermal testing dates back to the early 20th century when rudimentary thermal analysis techniques were first applied to clay minerals. Over subsequent decades, technological advancements have enabled increasingly sophisticated methods for analyzing thermal properties, from basic differential thermal analysis (DTA) to modern techniques such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and high-temperature X-ray diffraction (HT-XRD).
Recent technological trends indicate a growing interest in understanding the nanoscale thermal behavior of kaolinite, particularly as it relates to phase transformations, dehydroxylation processes, and structural reorganization under varying temperature conditions. The emergence of computational modeling approaches has further enhanced our ability to predict and interpret kaolinite's thermal responses at molecular and atomic levels.
The primary objectives of investigating kaolinite's response to temperature fluctuations encompass several dimensions. First, to establish comprehensive thermal property profiles across diverse kaolinite samples, accounting for variations in crystallinity, particle size, and impurity content. Second, to develop standardized testing protocols that enable reliable and reproducible thermal characterization across different laboratory settings and equipment configurations.
Additionally, this research aims to correlate thermal behavior with structural changes, particularly focusing on the critical temperature thresholds that trigger significant transformations in kaolinite's properties. Understanding these relationships will facilitate the prediction of material performance under various thermal conditions and enable the development of tailored kaolinite-based materials with specific thermal characteristics.
From an industrial perspective, the goal is to optimize processing parameters for kaolinite-containing products, minimizing energy consumption while maximizing desired material properties. This includes identifying precise heating and cooling regimes that yield optimal results for specific applications, from traditional ceramics to advanced composite materials.
The environmental implications of kaolinite's thermal behavior also warrant investigation, particularly regarding energy efficiency in manufacturing processes and the potential for developing more sustainable production methods that leverage kaolinite's natural thermal properties.
The historical context of kaolinite thermal testing dates back to the early 20th century when rudimentary thermal analysis techniques were first applied to clay minerals. Over subsequent decades, technological advancements have enabled increasingly sophisticated methods for analyzing thermal properties, from basic differential thermal analysis (DTA) to modern techniques such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and high-temperature X-ray diffraction (HT-XRD).
Recent technological trends indicate a growing interest in understanding the nanoscale thermal behavior of kaolinite, particularly as it relates to phase transformations, dehydroxylation processes, and structural reorganization under varying temperature conditions. The emergence of computational modeling approaches has further enhanced our ability to predict and interpret kaolinite's thermal responses at molecular and atomic levels.
The primary objectives of investigating kaolinite's response to temperature fluctuations encompass several dimensions. First, to establish comprehensive thermal property profiles across diverse kaolinite samples, accounting for variations in crystallinity, particle size, and impurity content. Second, to develop standardized testing protocols that enable reliable and reproducible thermal characterization across different laboratory settings and equipment configurations.
Additionally, this research aims to correlate thermal behavior with structural changes, particularly focusing on the critical temperature thresholds that trigger significant transformations in kaolinite's properties. Understanding these relationships will facilitate the prediction of material performance under various thermal conditions and enable the development of tailored kaolinite-based materials with specific thermal characteristics.
From an industrial perspective, the goal is to optimize processing parameters for kaolinite-containing products, minimizing energy consumption while maximizing desired material properties. This includes identifying precise heating and cooling regimes that yield optimal results for specific applications, from traditional ceramics to advanced composite materials.
The environmental implications of kaolinite's thermal behavior also warrant investigation, particularly regarding energy efficiency in manufacturing processes and the potential for developing more sustainable production methods that leverage kaolinite's natural thermal properties.
Market Applications and Demand Analysis for Thermally Stable Kaolinite
The global market for thermally stable kaolinite has been experiencing significant growth, driven by increasing applications across multiple industries. The ceramic industry remains the largest consumer of kaolinite, accounting for approximately 40% of global demand, where thermal stability is crucial for maintaining structural integrity during firing processes. Recent market research indicates that manufacturers are increasingly seeking kaolinite variants that can withstand temperature fluctuations between 500°C and 1200°C without significant deformation or property changes.
