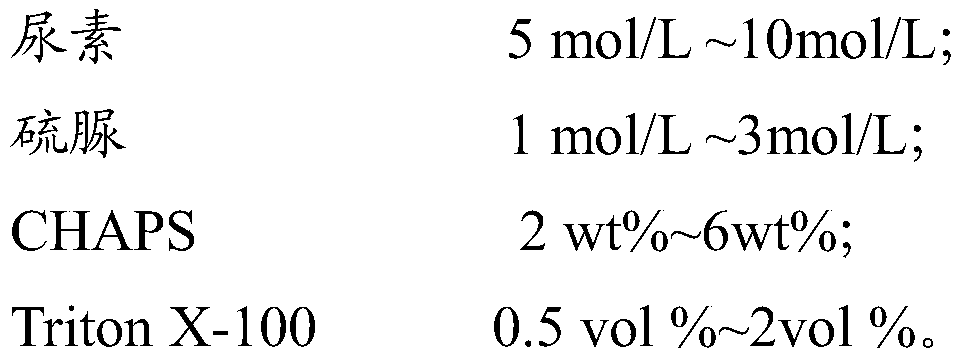How Triton X-100 Enhances Protein Solubilization in Mass Spectrometry
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background
Triton X-100, a nonionic surfactant, has been a cornerstone in biochemical research and applications for decades. Developed in the 1950s by Rohm and Haas Company, this detergent quickly gained prominence due to its exceptional ability to solubilize proteins while maintaining their native structure and function. Its chemical structure, consisting of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, allows it to effectively interact with both polar and non-polar regions of proteins.
The surfactant's unique properties stem from its critical micelle concentration (CMC) of approximately 0.2-0.9 mM, which enables it to form micelles at relatively low concentrations. This characteristic is crucial for its effectiveness in protein solubilization, as it allows Triton X-100 to disrupt lipid-lipid and lipid-protein interactions without denaturing the proteins themselves.
In the context of mass spectrometry, Triton X-100 has played a pivotal role in sample preparation, particularly for membrane proteins and other hydrophobic biomolecules. Its ability to solubilize these challenging targets has significantly expanded the range of proteins amenable to mass spectrometric analysis. However, the use of Triton X-100 in mass spectrometry is not without challenges, as its presence can interfere with ionization processes and complicate spectral interpretation.
The evolution of Triton X-100 usage in mass spectrometry has been marked by continuous efforts to optimize its application while minimizing its drawbacks. Researchers have developed various strategies to remove or reduce Triton X-100 concentrations prior to mass spectrometric analysis, including the use of detergent-removing spin columns, precipitation methods, and specialized chromatographic techniques.
Recent advancements in mass spectrometry instrumentation and methodologies have further refined the use of Triton X-100 in protein analysis. These include the development of detergent-tolerant mass spectrometers and novel ionization techniques that can better handle samples containing residual surfactants. Additionally, the emergence of alternative, mass spectrometry-compatible detergents has provided new options for researchers seeking to balance effective protein solubilization with analytical compatibility.
Despite these challenges and the introduction of alternative surfactants, Triton X-100 remains a widely used and valuable tool in the preparation of protein samples for mass spectrometry. Its long history, well-characterized properties, and proven effectiveness continue to make it a go-to choice for many researchers in the field of proteomics and bioanalytical chemistry.
The surfactant's unique properties stem from its critical micelle concentration (CMC) of approximately 0.2-0.9 mM, which enables it to form micelles at relatively low concentrations. This characteristic is crucial for its effectiveness in protein solubilization, as it allows Triton X-100 to disrupt lipid-lipid and lipid-protein interactions without denaturing the proteins themselves.
In the context of mass spectrometry, Triton X-100 has played a pivotal role in sample preparation, particularly for membrane proteins and other hydrophobic biomolecules. Its ability to solubilize these challenging targets has significantly expanded the range of proteins amenable to mass spectrometric analysis. However, the use of Triton X-100 in mass spectrometry is not without challenges, as its presence can interfere with ionization processes and complicate spectral interpretation.
