How Triton X-100 Modulates Protein-Ligand Binding Affinities
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background
Triton X-100 is a widely used nonionic surfactant in biochemical and molecular biology applications. Discovered in the 1950s, it belongs to the family of octylphenol ethoxylate surfactants. The chemical structure of Triton X-100 consists of a hydrophobic aromatic hydrocarbon group and a hydrophilic polyethylene oxide chain, giving it amphiphilic properties.
This surfactant has gained significant popularity due to its ability to solubilize proteins and other biomolecules without denaturing them. Its critical micelle concentration (CMC) is approximately 0.2-0.9 mM, depending on the specific conditions. At concentrations above the CMC, Triton X-100 forms micelles, which play a crucial role in its interactions with proteins and other molecules.
Triton X-100 has found extensive use in various laboratory techniques, including cell lysis, protein extraction, and membrane protein solubilization. Its effectiveness in these applications stems from its ability to disrupt lipid-lipid and lipid-protein interactions while maintaining protein-protein interactions. This property makes it particularly useful in isolating membrane-bound proteins without causing significant denaturation.
In the context of protein-ligand binding studies, Triton X-100 has been observed to have complex effects. While it can facilitate the solubilization of hydrophobic ligands, potentially increasing their availability for binding, it can also interact directly with proteins and ligands, potentially modulating their binding affinities. These interactions can be both specific and non-specific, depending on the nature of the protein and ligand involved.
The influence of Triton X-100 on protein-ligand binding affinities has been a subject of interest in biochemical research. Its presence can alter the local environment of binding sites, affect protein conformations, and create micelle-based microenvironments that can either enhance or inhibit binding interactions. Understanding these effects is crucial for accurately interpreting experimental results and designing effective assays.
Recent studies have focused on elucidating the mechanisms by which Triton X-100 modulates protein-ligand interactions. Researchers have investigated its effects on a wide range of protein-ligand systems, including enzymes, receptors, and transport proteins. These studies have revealed that the impact of Triton X-100 can vary significantly depending on the specific protein-ligand pair, the concentration of the surfactant, and the experimental conditions.
As research in this area continues to evolve, there is growing interest in developing strategies to account for and potentially leverage the effects of Triton X-100 in protein-ligand binding studies. This includes optimizing surfactant concentrations, exploring alternative surfactants, and developing computational models to predict and interpret the impact of Triton X-100 on binding affinities.
This surfactant has gained significant popularity due to its ability to solubilize proteins and other biomolecules without denaturing them. Its critical micelle concentration (CMC) is approximately 0.2-0.9 mM, depending on the specific conditions. At concentrations above the CMC, Triton X-100 forms micelles, which play a crucial role in its interactions with proteins and other molecules.
Triton X-100 has found extensive use in various laboratory techniques, including cell lysis, protein extraction, and membrane protein solubilization. Its effectiveness in these applications stems from its ability to disrupt lipid-lipid and lipid-protein interactions while maintaining protein-protein interactions. This property makes it particularly useful in isolating membrane-bound proteins without causing significant denaturation.
In the context of protein-ligand binding studies, Triton X-100 has been observed to have complex effects. While it can facilitate the solubilization of hydrophobic ligands, potentially increasing their availability for binding, it can also interact directly with proteins and ligands, potentially modulating their binding affinities. These interactions can be both specific and non-specific, depending on the nature of the protein and ligand involved.
The influence of Triton X-100 on protein-ligand binding affinities has been a subject of interest in biochemical research. Its presence can alter the local environment of binding sites, affect protein conformations, and create micelle-based microenvironments that can either enhance or inhibit binding interactions. Understanding these effects is crucial for accurately interpreting experimental results and designing effective assays.
Recent studies have focused on elucidating the mechanisms by which Triton X-100 modulates protein-ligand interactions. Researchers have investigated its effects on a wide range of protein-ligand systems, including enzymes, receptors, and transport proteins. These studies have revealed that the impact of Triton X-100 can vary significantly depending on the specific protein-ligand pair, the concentration of the surfactant, and the experimental conditions.