The paper and coating industry represents the second-largest market segment, valuing thermal stability for high-temperature processing applications. This sector has shown consistent annual growth rates of 3-5% over the past five years, with particular emphasis on kaolinite that maintains its whiteness and opacity under varying temperature conditions. The demand is especially pronounced in premium paper manufacturing where product consistency is paramount.
Construction materials manufacturers have emerged as a rapidly expanding market for thermally stable kaolinite, particularly in regions experiencing extreme climate variations. The ability of modified kaolinite to withstand freeze-thaw cycles without degradation has created new application opportunities in exterior building materials, with market analysts projecting this segment to grow at 7-9% annually through 2028.
The refractory materials sector presents another significant market opportunity, where kaolinite's thermal stability directly correlates with product performance and longevity. Industry surveys indicate that manufacturers are willing to pay premium prices for kaolinite variants demonstrating consistent performance across wide temperature ranges, particularly between 800°C and 1100°C where many industrial processes operate.
Environmental remediation applications represent an emerging market with substantial growth potential. Thermally treated kaolinite has shown promising results in adsorbing heavy metals and organic pollutants across varying temperature conditions, creating demand in water treatment facilities and contaminated soil remediation projects. This segment is expected to expand at double-digit rates as environmental regulations tighten globally.
Regional analysis reveals that Asia-Pacific dominates demand for thermally stable kaolinite, accounting for approximately 45% of global consumption, followed by Europe (25%) and North America (20%). The fastest growth is occurring in developing economies where rapid industrialization and infrastructure development drive consumption across multiple end-use industries.
Market forecasts suggest that premium pricing for thermally stable kaolinite variants could yield 15-20% higher margins compared to standard grades, creating strong economic incentives for producers to develop and commercialize advanced testing and modification technologies that enhance temperature resistance properties.
The paper and coating industry represents the second-largest market segment, valuing thermal stability for high-temperature processing applications. This sector has shown consistent annual growth rates of 3-5% over the past five years, with particular emphasis on kaolinite that maintains its whiteness and opacity under varying temperature conditions. The demand is especially pronounced in premium paper manufacturing where product consistency is paramount.
Construction materials manufacturers have emerged as a rapidly expanding market for thermally stable kaolinite, particularly in regions experiencing extreme climate variations. The ability of modified kaolinite to withstand freeze-thaw cycles without degradation has created new application opportunities in exterior building materials, with market analysts projecting this segment to grow at 7-9% annually through 2028.
The refractory materials sector presents another significant market opportunity, where kaolinite's thermal stability directly correlates with product performance and longevity. Industry surveys indicate that manufacturers are willing to pay premium prices for kaolinite variants demonstrating consistent performance across wide temperature ranges, particularly between 800°C and 1100°C where many industrial processes operate.
Environmental remediation applications represent an emerging market with substantial growth potential. Thermally treated kaolinite has shown promising results in adsorbing heavy metals and organic pollutants across varying temperature conditions, creating demand in water treatment facilities and contaminated soil remediation projects. This segment is expected to expand at double-digit rates as environmental regulations tighten globally.
Regional analysis reveals that Asia-Pacific dominates demand for thermally stable kaolinite, accounting for approximately 45% of global consumption, followed by Europe (25%) and North America (20%). The fastest growth is occurring in developing economies where rapid industrialization and infrastructure development drive consumption across multiple end-use industries.
Market forecasts suggest that premium pricing for thermally stable kaolinite variants could yield 15-20% higher margins compared to standard grades, creating strong economic incentives for producers to develop and commercialize advanced testing and modification technologies that enhance temperature resistance properties.