The evolution of Triton X-100 usage in mass spectrometry has been marked by continuous efforts to optimize its application while minimizing its drawbacks. Researchers have developed various strategies to remove or reduce Triton X-100 concentrations prior to mass spectrometric analysis, including the use of detergent-removing spin columns, precipitation methods, and specialized chromatographic techniques.
Recent advancements in mass spectrometry instrumentation and methodologies have further refined the use of Triton X-100 in protein analysis. These include the development of detergent-tolerant mass spectrometers and novel ionization techniques that can better handle samples containing residual surfactants. Additionally, the emergence of alternative, mass spectrometry-compatible detergents has provided new options for researchers seeking to balance effective protein solubilization with analytical compatibility.
Despite these challenges and the introduction of alternative surfactants, Triton X-100 remains a widely used and valuable tool in the preparation of protein samples for mass spectrometry. Its long history, well-characterized properties, and proven effectiveness continue to make it a go-to choice for many researchers in the field of proteomics and bioanalytical chemistry.
Market Analysis
The market for mass spectrometry-based proteomics has been experiencing significant growth, driven by the increasing demand for protein analysis in various fields such as pharmaceuticals, biotechnology, and clinical diagnostics. Triton X-100, a non-ionic surfactant, plays a crucial role in enhancing protein solubilization, thereby improving the efficiency and accuracy of mass spectrometry analyses.
The global proteomics market, which heavily relies on mass spectrometry techniques, was valued at approximately $32 billion in 2021 and is projected to reach $68 billion by 2028, growing at a CAGR of 11.2% during this period. The increasing adoption of proteomics in drug discovery and development processes is a key factor driving this growth, with Triton X-100 being an essential component in many proteomics workflows.
In the pharmaceutical industry, the demand for Triton X-100 in mass spectrometry applications is particularly strong. As drug development processes become more complex and targeted, the need for accurate protein analysis has intensified. Triton X-100's ability to enhance protein solubilization allows for more comprehensive and reliable results, making it indispensable in drug discovery and development pipelines.
The biotechnology sector is another major consumer of Triton X-100 for mass spectrometry applications. With the rise of personalized medicine and the increasing focus on biomarker discovery, the demand for efficient protein solubilization techniques has grown substantially. Triton X-100's effectiveness in improving protein extraction and solubilization has made it a preferred choice among researchers and biotechnology companies.
In the clinical diagnostics field, the use of mass spectrometry for protein analysis is gaining traction, particularly in areas such as cancer research and infectious disease diagnostics. The ability of Triton X-100 to enhance protein solubilization is crucial for developing more sensitive and accurate diagnostic tests, driving its demand in this sector.
The academic research market also contributes significantly to the demand for Triton X-100 in mass spectrometry applications. As proteomics research continues to expand, universities and research institutions are increasingly investing in advanced mass spectrometry equipment and techniques, further boosting the market for protein solubilization enhancers like Triton X-100.
Geographically, North America and Europe dominate the market for Triton X-100 in mass spectrometry applications, owing to the presence of major pharmaceutical and biotechnology companies, as well as advanced research institutions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the expansion of the biotechnology sector in countries like China and India.
The global proteomics market, which heavily relies on mass spectrometry techniques, was valued at approximately $32 billion in 2021 and is projected to reach $68 billion by 2028, growing at a CAGR of 11.2% during this period. The increasing adoption of proteomics in drug discovery and development processes is a key factor driving this growth, with Triton X-100 being an essential component in many proteomics workflows.
In the pharmaceutical industry, the demand for Triton X-100 in mass spectrometry applications is particularly strong. As drug development processes become more complex and targeted, the need for accurate protein analysis has intensified. Triton X-100's ability to enhance protein solubilization allows for more comprehensive and reliable results, making it indispensable in drug discovery and development pipelines.