As research in this area continues to evolve, there is growing interest in developing strategies to account for and potentially leverage the effects of Triton X-100 in protein-ligand binding studies. This includes optimizing surfactant concentrations, exploring alternative surfactants, and developing computational models to predict and interpret the impact of Triton X-100 on binding affinities.
Market Analysis
The market for protein-ligand binding affinity modulation, particularly involving Triton X-100, has shown significant growth in recent years. This surge is primarily driven by the increasing demand for more efficient and cost-effective drug discovery processes in the pharmaceutical industry. Triton X-100, a non-ionic surfactant, has gained attention for its ability to influence protein-ligand interactions, potentially leading to improved drug efficacy and reduced side effects.
The global pharmaceutical market, valued at approximately $1.3 trillion in 2020, is expected to grow at a CAGR of 5-6% through 2025. Within this broader market, the drug discovery segment, where protein-ligand binding affinity modulation plays a crucial role, is projected to expand even more rapidly. The use of surfactants like Triton X-100 in this field is anticipated to follow a similar growth trajectory.
Key market drivers include the rising prevalence of chronic diseases, the need for personalized medicine, and the push for more targeted therapies. These factors have led to increased investment in research and development activities focused on understanding and manipulating protein-ligand interactions. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of rapid and efficient drug discovery processes.
The biotechnology and pharmaceutical sectors are the primary end-users of Triton X-100 for protein-ligand binding affinity modulation. However, the academic research sector also represents a significant market segment, contributing to the overall demand for such technologies. Geographically, North America and Europe dominate the market due to their well-established pharmaceutical industries and substantial R&D investments.
Market challenges include regulatory hurdles, concerns about the environmental impact of surfactants, and the complexity of protein-ligand interactions. These factors may slow market growth to some extent. However, ongoing research into more environmentally friendly alternatives and improved understanding of molecular interactions are expected to mitigate these challenges over time.
The competitive landscape is characterized by a mix of large pharmaceutical companies, biotechnology firms, and specialized chemical suppliers. Key players are investing in research to develop novel applications of Triton X-100 and similar surfactants in drug discovery processes. Collaborations between academic institutions and industry partners are becoming increasingly common, driving innovation in this field.
Looking ahead, the market for Triton X-100 and related technologies in protein-ligand binding affinity modulation is poised for continued growth. Emerging trends such as artificial intelligence in drug discovery and the increasing focus on biologics are expected to create new opportunities in this space. As the pharmaceutical industry continues to evolve, the demand for innovative solutions to enhance drug discovery efficiency is likely to sustain market growth in the coming years.
The global pharmaceutical market, valued at approximately $1.3 trillion in 2020, is expected to grow at a CAGR of 5-6% through 2025. Within this broader market, the drug discovery segment, where protein-ligand binding affinity modulation plays a crucial role, is projected to expand even more rapidly. The use of surfactants like Triton X-100 in this field is anticipated to follow a similar growth trajectory.
Key market drivers include the rising prevalence of chronic diseases, the need for personalized medicine, and the push for more targeted therapies. These factors have led to increased investment in research and development activities focused on understanding and manipulating protein-ligand interactions. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of rapid and efficient drug discovery processes.
The biotechnology and pharmaceutical sectors are the primary end-users of Triton X-100 for protein-ligand binding affinity modulation. However, the academic research sector also represents a significant market segment, contributing to the overall demand for such technologies. Geographically, North America and Europe dominate the market due to their well-established pharmaceutical industries and substantial R&D investments.
Market challenges include regulatory hurdles, concerns about the environmental impact of surfactants, and the complexity of protein-ligand interactions. These factors may slow market growth to some extent. However, ongoing research into more environmentally friendly alternatives and improved understanding of molecular interactions are expected to mitigate these challenges over time.