Current Testing Methods and Technical Challenges
Testing kaolinite's response to temperature fluctuations currently employs several established methodologies, each with specific advantages and limitations. Thermogravimetric Analysis (TGA) stands as a primary technique, measuring mass changes as temperature varies. This method provides precise data on dehydration, dehydroxylation, and phase transformation points, typically operating between ambient temperature and 1000°C. However, TGA equipment requires significant investment and specialized expertise for accurate data interpretation.
Differential Scanning Calorimetry (DSC) complements TGA by measuring heat flow differences between kaolinite samples and reference materials during controlled temperature programs. This technique effectively identifies endothermic and exothermic reactions, revealing critical information about structural changes. The limitation lies in sample preparation requirements and potential interference from impurities.
X-ray Diffraction (XRD) analysis conducted at various temperature points offers insights into crystalline structure modifications. By examining diffraction patterns at incremental temperature stages, researchers can track structural transformations. The challenge here involves maintaining precise temperature control during measurements and the need for specialized high-temperature XRD equipment.
Fourier Transform Infrared Spectroscopy (FTIR) with temperature-controlled sample chambers allows monitoring of hydroxyl group changes and other molecular transformations. While providing valuable molecular-level information, this method struggles with quantitative analysis and requires careful baseline corrections.
Dilatometry measures dimensional changes in kaolinite samples during heating and cooling cycles, offering direct observation of thermal expansion and contraction behaviors. However, sample preparation must be extremely precise to avoid introducing measurement artifacts.
Several technical challenges persist across these methodologies. Temperature control precision represents a fundamental issue, as even minor fluctuations can significantly impact results, particularly during critical phase transition points. Most commercial equipment offers ±1-2°C accuracy, which proves insufficient for studying subtle transitions in kaolinite.
Sample heterogeneity presents another major challenge, as natural kaolinite deposits contain varying impurity levels that can dramatically alter thermal response patterns. Researchers must develop standardized purification protocols to ensure comparable results across studies.
The rate of temperature change significantly influences kaolinite's response, with rapid heating potentially causing different transformation pathways compared to gradual temperature increases. Current testing protocols lack standardization regarding optimal heating/cooling rates for specific research questions.
Additionally, environmental factors such as atmospheric composition and humidity levels during testing introduce variables that remain difficult to control consistently across different laboratory settings, complicating result comparability and reproducibility in the field.
Differential Scanning Calorimetry (DSC) complements TGA by measuring heat flow differences between kaolinite samples and reference materials during controlled temperature programs. This technique effectively identifies endothermic and exothermic reactions, revealing critical information about structural changes. The limitation lies in sample preparation requirements and potential interference from impurities.
X-ray Diffraction (XRD) analysis conducted at various temperature points offers insights into crystalline structure modifications. By examining diffraction patterns at incremental temperature stages, researchers can track structural transformations. The challenge here involves maintaining precise temperature control during measurements and the need for specialized high-temperature XRD equipment.
Fourier Transform Infrared Spectroscopy (FTIR) with temperature-controlled sample chambers allows monitoring of hydroxyl group changes and other molecular transformations. While providing valuable molecular-level information, this method struggles with quantitative analysis and requires careful baseline corrections.
Dilatometry measures dimensional changes in kaolinite samples during heating and cooling cycles, offering direct observation of thermal expansion and contraction behaviors. However, sample preparation must be extremely precise to avoid introducing measurement artifacts.
Several technical challenges persist across these methodologies. Temperature control precision represents a fundamental issue, as even minor fluctuations can significantly impact results, particularly during critical phase transition points. Most commercial equipment offers ±1-2°C accuracy, which proves insufficient for studying subtle transitions in kaolinite.
Sample heterogeneity presents another major challenge, as natural kaolinite deposits contain varying impurity levels that can dramatically alter thermal response patterns. Researchers must develop standardized purification protocols to ensure comparable results across studies.
The rate of temperature change significantly influences kaolinite's response, with rapid heating potentially causing different transformation pathways compared to gradual temperature increases. Current testing protocols lack standardization regarding optimal heating/cooling rates for specific research questions.