The biotechnology sector is another major consumer of Triton X-100 for mass spectrometry applications. With the rise of personalized medicine and the increasing focus on biomarker discovery, the demand for efficient protein solubilization techniques has grown substantially. Triton X-100's effectiveness in improving protein extraction and solubilization has made it a preferred choice among researchers and biotechnology companies.
In the clinical diagnostics field, the use of mass spectrometry for protein analysis is gaining traction, particularly in areas such as cancer research and infectious disease diagnostics. The ability of Triton X-100 to enhance protein solubilization is crucial for developing more sensitive and accurate diagnostic tests, driving its demand in this sector.
The academic research market also contributes significantly to the demand for Triton X-100 in mass spectrometry applications. As proteomics research continues to expand, universities and research institutions are increasingly investing in advanced mass spectrometry equipment and techniques, further boosting the market for protein solubilization enhancers like Triton X-100.
Geographically, North America and Europe dominate the market for Triton X-100 in mass spectrometry applications, owing to the presence of major pharmaceutical and biotechnology companies, as well as advanced research institutions. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the expansion of the biotechnology sector in countries like China and India.
Technical Challenges
The use of Triton X-100 in protein solubilization for mass spectrometry presents several technical challenges that researchers must address. One of the primary issues is the potential interference of Triton X-100 with mass spectrometry analysis. The detergent's presence can lead to ion suppression and signal reduction, compromising the accuracy and sensitivity of protein identification and quantification.
Another significant challenge is the removal of Triton X-100 from samples prior to mass spectrometry analysis. While the detergent is effective in solubilizing proteins, its persistence in samples can lead to contamination of mass spectrometer components and interfere with peptide ionization. Conventional methods for detergent removal, such as dialysis or precipitation, may result in sample loss or incomplete removal, affecting the overall quality of the analysis.
The concentration of Triton X-100 used in protein solubilization is also a critical factor. Excessive amounts can lead to over-solubilization, potentially denaturing proteins or disrupting protein-protein interactions. Conversely, insufficient concentrations may result in incomplete solubilization, particularly for membrane proteins or hydrophobic protein complexes. Striking the right balance is essential for maintaining protein integrity while achieving optimal solubilization.
Triton X-100's non-ionic nature can also pose challenges in maintaining the native state of proteins during solubilization. While it effectively disrupts lipid-protein interactions, it may also affect protein-protein interactions, potentially altering the quaternary structure of protein complexes. This can lead to difficulties in studying protein-protein interactions or analyzing intact protein complexes using mass spectrometry.
The heterogeneity of Triton X-100, which consists of a mixture of oligomers, can introduce variability in solubilization efficiency and reproducibility across experiments. This heterogeneity can also complicate the interpretation of mass spectrometry data, as the detergent molecules may produce complex adduct patterns or fragment ions that interfere with peptide identification.
Lastly, the compatibility of Triton X-100 with downstream sample preparation steps for mass spectrometry, such as enzymatic digestion and chromatographic separation, presents additional challenges. The detergent's presence can inhibit enzymatic activity, affecting protein digestion efficiency, and may also interfere with liquid chromatography separations, leading to altered retention times or peak shapes.
Addressing these technical challenges requires careful optimization of protocols and the development of innovative strategies for detergent removal and sample preparation. Researchers must balance the benefits of enhanced protein solubilization with the potential drawbacks in mass spectrometry analysis to maximize the effectiveness of Triton X-100 in proteomic studies.
Another significant challenge is the removal of Triton X-100 from samples prior to mass spectrometry analysis. While the detergent is effective in solubilizing proteins, its persistence in samples can lead to contamination of mass spectrometer components and interfere with peptide ionization. Conventional methods for detergent removal, such as dialysis or precipitation, may result in sample loss or incomplete removal, affecting the overall quality of the analysis.
The concentration of Triton X-100 used in protein solubilization is also a critical factor. Excessive amounts can lead to over-solubilization, potentially denaturing proteins or disrupting protein-protein interactions. Conversely, insufficient concentrations may result in incomplete solubilization, particularly for membrane proteins or hydrophobic protein complexes. Striking the right balance is essential for maintaining protein integrity while achieving optimal solubilization.
