How to Utilize PEMF Therapy for Improved Joint Health?
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment for various musculoskeletal conditions, particularly in the realm of joint health. This technology harnesses the power of electromagnetic fields to stimulate cellular repair and regeneration, offering potential benefits for individuals suffering from joint-related issues.
The history of PEMF therapy can be traced back to the mid-20th century, with early applications in bone healing. Over the decades, research and technological advancements have expanded its potential uses, leading to a growing interest in its application for joint health. The therapy works by delivering pulsed electromagnetic fields to targeted areas, influencing cellular behavior and promoting natural healing processes.
In recent years, the focus on PEMF therapy for joint health has intensified due to the increasing prevalence of joint-related disorders, such as osteoarthritis and rheumatoid arthritis. These conditions affect millions of people worldwide, leading to pain, reduced mobility, and decreased quality of life. Traditional treatments often involve medication or invasive procedures, which may have limitations or side effects. PEMF therapy offers a complementary or alternative approach that addresses the underlying cellular mechanisms of joint health.
The primary objective of utilizing PEMF therapy for improved joint health is to alleviate pain, reduce inflammation, and enhance overall joint function. By stimulating cellular activity, PEMF therapy aims to accelerate the body's natural healing processes, potentially slowing down the progression of degenerative joint conditions and improving the overall health of joint tissues.
Current research in this field is focused on optimizing PEMF parameters, such as frequency, intensity, and duration, to maximize therapeutic benefits for specific joint conditions. Additionally, there is a growing interest in understanding the long-term effects of PEMF therapy on joint health and its potential role in preventive care.
As the technology continues to evolve, researchers and clinicians are exploring innovative ways to integrate PEMF therapy into comprehensive joint health management strategies. This includes investigating its synergistic effects with other treatment modalities, developing more targeted and personalized PEMF protocols, and exploring its potential in regenerative medicine approaches for joint repair and restoration.
The ultimate goal of PEMF therapy research in joint health is to establish evidence-based protocols that can be widely adopted in clinical practice, offering patients a safe, effective, and non-invasive option for managing joint-related issues and improving their overall quality of life. As the field progresses, it holds promise for revolutionizing the approach to joint health management and potentially reducing the reliance on more invasive or pharmacological interventions.
The history of PEMF therapy can be traced back to the mid-20th century, with early applications in bone healing. Over the decades, research and technological advancements have expanded its potential uses, leading to a growing interest in its application for joint health. The therapy works by delivering pulsed electromagnetic fields to targeted areas, influencing cellular behavior and promoting natural healing processes.
In recent years, the focus on PEMF therapy for joint health has intensified due to the increasing prevalence of joint-related disorders, such as osteoarthritis and rheumatoid arthritis. These conditions affect millions of people worldwide, leading to pain, reduced mobility, and decreased quality of life. Traditional treatments often involve medication or invasive procedures, which may have limitations or side effects. PEMF therapy offers a complementary or alternative approach that addresses the underlying cellular mechanisms of joint health.
The primary objective of utilizing PEMF therapy for improved joint health is to alleviate pain, reduce inflammation, and enhance overall joint function. By stimulating cellular activity, PEMF therapy aims to accelerate the body's natural healing processes, potentially slowing down the progression of degenerative joint conditions and improving the overall health of joint tissues.
Current research in this field is focused on optimizing PEMF parameters, such as frequency, intensity, and duration, to maximize therapeutic benefits for specific joint conditions. Additionally, there is a growing interest in understanding the long-term effects of PEMF therapy on joint health and its potential role in preventive care.
As the technology continues to evolve, researchers and clinicians are exploring innovative ways to integrate PEMF therapy into comprehensive joint health management strategies. This includes investigating its synergistic effects with other treatment modalities, developing more targeted and personalized PEMF protocols, and exploring its potential in regenerative medicine approaches for joint repair and restoration.
The ultimate goal of PEMF therapy research in joint health is to establish evidence-based protocols that can be widely adopted in clinical practice, offering patients a safe, effective, and non-invasive option for managing joint-related issues and improving their overall quality of life. As the field progresses, it holds promise for revolutionizing the approach to joint health management and potentially reducing the reliance on more invasive or pharmacological interventions.
Market Analysis for Joint Health Solutions
The global market for joint health solutions has been experiencing significant growth, driven by an aging population, increasing awareness of preventive healthcare, and a rise in sports-related injuries. The joint health market encompasses a wide range of products and therapies, including dietary supplements, pharmaceuticals, medical devices, and alternative treatments like PEMF therapy.
In recent years, the joint health market has shown a steady increase, with a compound annual growth rate (CAGR) of around 7% expected over the next five years. This growth is primarily attributed to the rising prevalence of joint disorders such as osteoarthritis, rheumatoid arthritis, and other musculoskeletal conditions. The North American region currently holds the largest market share, followed by Europe and Asia-Pacific.