Additionally, environmental factors such as atmospheric composition and humidity levels during testing introduce variables that remain difficult to control consistently across different laboratory settings, complicating result comparability and reproducibility in the field.
Standard Protocols for Kaolinite Temperature Response Testing
01 Thermal stability and structural changes of kaolinite
Kaolinite undergoes significant structural changes when exposed to temperature fluctuations. At elevated temperatures, kaolinite may experience dehydroxylation, phase transformation, and crystallinity changes. These transformations affect its physical and chemical properties, including surface area, porosity, and reactivity. Understanding these changes is crucial for applications where kaolinite is exposed to varying thermal conditions.- Thermal stability and structural changes of kaolinite: Kaolinite undergoes significant structural changes when exposed to temperature fluctuations. At elevated temperatures, kaolinite may experience dehydroxylation, phase transformation, and crystallinity changes. These structural modifications affect its physical and chemical properties, including surface area, reactivity, and adsorption capacity. Understanding these changes is crucial for applications where kaolinite is exposed to varying thermal conditions.
- Kaolinite in thermal insulation and temperature regulation systems: Kaolinite is utilized in thermal insulation and temperature regulation systems due to its ability to respond to temperature fluctuations. Its low thermal conductivity and high specific heat capacity make it an effective material for maintaining stable temperatures in various applications. Kaolinite-based composites can be engineered to absorb, store, and release thermal energy, helping to mitigate the effects of temperature variations in building materials, electronic components, and industrial processes.
- Kaolinite modification for enhanced thermal performance: Various modification techniques can enhance kaolinite's response to temperature fluctuations. These include intercalation with organic compounds, surface functionalization, and the creation of kaolinite-polymer composites. Modified kaolinite exhibits improved thermal stability, controlled expansion/contraction behavior, and enhanced heat transfer properties. These modifications extend the temperature range in which kaolinite can effectively function and improve its performance in extreme thermal conditions.
- Kaolinite in ceramic and refractory applications under thermal stress: Kaolinite serves as a key component in ceramics and refractories subjected to thermal stress. When exposed to temperature fluctuations, kaolinite-based materials demonstrate specific thermal expansion behaviors, crack resistance, and thermal shock tolerance. The transformation of kaolinite to metakaolin and ultimately to mullite during firing contributes to the development of high-temperature resistant materials. These properties make kaolinite valuable in applications requiring materials to withstand repeated heating and cooling cycles.
- Monitoring and measurement of kaolinite's thermal response: Advanced techniques have been developed to monitor and measure kaolinite's response to temperature fluctuations. These include thermal analysis methods, in-situ X-ray diffraction, spectroscopic techniques, and computational modeling. These approaches provide insights into the kinetics of thermal transformations, phase changes, and structural rearrangements in kaolinite under varying temperature conditions. The data obtained helps optimize kaolinite-based materials for specific thermal applications and predict their behavior under temperature stress.