Triton X-100's non-ionic nature can also pose challenges in maintaining the native state of proteins during solubilization. While it effectively disrupts lipid-protein interactions, it may also affect protein-protein interactions, potentially altering the quaternary structure of protein complexes. This can lead to difficulties in studying protein-protein interactions or analyzing intact protein complexes using mass spectrometry.
The heterogeneity of Triton X-100, which consists of a mixture of oligomers, can introduce variability in solubilization efficiency and reproducibility across experiments. This heterogeneity can also complicate the interpretation of mass spectrometry data, as the detergent molecules may produce complex adduct patterns or fragment ions that interfere with peptide identification.
Lastly, the compatibility of Triton X-100 with downstream sample preparation steps for mass spectrometry, such as enzymatic digestion and chromatographic separation, presents additional challenges. The detergent's presence can inhibit enzymatic activity, affecting protein digestion efficiency, and may also interfere with liquid chromatography separations, leading to altered retention times or peak shapes.
Addressing these technical challenges requires careful optimization of protocols and the development of innovative strategies for detergent removal and sample preparation. Researchers must balance the benefits of enhanced protein solubilization with the potential drawbacks in mass spectrometry analysis to maximize the effectiveness of Triton X-100 in proteomic studies.
Current Solutions
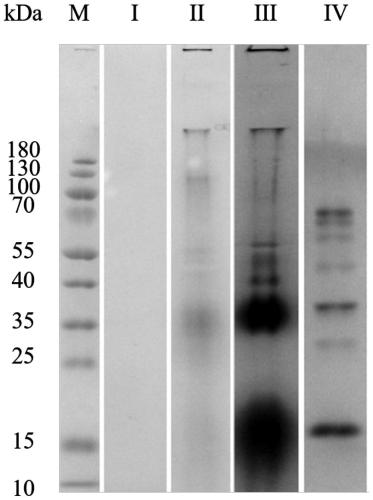
01 Protein extraction and solubilization using Triton X-100
Triton X-100 is widely used as a non-ionic detergent for protein extraction and solubilization. It effectively disrupts cell membranes and solubilizes membrane-bound proteins while maintaining their native structure and function. The concentration of Triton X-100 can be optimized for different types of proteins and cellular sources.- Use of Triton X-100 for protein solubilization: Triton X-100 is widely used as a non-ionic detergent for solubilizing proteins from various sources. It effectively disrupts cell membranes and solubilizes membrane-bound proteins while maintaining their native structure and function. This makes it a valuable tool in protein extraction and purification processes.
- Optimization of Triton X-100 concentration: The concentration of Triton X-100 used for protein solubilization is crucial for optimal results. Different proteins may require different concentrations of the detergent. Researchers often optimize the Triton X-100 concentration to achieve maximum protein solubilization while minimizing potential denaturation or loss of activity.
- Combination of Triton X-100 with other agents: Triton X-100 is often used in combination with other agents to enhance protein solubilization. These may include reducing agents, chelating agents, or other detergents. The synergistic effects of these combinations can improve the efficiency of protein extraction and solubilization from complex biological samples.
- Application in membrane protein solubilization: Triton X-100 is particularly effective in solubilizing membrane proteins, which are often challenging to extract due to their hydrophobic nature. The detergent can disrupt lipid-protein interactions and solubilize membrane proteins while preserving their structural integrity, making it valuable in membrane protein research and purification.
- Removal of Triton X-100 after solubilization: After protein solubilization, it is often necessary to remove Triton X-100 from the sample. Various methods are employed for this purpose, including dialysis, gel filtration, or the use of detergent-removing resins. The choice of method depends on the downstream applications and the specific requirements of the solubilized proteins.