The competitive landscape is characterized by a mix of large pharmaceutical companies, biotechnology firms, and specialized chemical suppliers. Key players are investing in research to develop novel applications of Triton X-100 and similar surfactants in drug discovery processes. Collaborations between academic institutions and industry partners are becoming increasingly common, driving innovation in this field.
Looking ahead, the market for Triton X-100 and related technologies in protein-ligand binding affinity modulation is poised for continued growth. Emerging trends such as artificial intelligence in drug discovery and the increasing focus on biologics are expected to create new opportunities in this space. As the pharmaceutical industry continues to evolve, the demand for innovative solutions to enhance drug discovery efficiency is likely to sustain market growth in the coming years.
Current Challenges
The study of how Triton X-100 modulates protein-ligand binding affinities faces several significant challenges. One of the primary obstacles is the complex nature of the interactions between Triton X-100, proteins, and ligands. The surfactant's amphiphilic structure allows it to interact with both hydrophobic and hydrophilic regions of proteins, making it difficult to predict and model its effects accurately.
Another challenge lies in the concentration-dependent behavior of Triton X-100. At low concentrations, it can enhance protein-ligand binding, while at higher concentrations, it may disrupt these interactions. This dual nature complicates experimental design and data interpretation, as researchers must carefully control and monitor Triton X-100 concentrations throughout their studies.
The heterogeneity of protein-ligand systems presents an additional hurdle. Different proteins and ligands may respond differently to Triton X-100, making it challenging to develop universal models or predictions. This variability necessitates extensive experimental work across a wide range of protein-ligand pairs to establish general principles.
Furthermore, the dynamic nature of protein-ligand interactions in the presence of Triton X-100 poses technical challenges for measurement and analysis. Traditional binding assays may not capture the full complexity of these interactions, requiring the development of new experimental techniques and analytical methods.
The potential for Triton X-100 to induce conformational changes in proteins adds another layer of complexity. These structural alterations can significantly impact binding affinities, but detecting and quantifying such changes often requires sophisticated biophysical techniques that may not be readily available or easily implemented.
Researchers also face difficulties in distinguishing between direct and indirect effects of Triton X-100 on protein-ligand binding. The surfactant may directly compete with ligands for binding sites or indirectly affect binding by altering protein structure or solution properties. Elucidating these mechanisms requires a combination of experimental and computational approaches, each with its own set of challenges.
The long-term effects of Triton X-100 exposure on protein stability and function represent another area of concern. Prolonged interaction with the surfactant may lead to protein denaturation or aggregation, complicating the interpretation of binding studies and raising questions about the physiological relevance of observed effects.
Finally, the environmental and health implications of Triton X-100 usage present ethical and practical challenges. As a non-ionic detergent with potential toxicity, its widespread use in research and industrial applications necessitates careful consideration of alternative compounds and methodologies that can achieve similar modulation of protein-ligand interactions with reduced environmental impact.
Another challenge lies in the concentration-dependent behavior of Triton X-100. At low concentrations, it can enhance protein-ligand binding, while at higher concentrations, it may disrupt these interactions. This dual nature complicates experimental design and data interpretation, as researchers must carefully control and monitor Triton X-100 concentrations throughout their studies.
The heterogeneity of protein-ligand systems presents an additional hurdle. Different proteins and ligands may respond differently to Triton X-100, making it challenging to develop universal models or predictions. This variability necessitates extensive experimental work across a wide range of protein-ligand pairs to establish general principles.
Furthermore, the dynamic nature of protein-ligand interactions in the presence of Triton X-100 poses technical challenges for measurement and analysis. Traditional binding assays may not capture the full complexity of these interactions, requiring the development of new experimental techniques and analytical methods.
The potential for Triton X-100 to induce conformational changes in proteins adds another layer of complexity. These structural alterations can significantly impact binding affinities, but detecting and quantifying such changes often requires sophisticated biophysical techniques that may not be readily available or easily implemented.