02 Kaolinite in thermal insulation applications
Kaolinite is utilized in thermal insulation materials due to its response to temperature fluctuations. When properly formulated, kaolinite-based materials can provide stable thermal performance across varying temperatures. These materials often incorporate kaolinite with other components to enhance thermal resistance properties, creating effective insulation systems that maintain their integrity during temperature changes.Expand Specific Solutions03 Kaolinite in heat transfer and energy storage systems
Kaolinite's response to temperature fluctuations makes it valuable in heat transfer and energy storage applications. Systems incorporating kaolinite can absorb, store, and release thermal energy in response to temperature changes. This property is utilized in various thermal management solutions where controlled heat absorption and dissipation are required, enhancing energy efficiency in different environmental conditions.Expand Specific Solutions04 Modified kaolinite composites for improved thermal performance
Kaolinite can be modified or combined with other materials to enhance its response to temperature fluctuations. These modifications may include surface treatments, intercalation with organic or inorganic compounds, or formation of composite materials. The resulting materials exhibit improved thermal stability, reduced thermal expansion, or enhanced heat resistance compared to unmodified kaolinite, making them suitable for applications in extreme or fluctuating temperature environments.Expand Specific Solutions05 Kaolinite in electronic and industrial applications under thermal stress
Kaolinite is incorporated into electronic components and industrial materials that experience thermal stress. Its ability to maintain dimensional stability during temperature fluctuations makes it valuable in applications where thermal expansion could cause failure. Kaolinite-containing materials are used in electronic substrates, circuit boards, and industrial coatings where consistent performance across varying temperatures is essential for operational reliability and longevity.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The kaolinite temperature fluctuation testing market is in a growth phase, with increasing demand driven by petrochemical, ceramic, and advanced materials applications. The market size is expanding due to rising industrial needs for thermal stability analysis. Technologically, the field shows moderate maturity with established players like Sinopec Research Institute and BASF leading industrial applications, while academic institutions including China University of Geosciences, University of Tokyo, and CNRS contribute significant research advancements. Companies like China Resources Chemical Innovative Materials and Jiangsu Honglingda Technology represent emerging commercial players developing specialized testing methodologies and equipment for temperature-responsive kaolinite applications in various industrial sectors.
Sinopec Research Institute of Petroleum Processing
Technical Solution: Sinopec RIPP has developed advanced thermal analysis protocols for kaolinite characterization using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Their approach involves precise temperature control systems (±0.1°C) that can simulate rapid temperature fluctuations between -40°C and 1000°C while monitoring structural changes in real-time. The institute has pioneered a multi-cycle thermal stress testing methodology that evaluates kaolinite's stability under repeated heating-cooling cycles, particularly relevant for catalytic applications in petroleum refining. Their proprietary software analyzes dehydroxylation kinetics during temperature changes, providing quantitative data on structural transformation rates and activation energies that predict long-term performance under variable temperature conditions.
Strengths: Exceptional precision in temperature control; comprehensive data analysis capabilities; direct industrial application expertise in petroleum catalysis. Weakness: Testing protocols are primarily optimized for petroleum applications rather than broader geological or materials science contexts.
BASF SE
Technical Solution: BASF has engineered a sophisticated thermal cycling chamber specifically designed for clay mineral testing that can simulate industrial processing conditions with temperature fluctuations between -50°C and 1200°C. Their methodology incorporates in-situ X-ray diffraction (XRD) during thermal cycling to monitor crystallographic changes in kaolinite structure in real-time. BASF's approach includes humidity-controlled thermal testing environments that allow for simultaneous monitoring of moisture interaction effects during temperature fluctuations, critical for understanding kaolinite behavior in variable environmental conditions. The company has developed specialized software algorithms that can predict kaolinite's thermal expansion coefficients across different temperature ranges based on empirical testing data, enabling precise material performance forecasting for industrial applications.
Strengths: Comprehensive testing infrastructure combining multiple analytical techniques; ability to simulate complex industrial conditions; advanced predictive modeling capabilities. Weakness: Proprietary nature of testing protocols limits broader scientific accessibility and standardization across the industry.
Critical Parameters and Measurement Technologies
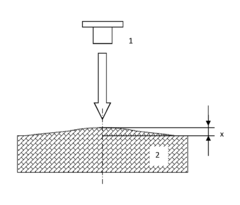
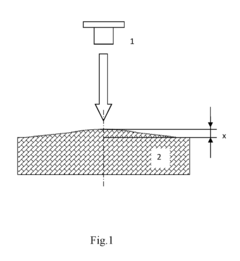
Method for determining a coefficient of thermal linear expansion of a material and a device for implementing the same
PatentInactiveUS20170074812A1
Innovation
- A method involving transient heating of a material's surface with periodic changes in heating power density, measuring surface deformation amplitude, and accounting for volumetric heat capacity and bulk modulus, using a device with a heating source and sensors for relative movement and deformation measurement, allowing for contactless measurement of CLTE with improved accuracy and efficiency.