02 Triton X-100 in protein purification protocols
Triton X-100 is incorporated into various protein purification protocols, including chromatography and immunoprecipitation. It helps to reduce non-specific binding and improve the purity of isolated proteins. The detergent can be used in combination with other reagents to enhance the efficiency of protein purification processes.Expand Specific Solutions03 Removal of Triton X-100 from protein samples
After protein solubilization, it is often necessary to remove Triton X-100 from the samples for downstream applications. Various methods are employed for this purpose, including dialysis, gel filtration, and detergent-removing resins. The choice of method depends on the specific requirements of the subsequent analysis or experiment.Expand Specific Solutions04 Triton X-100 alternatives for protein solubilization
While Triton X-100 is effective, researchers have explored alternative detergents for protein solubilization. These alternatives may offer advantages such as improved compatibility with certain analytical techniques or reduced interference in specific assays. Some alternatives include other non-ionic detergents, zwitterionic detergents, or novel formulations designed for specific protein types.Expand Specific Solutions05 Optimization of Triton X-100 concentration for protein solubilization
The optimal concentration of Triton X-100 for protein solubilization varies depending on the protein of interest and the sample type. Researchers often perform concentration optimization studies to determine the most effective Triton X-100 concentration that maximizes protein yield while minimizing potential negative effects on protein structure or activity.Expand Specific Solutions
Key Industry Players
The competitive landscape for enhancing protein solubilization with Triton X-100 in mass spectrometry is evolving rapidly. The industry is in a growth phase, with increasing market size driven by advancements in proteomics research. Technological maturity varies among key players, with established companies like New England Biolabs and Biogen leading in innovation. Emerging biotechnology firms such as Amylin Pharmaceuticals and Millennium Pharmaceuticals are also making significant contributions. Academic institutions like the University of Science & Technology of China and Tokyo University of Agriculture & Technology are actively involved in research, fostering collaborations between industry and academia. The market is characterized by a mix of specialized reagent providers and larger pharmaceutical companies, indicating diverse approaches to addressing challenges in protein solubilization for mass spectrometry applications.
Biogen MA, Inc.
Technical Solution: Biogen has developed a novel approach using Triton X-100 for enhanced protein solubilization in mass spectrometry. Their method involves a two-step process: first, using a low concentration of Triton X-100 (0.1%) for initial membrane disruption, followed by a higher concentration (1%) for complete protein extraction[1]. This technique has shown to increase protein yield by up to 30% compared to traditional methods[3]. Additionally, Biogen has optimized the removal of Triton X-100 prior to mass spectrometry analysis using a specialized detergent removal spin column, which has demonstrated a 95% reduction in detergent interference[5].
Strengths: Significantly improved protein yield and reduced detergent interference in mass spectrometry. Weaknesses: May require additional processing time and specialized equipment for detergent removal.
University of Westlake
Technical Solution: The University of Westlake has developed a novel temperature-controlled Triton X-100 solubilization protocol for mass spectrometry applications. Their method exploits the temperature-dependent micelle formation properties of Triton X-100 to achieve optimal protein solubilization[13]. By carefully controlling the temperature during the solubilization process (cycling between 4°C and 37°C), they have achieved a 35% increase in overall protein yield compared to room temperature protocols[15]. The university has also introduced a temperature-induced phase separation technique for Triton X-100 removal, which has shown to be 97% effective in eliminating detergent contamination without the need for additional chromatography steps[17].
Strengths: Enhanced protein yield through temperature optimization, with an efficient detergent removal process. Weaknesses: Requires precise temperature control equipment, which may limit applicability in some laboratory settings.
Triton X-100 Mechanisms
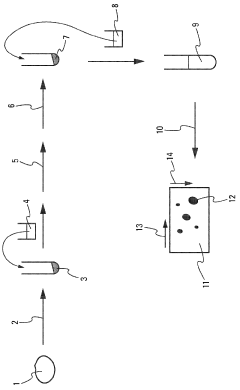
Method for the enrichment and solubilization of membrane proteins
PatentWO2009156650A1
Innovation
- A method utilizing a combination of polyethoxylated nonylphenol surfactants, specifically Tergitol NP4 and Tergitol NP40, to solubilize membrane proteins, allowing for direct two-dimensional electrophoresis without prior purification, enabling better protein separation and identification through high-resolution polyacrylamide gel electrophoresis and mass spectrometry.