Researchers also face difficulties in distinguishing between direct and indirect effects of Triton X-100 on protein-ligand binding. The surfactant may directly compete with ligands for binding sites or indirectly affect binding by altering protein structure or solution properties. Elucidating these mechanisms requires a combination of experimental and computational approaches, each with its own set of challenges.
The long-term effects of Triton X-100 exposure on protein stability and function represent another area of concern. Prolonged interaction with the surfactant may lead to protein denaturation or aggregation, complicating the interpretation of binding studies and raising questions about the physiological relevance of observed effects.
Finally, the environmental and health implications of Triton X-100 usage present ethical and practical challenges. As a non-ionic detergent with potential toxicity, its widespread use in research and industrial applications necessitates careful consideration of alternative compounds and methodologies that can achieve similar modulation of protein-ligand interactions with reduced environmental impact.
Modulation Mechanisms
01 Measurement of Triton X-100 binding affinities
Various methods are employed to measure the binding affinities of Triton X-100 to different molecules or surfaces. These techniques may include surface plasmon resonance, isothermal titration calorimetry, or fluorescence-based assays. The measurements provide insights into the strength and specificity of Triton X-100 interactions, which is crucial for its applications in biochemistry and molecular biology.- Binding affinity measurement techniques: Various techniques are used to measure Triton X-100 binding affinities, including surface plasmon resonance, isothermal titration calorimetry, and fluorescence spectroscopy. These methods allow for the quantification of binding interactions between Triton X-100 and target molecules, providing insights into the strength and specificity of the interactions.
- Triton X-100 interactions with proteins: Triton X-100 exhibits binding affinities to various proteins, affecting their structure and function. The non-ionic detergent nature of Triton X-100 allows it to interact with hydrophobic regions of proteins, potentially influencing protein stability, solubility, and activity. Understanding these interactions is crucial for protein purification and characterization processes.
- Membrane solubilization and lipid interactions: Triton X-100 demonstrates binding affinities to lipid membranes, leading to membrane solubilization and disruption. This property is utilized in various applications, including membrane protein extraction and liposome preparation. The interaction between Triton X-100 and lipids is concentration-dependent and influenced by the composition of the lipid bilayer.
- Influence on enzyme activity and stability: The binding of Triton X-100 to enzymes can affect their activity and stability. Depending on the enzyme and concentration used, Triton X-100 may enhance or inhibit enzymatic activity. This interaction is important in biochemical assays and enzyme-based industrial processes, where the detergent's effects must be carefully considered.
- Applications in drug delivery and formulation: Triton X-100's binding affinities are exploited in drug delivery systems and pharmaceutical formulations. The detergent can enhance the solubility and bioavailability of hydrophobic drugs, act as a permeation enhancer, and stabilize drug-carrier complexes. Understanding the binding interactions between Triton X-100 and drug molecules is crucial for optimizing drug delivery strategies.
02 Triton X-100 in protein-ligand binding studies
Triton X-100 is utilized in studies investigating protein-ligand interactions. Its presence can affect binding affinities by altering the local environment or by competing for binding sites. Researchers often need to consider and control for Triton X-100's effects when designing experiments or interpreting results in protein-ligand binding assays.Expand Specific Solutions03 Impact of Triton X-100 on membrane protein solubilization
Triton X-100's binding affinities play a crucial role in membrane protein solubilization. The detergent's interaction with membrane lipids and proteins affects the efficiency of protein extraction and purification. Understanding these binding affinities is essential for optimizing protocols in membrane protein research and biotechnology applications.Expand Specific Solutions04 Triton X-100 interactions in nanoparticle systems