Environmental Factors Affecting Kaolinite Thermal Behavior
Kaolinite's thermal behavior is significantly influenced by various environmental factors that can alter its response to temperature fluctuations. Humidity levels play a crucial role in this interaction, as water molecules intercalated between kaolinite layers can dramatically change the material's thermal properties. Higher ambient humidity typically increases the water content within kaolinite structures, which subsequently affects dehydroxylation temperatures and thermal expansion characteristics during testing procedures.
Atmospheric composition represents another critical environmental variable. The presence of oxygen, carbon dioxide, or other gases can catalyze or inhibit specific thermal reactions in kaolinite. For instance, testing conducted in oxygen-rich environments may accelerate oxidation reactions of any organic impurities present in the kaolinite sample, potentially skewing thermal analysis results and creating misleading data regarding the clay's intrinsic thermal behavior.
Pressure conditions during thermal testing also significantly impact kaolinite's response patterns. Higher pressures can alter phase transformation temperatures and mechanisms, particularly affecting the metakaolin formation process that occurs during kaolinite's thermal decomposition. Researchers have documented pressure-dependent shifts in transformation temperatures by as much as 15-30°C under varying atmospheric conditions.
The presence of impurities and associated minerals constitutes a major environmental factor affecting thermal behavior. Natural kaolinite deposits typically contain varying amounts of quartz, feldspar, mica, and iron compounds that can dramatically alter thermal response patterns. Iron impurities, in particular, can catalyze certain reactions and lower transformation temperatures, while silica impurities may increase thermal stability in specific temperature ranges.
Particle size distribution and aggregation state represent physical environmental factors that influence heat transfer efficiency and reaction kinetics. Finer kaolinite particles generally exhibit more rapid thermal responses due to increased surface area, while aggregated structures may display thermal behavior that differs significantly from dispersed particles due to heat transfer limitations and localized reaction environments.
Pre-treatment history of kaolinite samples, including previous exposure to chemicals, mechanical processing, or thermal cycling, can permanently alter the material's subsequent thermal behavior. This "environmental memory" effect must be carefully considered when designing testing protocols, as it may introduce variables that confound accurate assessment of temperature fluctuation responses.
Atmospheric composition represents another critical environmental variable. The presence of oxygen, carbon dioxide, or other gases can catalyze or inhibit specific thermal reactions in kaolinite. For instance, testing conducted in oxygen-rich environments may accelerate oxidation reactions of any organic impurities present in the kaolinite sample, potentially skewing thermal analysis results and creating misleading data regarding the clay's intrinsic thermal behavior.
Pressure conditions during thermal testing also significantly impact kaolinite's response patterns. Higher pressures can alter phase transformation temperatures and mechanisms, particularly affecting the metakaolin formation process that occurs during kaolinite's thermal decomposition. Researchers have documented pressure-dependent shifts in transformation temperatures by as much as 15-30°C under varying atmospheric conditions.
The presence of impurities and associated minerals constitutes a major environmental factor affecting thermal behavior. Natural kaolinite deposits typically contain varying amounts of quartz, feldspar, mica, and iron compounds that can dramatically alter thermal response patterns. Iron impurities, in particular, can catalyze certain reactions and lower transformation temperatures, while silica impurities may increase thermal stability in specific temperature ranges.
Particle size distribution and aggregation state represent physical environmental factors that influence heat transfer efficiency and reaction kinetics. Finer kaolinite particles generally exhibit more rapid thermal responses due to increased surface area, while aggregated structures may display thermal behavior that differs significantly from dispersed particles due to heat transfer limitations and localized reaction environments.