Within this market, PEMF therapy is emerging as a promising non-invasive treatment option for joint health. The PEMF therapy segment is projected to grow at a faster rate compared to traditional joint health solutions, driven by increasing clinical evidence supporting its efficacy and growing patient preference for non-pharmacological interventions.
Consumer demand for joint health solutions is shifting towards more natural and holistic approaches, with a focus on preventive care and long-term management of joint conditions. This trend aligns well with PEMF therapy, which offers a non-invasive and drug-free alternative to conventional treatments.
The competitive landscape of the joint health market is diverse, with both established pharmaceutical companies and innovative medical device manufacturers vying for market share. Key players in the PEMF therapy segment include OMI, BEMER Group, and Orthofix Medical. These companies are investing heavily in research and development to enhance the efficacy and user-friendliness of PEMF devices for joint health applications.
Regulatory factors play a crucial role in shaping the market for joint health solutions, particularly for medical devices like PEMF therapy equipment. The FDA's clearance of several PEMF devices for various indications, including joint health, has provided a significant boost to market growth and consumer acceptance.
Looking ahead, the joint health solutions market is expected to continue its upward trajectory, with PEMF therapy positioned as a key growth driver. Factors such as increasing healthcare expenditure, growing adoption of wearable medical devices, and advancements in PEMF technology are likely to fuel market expansion. However, challenges such as high initial costs of PEMF devices and the need for more extensive clinical validation may impact adoption rates in certain market segments.
In recent years, the joint health market has shown a steady increase, with a compound annual growth rate (CAGR) of around 7% expected over the next five years. This growth is primarily attributed to the rising prevalence of joint disorders such as osteoarthritis, rheumatoid arthritis, and other musculoskeletal conditions. The North American region currently holds the largest market share, followed by Europe and Asia-Pacific.
Within this market, PEMF therapy is emerging as a promising non-invasive treatment option for joint health. The PEMF therapy segment is projected to grow at a faster rate compared to traditional joint health solutions, driven by increasing clinical evidence supporting its efficacy and growing patient preference for non-pharmacological interventions.
Consumer demand for joint health solutions is shifting towards more natural and holistic approaches, with a focus on preventive care and long-term management of joint conditions. This trend aligns well with PEMF therapy, which offers a non-invasive and drug-free alternative to conventional treatments.
The competitive landscape of the joint health market is diverse, with both established pharmaceutical companies and innovative medical device manufacturers vying for market share. Key players in the PEMF therapy segment include OMI, BEMER Group, and Orthofix Medical. These companies are investing heavily in research and development to enhance the efficacy and user-friendliness of PEMF devices for joint health applications.
Regulatory factors play a crucial role in shaping the market for joint health solutions, particularly for medical devices like PEMF therapy equipment. The FDA's clearance of several PEMF devices for various indications, including joint health, has provided a significant boost to market growth and consumer acceptance.
Looking ahead, the joint health solutions market is expected to continue its upward trajectory, with PEMF therapy positioned as a key growth driver. Factors such as increasing healthcare expenditure, growing adoption of wearable medical devices, and advancements in PEMF technology are likely to fuel market expansion. However, challenges such as high initial costs of PEMF devices and the need for more extensive clinical validation may impact adoption rates in certain market segments.
PEMF Technology: Current Status and Challenges
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a non-invasive treatment for various musculoskeletal conditions, particularly in the realm of joint health. The current status of PEMF technology reflects a growing body of research and clinical applications, yet it also faces several challenges that need to be addressed for wider adoption and efficacy.
The primary mechanism of PEMF therapy involves the application of low-frequency electromagnetic fields to stimulate cellular repair and reduce inflammation. Current PEMF devices range from portable, home-use units to more sophisticated clinical systems. These devices generate electromagnetic fields with varying frequencies, intensities, and waveforms, tailored to specific therapeutic goals.
One of the main challenges in PEMF technology is the lack of standardization in treatment protocols. Different studies and clinical applications use varying parameters, making it difficult to establish a consensus on the most effective settings for specific joint conditions. This variability also complicates the comparison of research results and the development of evidence-based guidelines for PEMF therapy in joint health.
Another significant challenge is the need for more robust, long-term clinical studies. While many short-term studies have shown promising results in reducing pain and improving joint function, there is a scarcity of large-scale, randomized controlled trials that demonstrate the long-term efficacy and safety of PEMF therapy for joint health. This gap in research hinders the widespread acceptance of PEMF technology in mainstream medical practice.
The optimization of PEMF devices for joint-specific applications presents another technical challenge. Current technology often employs a one-size-fits-all approach, which may not be ideal for targeting specific joint structures or addressing individual patient needs. Developing more precise and customizable PEMF devices that can target specific joint areas with optimal field parameters remains an area of ongoing research and development.