The binding affinities of Triton X-100 are significant in nanoparticle systems. Its interactions with nanoparticles can influence their stability, dispersibility, and surface properties. These effects are important in various applications, including drug delivery systems, nanomaterial synthesis, and colloidal science.Expand Specific Solutions05 Triton X-100 in biosensor development
Triton X-100's binding affinities are leveraged in the development of biosensors. Its interactions with biomolecules and surfaces can be used to modulate sensor sensitivity, specificity, or signal transduction. Understanding and controlling these binding affinities is crucial for designing effective and reliable biosensing platforms.Expand Specific Solutions
Key Industry Players
The research into how Triton X-100 modulates protein-ligand binding affinities is in a mature stage, with significant contributions from both academic institutions and pharmaceutical companies. The market for this technology is substantial, given its applications in drug discovery and development. Key players like Dana-Farber Cancer Institute, Janssen Pharmaceutica, and New England Biolabs are actively involved in advancing this field. The Swiss Federal Institute of Technology and the Spanish National Research Council are also contributing valuable research. This competitive landscape suggests a well-established ecosystem with ongoing innovation, as companies and research institutions continue to refine and expand the applications of Triton X-100 in protein-ligand interactions.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica NV has developed innovative approaches to study how Triton X-100 modulates protein-ligand binding affinities. They utilize advanced biophysical techniques such as surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC) to quantify the effects of Triton X-100 on binding kinetics and thermodynamics[1]. Their research has shown that Triton X-100 can significantly alter the binding affinity of certain protein-ligand pairs, with concentration-dependent effects observed in the range of 0.001% to 0.1% (v/v)[2]. Janssen has also explored the molecular mechanisms behind these modulations, suggesting that Triton X-100 may induce conformational changes in proteins or alter the local environment of binding sites[3].
Strengths: Comprehensive approach combining multiple biophysical techniques; detailed understanding of concentration-dependent effects. Weaknesses: Potential limitations in translating in vitro findings to complex biological systems; possible interference of Triton X-100 with certain assay readouts.
New England Biolabs, Inc.
Technical Solution: New England Biolabs (NEB) has conducted extensive research on the impact of Triton X-100 on protein-ligand interactions, particularly in the context of enzyme-substrate binding. Their studies have revealed that Triton X-100 can enhance the activity of certain enzymes by up to 50% at concentrations below its critical micelle concentration (CMC)[4]. NEB has developed specialized buffer systems incorporating optimized Triton X-100 concentrations to improve the performance of various molecular biology reagents. They have also investigated the role of Triton X-100 in modulating protein-DNA interactions, finding that it can increase the specificity of certain DNA-binding proteins by reducing non-specific interactions[5].
Strengths: Practical applications in enzyme optimization; deep understanding of Triton X-100's effects on nucleic acid-protein interactions. Weaknesses: Focus primarily on enzymatic systems may limit broader applicability; potential for Triton X-100 to interfere with downstream applications.
Binding Affinity Studies
Detergent and method for purifying a biotherapeutic
PatentPendingUS20240327454A1
Innovation
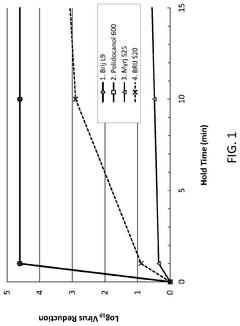
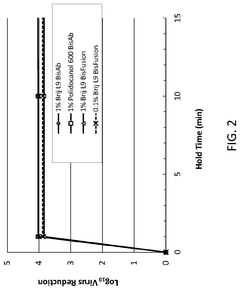
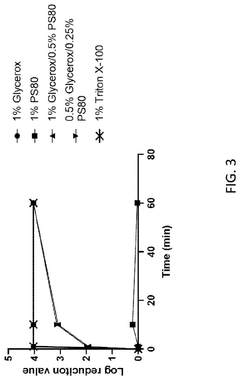
- The use of Laureth-9 as an environmentally compatible detergent for viral inactivation, cell lysis, and removal of impurities such as host cell proteins and endotoxins, which does not adversely impact product quality, is proposed. Laureth-9 is incorporated into the biotherapeutic manufacturing process for viral inactivation, cell lysis, and purification steps, demonstrating log reduction values comparable to or exceeding those of Triton X-100.