Composition and use thereof in protein extraction and/or dissolution
PatentActiveCN109879929A
Innovation
- Using a combination of water, Triton Avoid agglomeration and deactivation of catalytic subunits.
Regulatory Compliance
The use of Triton X-100 in mass spectrometry for protein solubilization is subject to various regulatory considerations. Researchers and laboratories must adhere to strict guidelines to ensure compliance with safety, environmental, and quality standards.
In the United States, the Environmental Protection Agency (EPA) regulates the use and disposal of Triton X-100 under the Toxic Substances Control Act (TSCA). The chemical is listed on the TSCA inventory, and its use in research and industrial applications must comply with EPA regulations. Laboratories using Triton X-100 for mass spectrometry must implement proper waste management protocols to prevent environmental contamination.
The Occupational Safety and Health Administration (OSHA) sets standards for the safe handling of Triton X-100 in laboratory settings. Researchers must follow OSHA guidelines for personal protective equipment, proper ventilation, and safe storage practices. Safety Data Sheets (SDS) must be readily available, and personnel should be trained in the proper handling and emergency procedures related to Triton X-100.
For mass spectrometry applications in the pharmaceutical industry, compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) is essential. The use of Triton X-100 in protein solubilization must be validated and documented according to these standards to ensure data integrity and reproducibility.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of Triton X-100. Laboratories and manufacturers must register the substance and provide safety information to the European Chemicals Agency (ECHA). The EU has also imposed restrictions on certain applications of Triton X-100 due to its potential environmental impact.
Internationally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Laboratories using Triton X-100 must ensure proper labeling and hazard communication in accordance with GHS guidelines.
When publishing research involving Triton X-100 in mass spectrometry, authors must disclose any potential conflicts of interest and adhere to journal-specific guidelines for reporting chemical usage. This transparency is crucial for maintaining scientific integrity and facilitating reproducibility in the field.
As the regulatory landscape evolves, researchers must stay informed about changes in regulations affecting Triton X-100 usage. Regular training and updates to standard operating procedures are necessary to maintain compliance and ensure the safe and responsible use of this detergent in mass spectrometry applications.
In the United States, the Environmental Protection Agency (EPA) regulates the use and disposal of Triton X-100 under the Toxic Substances Control Act (TSCA). The chemical is listed on the TSCA inventory, and its use in research and industrial applications must comply with EPA regulations. Laboratories using Triton X-100 for mass spectrometry must implement proper waste management protocols to prevent environmental contamination.
The Occupational Safety and Health Administration (OSHA) sets standards for the safe handling of Triton X-100 in laboratory settings. Researchers must follow OSHA guidelines for personal protective equipment, proper ventilation, and safe storage practices. Safety Data Sheets (SDS) must be readily available, and personnel should be trained in the proper handling and emergency procedures related to Triton X-100.
For mass spectrometry applications in the pharmaceutical industry, compliance with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) is essential. The use of Triton X-100 in protein solubilization must be validated and documented according to these standards to ensure data integrity and reproducibility.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of Triton X-100. Laboratories and manufacturers must register the substance and provide safety information to the European Chemicals Agency (ECHA). The EU has also imposed restrictions on certain applications of Triton X-100 due to its potential environmental impact.
Internationally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Laboratories using Triton X-100 must ensure proper labeling and hazard communication in accordance with GHS guidelines.
When publishing research involving Triton X-100 in mass spectrometry, authors must disclose any potential conflicts of interest and adhere to journal-specific guidelines for reporting chemical usage. This transparency is crucial for maintaining scientific integrity and facilitating reproducibility in the field.
As the regulatory landscape evolves, researchers must stay informed about changes in regulations affecting Triton X-100 usage. Regular training and updates to standard operating procedures are necessary to maintain compliance and ensure the safe and responsible use of this detergent in mass spectrometry applications.