Regulatory hurdles also pose a challenge to the advancement of PEMF technology. The classification and approval processes for PEMF devices vary across different countries, leading to inconsistencies in availability and usage guidelines. Navigating these regulatory landscapes while ensuring safety and efficacy standards are met is crucial for the global adoption of PEMF therapy in joint health management.
Despite these challenges, PEMF technology continues to evolve, with ongoing research focusing on refining treatment protocols, improving device design, and expanding the understanding of the biological mechanisms underlying its effects on joint health. As the field progresses, addressing these challenges will be critical in realizing the full potential of PEMF therapy for improving joint health and quality of life for patients with various musculoskeletal conditions.
The primary mechanism of PEMF therapy involves the application of low-frequency electromagnetic fields to stimulate cellular repair and reduce inflammation. Current PEMF devices range from portable, home-use units to more sophisticated clinical systems. These devices generate electromagnetic fields with varying frequencies, intensities, and waveforms, tailored to specific therapeutic goals.
One of the main challenges in PEMF technology is the lack of standardization in treatment protocols. Different studies and clinical applications use varying parameters, making it difficult to establish a consensus on the most effective settings for specific joint conditions. This variability also complicates the comparison of research results and the development of evidence-based guidelines for PEMF therapy in joint health.
Another significant challenge is the need for more robust, long-term clinical studies. While many short-term studies have shown promising results in reducing pain and improving joint function, there is a scarcity of large-scale, randomized controlled trials that demonstrate the long-term efficacy and safety of PEMF therapy for joint health. This gap in research hinders the widespread acceptance of PEMF technology in mainstream medical practice.
The optimization of PEMF devices for joint-specific applications presents another technical challenge. Current technology often employs a one-size-fits-all approach, which may not be ideal for targeting specific joint structures or addressing individual patient needs. Developing more precise and customizable PEMF devices that can target specific joint areas with optimal field parameters remains an area of ongoing research and development.
Regulatory hurdles also pose a challenge to the advancement of PEMF technology. The classification and approval processes for PEMF devices vary across different countries, leading to inconsistencies in availability and usage guidelines. Navigating these regulatory landscapes while ensuring safety and efficacy standards are met is crucial for the global adoption of PEMF therapy in joint health management.
Despite these challenges, PEMF technology continues to evolve, with ongoing research focusing on refining treatment protocols, improving device design, and expanding the understanding of the biological mechanisms underlying its effects on joint health. As the field progresses, addressing these challenges will be critical in realizing the full potential of PEMF therapy for improving joint health and quality of life for patients with various musculoskeletal conditions.
Existing PEMF Solutions for Joint Health
01 PEMF devices for joint health treatment
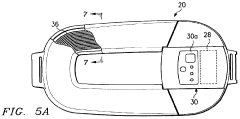
Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating joint health issues. These devices generate electromagnetic fields that penetrate the body, stimulating cellular activity and promoting healing in joints. They can be used to address various joint conditions, including arthritis, inflammation, and pain.- PEMF devices for joint health treatment: Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating joint health issues. These devices generate electromagnetic fields that penetrate the body, stimulating cellular repair and reducing inflammation in joints. The therapy can be applied to various joints, including knees, hips, and shoulders, to alleviate pain and improve mobility.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other treatment modalities to enhance its effectiveness in improving joint health. This may include integration with physical therapy exercises, heat therapy, or other complementary treatments. The synergistic approach aims to provide more comprehensive care for joint-related issues.
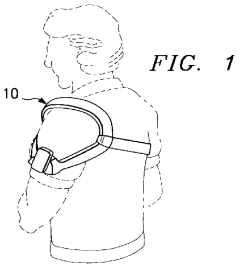
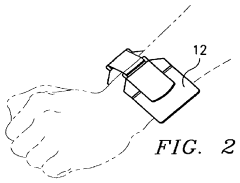
- Wearable PEMF devices for continuous treatment: Wearable PEMF devices allow for continuous or extended treatment of joint issues. These devices can be worn on specific body parts, providing targeted therapy throughout the day. The portability and ease of use of these wearable devices make it convenient for patients to receive consistent PEMF therapy for joint health maintenance.
- Customizable PEMF protocols for joint health: PEMF therapy systems with customizable treatment protocols are developed to address specific joint health needs. These systems allow for adjustments in frequency, intensity, and duration of the electromagnetic pulses based on the patient's condition and response to treatment. The ability to tailor the therapy enhances its effectiveness in managing various joint health issues.
- PEMF therapy for post-surgical joint recovery: PEMF therapy is utilized in post-surgical recovery for joint procedures. The therapy aids in reducing inflammation, promoting healing, and accelerating the rehabilitation process after joint surgeries. This application of PEMF can help patients regain mobility and strength in the affected joints more quickly.