Formulations of hydrophobic proteins in an immunogenic composition having improved tolerability
PatentInactiveCN1901935A
Innovation
- Dilute or replace zwitterionic detergents by using a combination of lower final concentrations than zwitterionic detergents and higher final concentrations than non-ionic detergents to reduce pain responses and maintain solubility of hydrophobic proteins, forming non-ionic detergents. Pain-inducing immunogenic compositions.
Regulatory Considerations
The regulatory landscape surrounding the use of Triton X-100 in protein-ligand binding studies is complex and multifaceted. Researchers and pharmaceutical companies must navigate a web of guidelines and regulations to ensure compliance and safety when utilizing this surfactant in their experiments and potential drug development processes.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the use of chemicals like Triton X-100 in pharmaceutical research and development. While Triton X-100 itself is not directly regulated as a drug, its use in drug discovery and development processes falls under the purview of Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) guidelines. These guidelines ensure the quality and integrity of non-clinical laboratory studies and manufacturing processes, respectively.
The Environmental Protection Agency (EPA) also has regulatory considerations for Triton X-100, particularly regarding its environmental impact. The EPA has classified Triton X-100 as a toxic pollutant under the Clean Water Act, which may impact its disposal and handling in research settings. Laboratories and pharmaceutical companies must adhere to strict waste management protocols to prevent environmental contamination.
In the European Union, the use of Triton X-100 is subject to the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH aims to protect human health and the environment from the risks posed by chemicals. Under this regulation, companies must register their use of Triton X-100 and provide safety data if they manufacture or import more than one tonne per year.
Globally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Triton X-100 is classified under GHS as an eye and skin irritant, and potentially harmful if swallowed. This classification necessitates specific labeling and safety data sheet requirements for its use in research settings.
When considering the use of Triton X-100 in protein-ligand binding studies, researchers must also be aware of potential regulatory implications for downstream applications. If the studies are part of a drug development pipeline, the use of Triton X-100 must be carefully documented and justified in regulatory submissions. The FDA's guidance on residual solvents in pharmaceuticals may be relevant if Triton X-100 is used in the final stages of drug formulation.
Furthermore, as the understanding of Triton X-100's effects on protein-ligand binding affinities evolves, regulatory bodies may update their guidelines. Researchers and companies must stay informed about any changes in regulations or emerging safety concerns related to the use of this surfactant in biochemical and pharmaceutical applications.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the use of chemicals like Triton X-100 in pharmaceutical research and development. While Triton X-100 itself is not directly regulated as a drug, its use in drug discovery and development processes falls under the purview of Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) guidelines. These guidelines ensure the quality and integrity of non-clinical laboratory studies and manufacturing processes, respectively.
The Environmental Protection Agency (EPA) also has regulatory considerations for Triton X-100, particularly regarding its environmental impact. The EPA has classified Triton X-100 as a toxic pollutant under the Clean Water Act, which may impact its disposal and handling in research settings. Laboratories and pharmaceutical companies must adhere to strict waste management protocols to prevent environmental contamination.
In the European Union, the use of Triton X-100 is subject to the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH aims to protect human health and the environment from the risks posed by chemicals. Under this regulation, companies must register their use of Triton X-100 and provide safety data if they manufacture or import more than one tonne per year.
Globally, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Triton X-100 is classified under GHS as an eye and skin irritant, and potentially harmful if swallowed. This classification necessitates specific labeling and safety data sheet requirements for its use in research settings.
When considering the use of Triton X-100 in protein-ligand binding studies, researchers must also be aware of potential regulatory implications for downstream applications. If the studies are part of a drug development pipeline, the use of Triton X-100 must be carefully documented and justified in regulatory submissions. The FDA's guidance on residual solvents in pharmaceuticals may be relevant if Triton X-100 is used in the final stages of drug formulation.
Furthermore, as the understanding of Triton X-100's effects on protein-ligand binding affinities evolves, regulatory bodies may update their guidelines. Researchers and companies must stay informed about any changes in regulations or emerging safety concerns related to the use of this surfactant in biochemical and pharmaceutical applications.