Pre-treatment history of kaolinite samples, including previous exposure to chemicals, mechanical processing, or thermal cycling, can permanently alter the material's subsequent thermal behavior. This "environmental memory" effect must be carefully considered when designing testing protocols, as it may introduce variables that confound accurate assessment of temperature fluctuation responses.
Quality Control Standards and Certification Requirements
Quality control standards for testing kaolinite's response to temperature fluctuations must adhere to internationally recognized frameworks. The International Organization for Standardization (ISO) provides several relevant standards, including ISO 12677 for chemical analysis of refractory products and ISO 21587 specifically for clay materials. These standards establish precise protocols for sample preparation, testing methodologies, and data interpretation that ensure consistency across different testing facilities.
ASTM International offers complementary standards such as ASTM C1285 for leaching tests and ASTM D6316 for thermal analysis of clay minerals. These standards specify equipment calibration requirements, acceptable temperature ranges, and heating/cooling rates that must be maintained during testing. Laboratories conducting kaolinite temperature response tests must demonstrate compliance with these standards through regular audits and certification processes.
Certification requirements typically involve laboratory accreditation under ISO/IEC 17025, which establishes general requirements for the competence of testing and calibration laboratories. This certification ensures that facilities possess the technical capability and quality management systems necessary for reliable kaolinite testing. Additionally, personnel conducting these tests should hold relevant qualifications, such as Certified Thermal Analyst (CTA) or equivalent industry-recognized credentials.
Documentation requirements constitute another critical aspect of quality control. Test reports must include detailed information about sample origin, preparation methods, testing equipment specifications, calibration records, and environmental conditions during testing. This comprehensive documentation enables traceability and reproducibility of results, which are fundamental principles in quality assurance systems.
Statistical quality control methods must be implemented to validate test results. These include the use of control charts to monitor testing process stability, calculation of measurement uncertainty, and regular proficiency testing through interlaboratory comparisons. For kaolinite temperature fluctuation testing, acceptable tolerance limits typically range between ±1°C for temperature control and ±2% for dimensional or mass changes.
Industry-specific certifications may also apply depending on the end-use application of the kaolinite. For example, materials intended for pharmaceutical or food-contact applications require additional certifications such as USP <661.1> or EU 10/2011 compliance. Similarly, kaolinite used in construction materials may need to meet building code requirements and sustainability certifications like LEED or BREEAM.
ASTM International offers complementary standards such as ASTM C1285 for leaching tests and ASTM D6316 for thermal analysis of clay minerals. These standards specify equipment calibration requirements, acceptable temperature ranges, and heating/cooling rates that must be maintained during testing. Laboratories conducting kaolinite temperature response tests must demonstrate compliance with these standards through regular audits and certification processes.
Certification requirements typically involve laboratory accreditation under ISO/IEC 17025, which establishes general requirements for the competence of testing and calibration laboratories. This certification ensures that facilities possess the technical capability and quality management systems necessary for reliable kaolinite testing. Additionally, personnel conducting these tests should hold relevant qualifications, such as Certified Thermal Analyst (CTA) or equivalent industry-recognized credentials.
Documentation requirements constitute another critical aspect of quality control. Test reports must include detailed information about sample origin, preparation methods, testing equipment specifications, calibration records, and environmental conditions during testing. This comprehensive documentation enables traceability and reproducibility of results, which are fundamental principles in quality assurance systems.
Statistical quality control methods must be implemented to validate test results. These include the use of control charts to monitor testing process stability, calculation of measurement uncertainty, and regular proficiency testing through interlaboratory comparisons. For kaolinite temperature fluctuation testing, acceptable tolerance limits typically range between ±1°C for temperature control and ±2% for dimensional or mass changes.
Industry-specific certifications may also apply depending on the end-use application of the kaolinite. For example, materials intended for pharmaceutical or food-contact applications require additional certifications such as USP <661.1> or EU 10/2011 compliance. Similarly, kaolinite used in construction materials may need to meet building code requirements and sustainability certifications like LEED or BREEAM.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!