02 Wearable PEMF devices for continuous joint therapy
Wearable PEMF devices allow for continuous therapy application to specific joint areas. These devices are designed to be compact, portable, and easy to use, enabling patients to receive treatment while going about their daily activities. They can be worn on various body parts, such as knees, shoulders, or wrists, providing targeted therapy for joint health.Expand Specific Solutions03 Combination of PEMF therapy with other treatment modalities
PEMF therapy can be combined with other treatment modalities to enhance joint health outcomes. This may include integration with physical therapy exercises, heat therapy, or other complementary treatments. The synergistic effect of combining PEMF with other therapies can potentially lead to improved joint function and reduced pain.Expand Specific Solutions04 Customizable PEMF treatment protocols for joint health
Advanced PEMF devices offer customizable treatment protocols tailored to specific joint health needs. These systems allow for adjustments in frequency, intensity, and duration of electromagnetic pulses based on the patient's condition and response to therapy. This personalized approach aims to optimize the effectiveness of PEMF therapy for individual joint health concerns.Expand Specific Solutions05 PEMF therapy for post-surgical joint recovery
PEMF therapy is utilized in post-surgical joint recovery to accelerate healing and reduce complications. The electromagnetic fields can help reduce inflammation, promote tissue repair, and improve circulation in the affected joint area. This application of PEMF therapy aims to enhance the recovery process and restore joint function more quickly after surgical interventions.Expand Specific Solutions
Key Players in PEMF Device Industry
The PEMF therapy market for joint health is in a growth phase, with increasing adoption and technological advancements. The market size is expanding due to rising awareness of non-invasive treatments and an aging population. Technologically, PEMF therapy is maturing, with companies like Venus Concept Ltd., Regenesis Biomedical, Inc., and SofPulse, Inc. leading innovation. Research institutions such as the National University of Singapore and Swiss Federal Institute of Technology are contributing to scientific advancements. The competitive landscape includes established medical device manufacturers like Orthofix US LLC and Medtronic AF Luxembourg SARL, alongside specialized PEMF companies such as Biomagnetic Sciences LLC and OrthoCor Medical, Inc., indicating a diverse and dynamic market with potential for further growth and technological refinement.
Venus Concept Ltd.
Technical Solution: Venus Concept Ltd. has developed a proprietary PEMF technology called Venus Pulse™ for joint health improvement. Their approach combines PEMF with other modalities such as radiofrequency and magnetic pulse technology. The Venus Pulse™ system delivers low-frequency pulsed electromagnetic fields to stimulate cellular repair and regeneration in joint tissues. The therapy is designed to increase blood flow, reduce inflammation, and promote the production of growth factors in the affected areas [1][3]. The company's devices offer customizable treatment parameters, allowing practitioners to tailor the therapy to individual patient needs.
Strengths: Multi-modal approach combining PEMF with other technologies; customizable treatment parameters. Weaknesses: Limited to clinical settings; may require multiple sessions for optimal results.
Regenesis Biomedical, Inc.
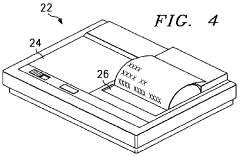
Technical Solution: Regenesis Biomedical has developed the Provant® Therapy System, a PEMF device specifically designed for joint health and pain management. The system utilizes a proprietary pulsed radiofrequency energy (PRFE) technology that emits non-thermal electromagnetic energy to stimulate cellular repair and reduce inflammation in joint tissues. The Provant® system operates at a frequency of 27.12 MHz, which has been shown to effectively penetrate deep into joint structures [2][4]. The therapy is non-invasive and can be self-administered by patients at home, typically for 30-minute sessions twice daily. Clinical studies have demonstrated significant improvements in joint pain, stiffness, and function, particularly in osteoarthritis patients.
Strengths: Home-use device; clinically proven efficacy; non-invasive treatment. Weaknesses: Requires consistent use for optimal results; may not be suitable for all types of joint conditions.