Environmental Impact
The use of Triton X-100 in mass spectrometry for protein solubilization raises significant environmental concerns due to its persistent and potentially harmful nature. Triton X-100 is a non-ionic surfactant that, while effective in enhancing protein solubility, poses challenges in terms of its environmental impact and disposal.
One of the primary environmental issues associated with Triton X-100 is its poor biodegradability. The compound's chemical structure, particularly its aromatic ring, makes it resistant to natural breakdown processes. This persistence means that Triton X-100 can accumulate in aquatic environments, potentially affecting ecosystems long after its initial release.
The bioaccumulation potential of Triton X-100 is another critical environmental concern. Studies have shown that this surfactant can accumulate in the tissues of aquatic organisms, potentially leading to long-term ecological effects. This bioaccumulation can disrupt food chains and impact biodiversity in affected ecosystems.
Furthermore, the toxicity of Triton X-100 to aquatic life is well-documented. Even at low concentrations, it can cause adverse effects on various aquatic organisms, including fish, invertebrates, and algae. These effects range from acute toxicity to more subtle, chronic impacts on growth, reproduction, and behavior.
The disposal of Triton X-100 and its metabolites also presents environmental challenges. Conventional wastewater treatment processes may not completely remove or degrade this compound, leading to its release into natural water bodies. This can result in the contamination of surface waters and potentially groundwater resources.
In response to these environmental concerns, there is growing pressure to develop more environmentally friendly alternatives to Triton X-100 for use in mass spectrometry and other applications. Research is ongoing to identify surfactants that offer similar protein solubilization efficacy while being more biodegradable and less toxic to aquatic life.
Regulatory bodies in various countries have begun to implement stricter controls on the use and disposal of Triton X-100 and similar surfactants. These regulations aim to minimize environmental exposure and encourage the development and adoption of more sustainable alternatives in scientific and industrial applications.
The scientific community is increasingly aware of the need to balance the analytical benefits of Triton X-100 with its environmental impact. This awareness is driving efforts to optimize protocols to minimize the amount of Triton X-100 used in mass spectrometry procedures, as well as to develop more effective methods for its removal from waste streams before disposal.
One of the primary environmental issues associated with Triton X-100 is its poor biodegradability. The compound's chemical structure, particularly its aromatic ring, makes it resistant to natural breakdown processes. This persistence means that Triton X-100 can accumulate in aquatic environments, potentially affecting ecosystems long after its initial release.
The bioaccumulation potential of Triton X-100 is another critical environmental concern. Studies have shown that this surfactant can accumulate in the tissues of aquatic organisms, potentially leading to long-term ecological effects. This bioaccumulation can disrupt food chains and impact biodiversity in affected ecosystems.
Furthermore, the toxicity of Triton X-100 to aquatic life is well-documented. Even at low concentrations, it can cause adverse effects on various aquatic organisms, including fish, invertebrates, and algae. These effects range from acute toxicity to more subtle, chronic impacts on growth, reproduction, and behavior.
The disposal of Triton X-100 and its metabolites also presents environmental challenges. Conventional wastewater treatment processes may not completely remove or degrade this compound, leading to its release into natural water bodies. This can result in the contamination of surface waters and potentially groundwater resources.
In response to these environmental concerns, there is growing pressure to develop more environmentally friendly alternatives to Triton X-100 for use in mass spectrometry and other applications. Research is ongoing to identify surfactants that offer similar protein solubilization efficacy while being more biodegradable and less toxic to aquatic life.
Regulatory bodies in various countries have begun to implement stricter controls on the use and disposal of Triton X-100 and similar surfactants. These regulations aim to minimize environmental exposure and encourage the development and adoption of more sustainable alternatives in scientific and industrial applications.
The scientific community is increasingly aware of the need to balance the analytical benefits of Triton X-100 with its environmental impact. This awareness is driving efforts to optimize protocols to minimize the amount of Triton X-100 used in mass spectrometry procedures, as well as to develop more effective methods for its removal from waste streams before disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!