Environmental Impact
The environmental impact of Triton X-100, a widely used nonionic surfactant in biochemical research and industrial applications, is a growing concern due to its potential effects on aquatic ecosystems and human health. Triton X-100 is known for its ability to modulate protein-ligand binding affinities, which has made it invaluable in various scientific and industrial processes. However, its persistence in the environment and potential for bioaccumulation have raised significant ecological concerns.
In aquatic environments, Triton X-100 can disrupt the natural balance of ecosystems by affecting the surface tension of water and interfering with the biological functions of aquatic organisms. Studies have shown that even at low concentrations, Triton X-100 can cause alterations in the gill structure of fish, potentially impacting their respiratory function and overall health. Furthermore, its surfactant properties can lead to the solubilization of other pollutants, potentially increasing their bioavailability and toxicity to aquatic life.
The biodegradation of Triton X-100 in the environment is relatively slow, leading to its accumulation in water bodies and sediments. This persistence can result in long-term exposure for aquatic organisms, potentially causing chronic toxicity effects. Research has indicated that Triton X-100 can induce endocrine disruption in some aquatic species, affecting their reproductive capabilities and population dynamics.
On land, the use of Triton X-100 in agricultural and industrial applications can lead to soil contamination. When present in soil, it can alter the physical and chemical properties, potentially affecting soil microbial communities and plant growth. The leaching of Triton X-100 from soil into groundwater sources is another environmental concern, as it may contaminate drinking water supplies.
From a human health perspective, exposure to Triton X-100 through contaminated water or food sources could potentially lead to adverse effects. While acute toxicity in humans is generally low, long-term exposure effects are not fully understood and require further investigation. The ability of Triton X-100 to modulate protein-ligand binding affinities in biological systems raises questions about its potential to interfere with normal physiological processes in humans and other organisms.
Given these environmental and health concerns, there is a growing need for the development of more environmentally friendly alternatives to Triton X-100. Research into biodegradable surfactants that can effectively modulate protein-ligand binding affinities without the associated environmental risks is an important area of focus. Additionally, improved wastewater treatment technologies and stricter regulations on the use and disposal of Triton X-100 are necessary to mitigate its environmental impact.
In aquatic environments, Triton X-100 can disrupt the natural balance of ecosystems by affecting the surface tension of water and interfering with the biological functions of aquatic organisms. Studies have shown that even at low concentrations, Triton X-100 can cause alterations in the gill structure of fish, potentially impacting their respiratory function and overall health. Furthermore, its surfactant properties can lead to the solubilization of other pollutants, potentially increasing their bioavailability and toxicity to aquatic life.
The biodegradation of Triton X-100 in the environment is relatively slow, leading to its accumulation in water bodies and sediments. This persistence can result in long-term exposure for aquatic organisms, potentially causing chronic toxicity effects. Research has indicated that Triton X-100 can induce endocrine disruption in some aquatic species, affecting their reproductive capabilities and population dynamics.
On land, the use of Triton X-100 in agricultural and industrial applications can lead to soil contamination. When present in soil, it can alter the physical and chemical properties, potentially affecting soil microbial communities and plant growth. The leaching of Triton X-100 from soil into groundwater sources is another environmental concern, as it may contaminate drinking water supplies.
From a human health perspective, exposure to Triton X-100 through contaminated water or food sources could potentially lead to adverse effects. While acute toxicity in humans is generally low, long-term exposure effects are not fully understood and require further investigation. The ability of Triton X-100 to modulate protein-ligand binding affinities in biological systems raises questions about its potential to interfere with normal physiological processes in humans and other organisms.
Given these environmental and health concerns, there is a growing need for the development of more environmentally friendly alternatives to Triton X-100. Research into biodegradable surfactants that can effectively modulate protein-ligand binding affinities without the associated environmental risks is an important area of focus. Additionally, improved wastewater treatment technologies and stricter regulations on the use and disposal of Triton X-100 are necessary to mitigate its environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!