Core PEMF Innovations for Joint Treatment
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
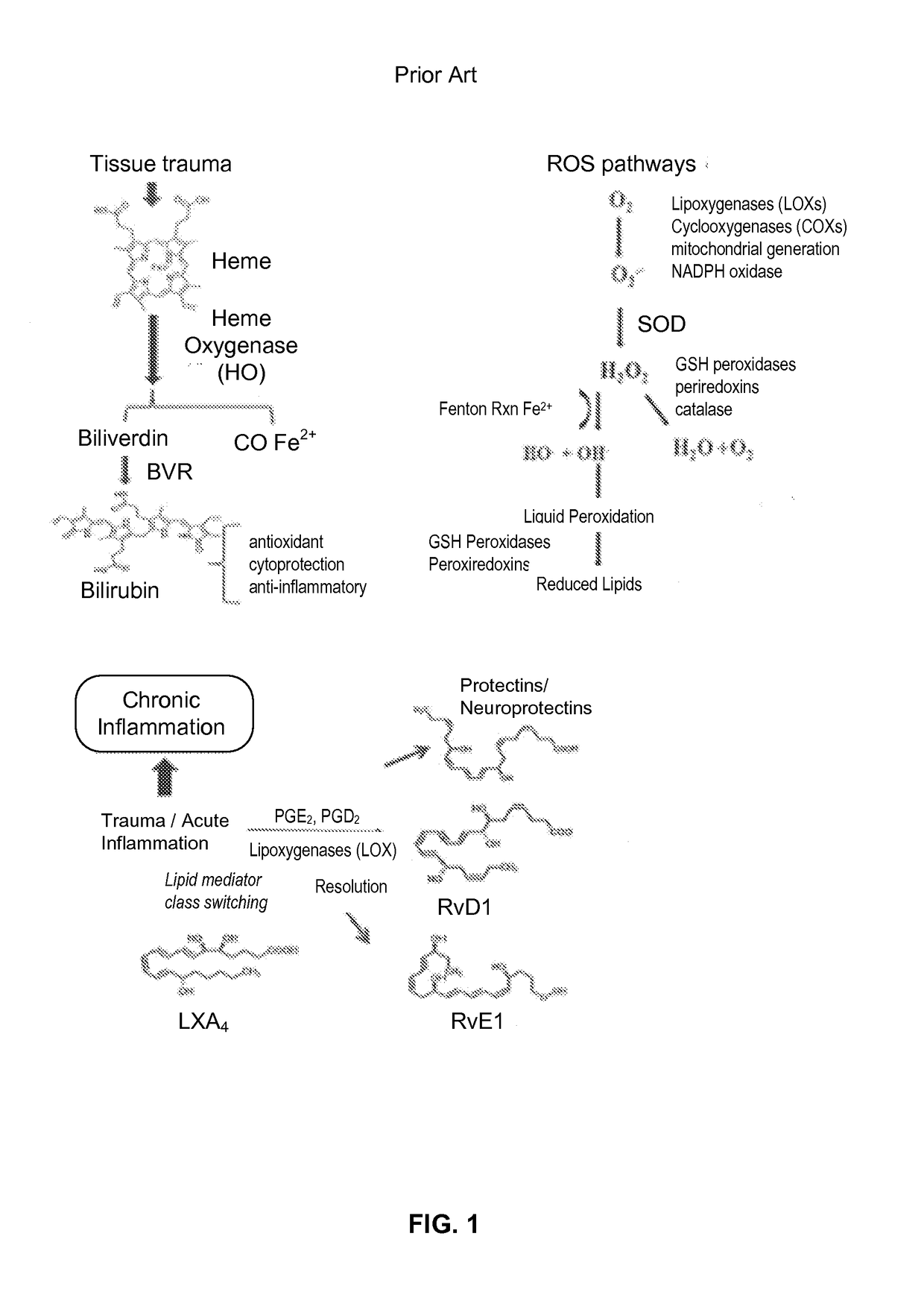
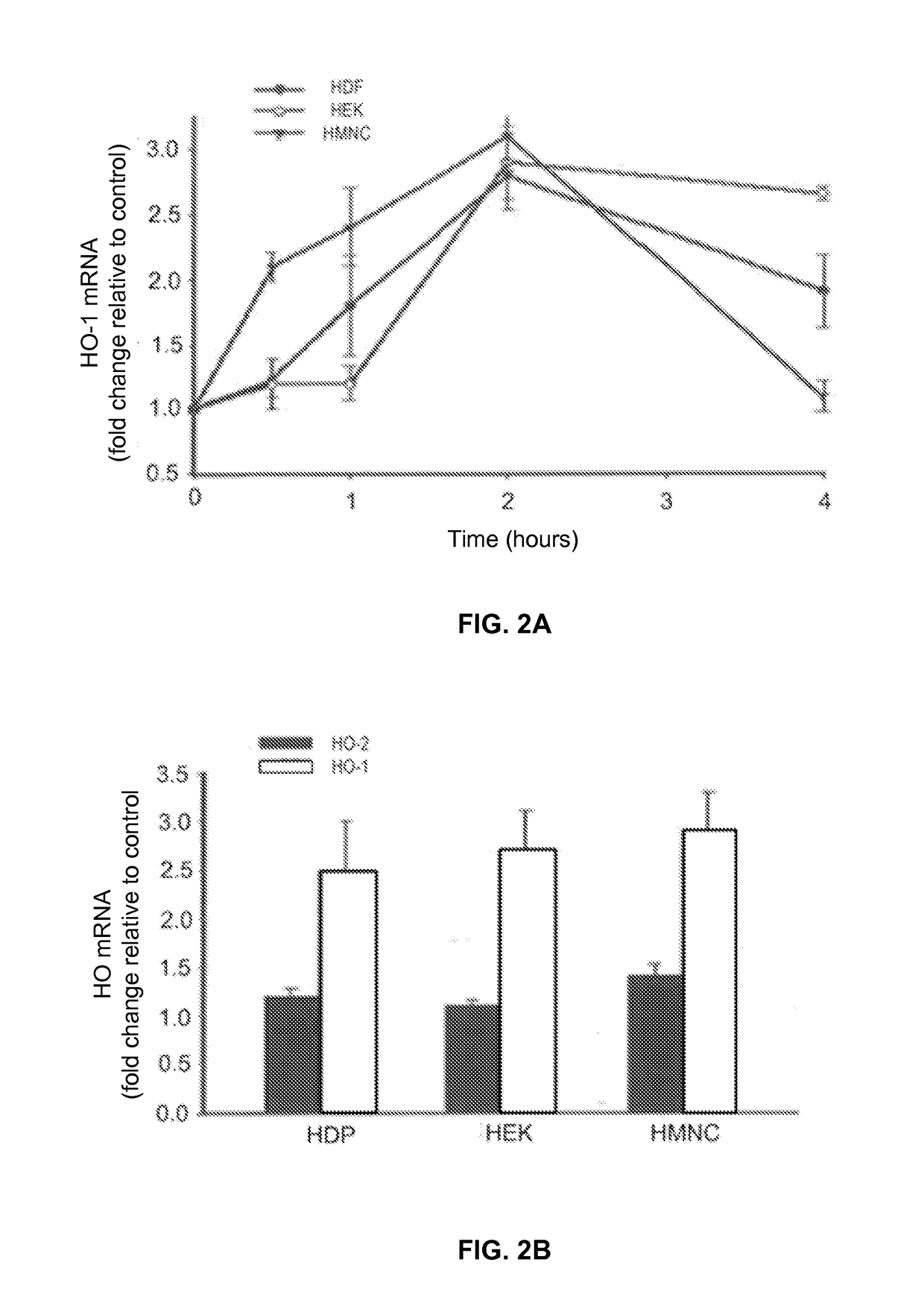
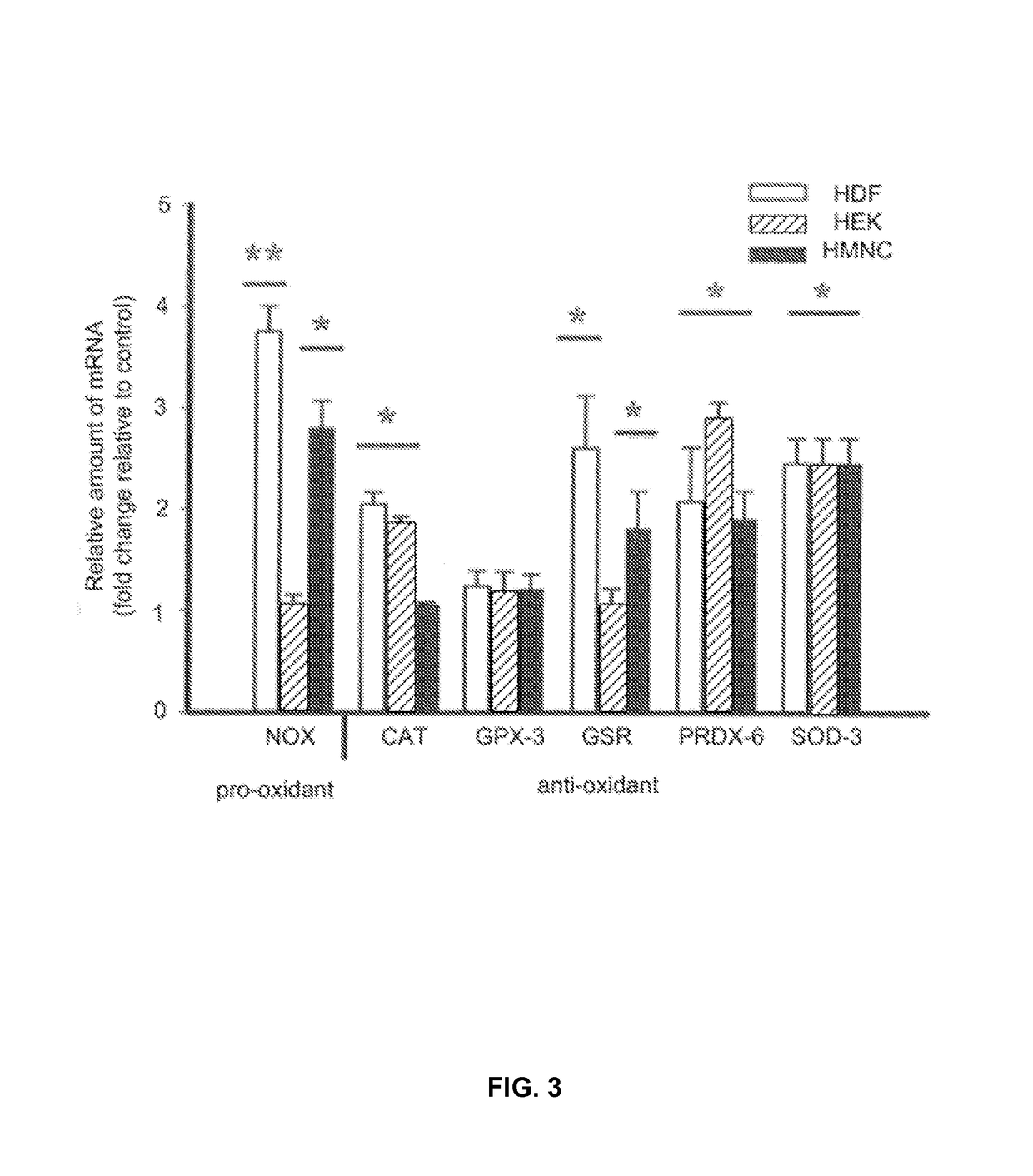
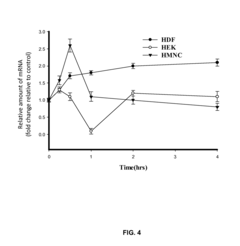
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Pulsed electromagnetic field (PEMF) stimulation therapy system with bi-phasic coil
PatentInactiveUS6132362A
Innovation
- A PEMF therapy system with a high-efficiency single-coil transducer that recovers flyback energy and uses an energy recovery capacitance circuit to sequence current in both directions, eliminating the need for a secondary coil, thereby reducing weight, power consumption, and manufacturing complexity.
Safety and Regulatory Considerations
The utilization of Pulsed Electromagnetic Field (PEMF) therapy for improved joint health requires careful consideration of safety and regulatory aspects. PEMF devices are classified as medical devices in many jurisdictions, necessitating compliance with stringent regulatory standards. In the United States, the Food and Drug Administration (FDA) oversees the approval and regulation of PEMF devices, categorizing them based on their intended use and potential risks.
FDA-cleared PEMF devices for joint health applications typically fall under Class II medical devices, requiring manufacturers to demonstrate safety and efficacy through clinical trials and rigorous testing. These devices must adhere to specific output parameters, including frequency ranges, magnetic field strengths, and exposure durations, to ensure patient safety while delivering therapeutic benefits.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Health Canada have established similar frameworks for PEMF device approval and use. Manufacturers must obtain CE marking in the European Union, demonstrating compliance with essential health and safety requirements.
Safety considerations for PEMF therapy extend beyond regulatory compliance. Practitioners and users must be aware of potential contraindications, such as pregnancy, active bleeding, or the presence of electronic implants like pacemakers. Proper training and education for healthcare providers and patients are crucial to ensure safe and effective use of PEMF devices for joint health improvement.
Long-term safety studies have generally shown PEMF therapy to be well-tolerated, with minimal reported side effects. However, ongoing research continues to evaluate potential long-term impacts, particularly for extended or high-intensity use. As with any medical intervention, individual patient factors must be considered, and treatment protocols should be tailored accordingly.
Quality control measures in PEMF device manufacturing play a vital role in maintaining safety standards. Regular calibration, maintenance, and testing of devices are essential to ensure consistent and accurate electromagnetic field delivery. Additionally, post-market surveillance and reporting systems help identify and address any unforeseen safety issues that may arise during widespread clinical use.
As PEMF technology evolves, regulatory frameworks must adapt to accommodate new device designs and treatment modalities. This includes addressing emerging concerns such as potential electromagnetic interference with other medical devices or electronic equipment in clinical settings. Collaborative efforts between manufacturers, regulatory bodies, and healthcare professionals are crucial to developing and implementing updated safety guidelines and best practices for PEMF therapy in joint health applications.
FDA-cleared PEMF devices for joint health applications typically fall under Class II medical devices, requiring manufacturers to demonstrate safety and efficacy through clinical trials and rigorous testing. These devices must adhere to specific output parameters, including frequency ranges, magnetic field strengths, and exposure durations, to ensure patient safety while delivering therapeutic benefits.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Health Canada have established similar frameworks for PEMF device approval and use. Manufacturers must obtain CE marking in the European Union, demonstrating compliance with essential health and safety requirements.
Safety considerations for PEMF therapy extend beyond regulatory compliance. Practitioners and users must be aware of potential contraindications, such as pregnancy, active bleeding, or the presence of electronic implants like pacemakers. Proper training and education for healthcare providers and patients are crucial to ensure safe and effective use of PEMF devices for joint health improvement.
Long-term safety studies have generally shown PEMF therapy to be well-tolerated, with minimal reported side effects. However, ongoing research continues to evaluate potential long-term impacts, particularly for extended or high-intensity use. As with any medical intervention, individual patient factors must be considered, and treatment protocols should be tailored accordingly.
Quality control measures in PEMF device manufacturing play a vital role in maintaining safety standards. Regular calibration, maintenance, and testing of devices are essential to ensure consistent and accurate electromagnetic field delivery. Additionally, post-market surveillance and reporting systems help identify and address any unforeseen safety issues that may arise during widespread clinical use.
As PEMF technology evolves, regulatory frameworks must adapt to accommodate new device designs and treatment modalities. This includes addressing emerging concerns such as potential electromagnetic interference with other medical devices or electronic equipment in clinical settings. Collaborative efforts between manufacturers, regulatory bodies, and healthcare professionals are crucial to developing and implementing updated safety guidelines and best practices for PEMF therapy in joint health applications.
Cost-Effectiveness Analysis
The cost-effectiveness analysis of PEMF therapy for improved joint health reveals a complex interplay between initial investment, long-term benefits, and potential healthcare savings. Initial costs for PEMF devices can range from $200 to $3000, depending on the quality and features of the equipment. However, these upfront expenses should be weighed against the potential reduction in long-term healthcare costs associated with joint problems.
Studies have shown that regular use of PEMF therapy can lead to a decrease in pain medication consumption, fewer doctor visits, and reduced need for invasive procedures such as joint injections or surgeries. For instance, a randomized controlled trial involving osteoarthritis patients demonstrated a 50% reduction in pain medication use over a 6-month period, translating to significant cost savings for both patients and healthcare systems.
When comparing PEMF therapy to traditional treatments, the cost-effectiveness becomes more apparent. While conventional treatments like physical therapy or regular pain medication may provide short-term relief, they often require ongoing expenses. In contrast, PEMF therapy offers a one-time investment with potentially years of benefits, making it more cost-effective in the long run.
Furthermore, the non-invasive nature of PEMF therapy reduces the risk of complications and associated healthcare costs. This is particularly relevant for elderly patients or those with comorbidities who may be at higher risk for surgical interventions.
From an employer's perspective, implementing PEMF therapy in workplace wellness programs could lead to reduced absenteeism and increased productivity among employees suffering from joint issues. A cost-benefit analysis conducted by a large corporation showed a return on investment of 3:1 over two years after introducing PEMF devices in their ergonomic support program.
However, it's important to note that the cost-effectiveness of PEMF therapy can vary depending on individual factors such as the severity of joint issues, frequency of use, and adherence to treatment protocols. Additionally, as the technology continues to evolve, we may see more affordable and efficient PEMF devices entering the market, further improving the cost-effectiveness ratio.
In conclusion, while the initial investment in PEMF therapy may seem substantial, the potential for long-term cost savings in medication, medical procedures, and improved quality of life makes it a compelling option for those seeking to improve joint health. As more research emerges and technology advances, the cost-effectiveness of PEMF therapy is likely to become even more favorable.
Studies have shown that regular use of PEMF therapy can lead to a decrease in pain medication consumption, fewer doctor visits, and reduced need for invasive procedures such as joint injections or surgeries. For instance, a randomized controlled trial involving osteoarthritis patients demonstrated a 50% reduction in pain medication use over a 6-month period, translating to significant cost savings for both patients and healthcare systems.
When comparing PEMF therapy to traditional treatments, the cost-effectiveness becomes more apparent. While conventional treatments like physical therapy or regular pain medication may provide short-term relief, they often require ongoing expenses. In contrast, PEMF therapy offers a one-time investment with potentially years of benefits, making it more cost-effective in the long run.
Furthermore, the non-invasive nature of PEMF therapy reduces the risk of complications and associated healthcare costs. This is particularly relevant for elderly patients or those with comorbidities who may be at higher risk for surgical interventions.
From an employer's perspective, implementing PEMF therapy in workplace wellness programs could lead to reduced absenteeism and increased productivity among employees suffering from joint issues. A cost-benefit analysis conducted by a large corporation showed a return on investment of 3:1 over two years after introducing PEMF devices in their ergonomic support program.
However, it's important to note that the cost-effectiveness of PEMF therapy can vary depending on individual factors such as the severity of joint issues, frequency of use, and adherence to treatment protocols. Additionally, as the technology continues to evolve, we may see more affordable and efficient PEMF devices entering the market, further improving the cost-effectiveness ratio.
In conclusion, while the initial investment in PEMF therapy may seem substantial, the potential for long-term cost savings in medication, medical procedures, and improved quality of life makes it a compelling option for those seeking to improve joint health. As more research emerges and technology advances, the cost-effectiveness of PEMF therapy is likely to become even more favorable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!