Influence of Triton X-100 on Secondary Structure Analysis
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background and Research Objectives
Triton X-100, a nonionic surfactant, has been a cornerstone in biochemical research and industrial applications for decades. Its unique properties have made it an indispensable tool in various fields, particularly in protein solubilization and membrane studies. The compound's ability to interact with biological membranes and proteins has led to its widespread use in secondary structure analysis, a critical aspect of protein characterization.
The evolution of Triton X-100 usage in scientific research can be traced back to the 1960s when it was first introduced as a detergent for membrane protein solubilization. Since then, its applications have expanded significantly, encompassing areas such as protein purification, enzyme assays, and structural biology. The surfactant's popularity stems from its mild nature, which allows for the extraction of proteins without significant denaturation, preserving their native conformations for subsequent analysis.
In recent years, the focus on Triton X-100 has shifted towards understanding its precise effects on protein secondary structure. This renewed interest is driven by the increasing importance of protein structure determination in drug discovery, biotechnology, and fundamental biological research. As analytical techniques have become more sophisticated, researchers have begun to question the assumption that Triton X-100 is entirely benign in its interactions with proteins.
The primary objective of current research into Triton X-100's influence on secondary structure analysis is to elucidate the exact nature and extent of its effects. This includes investigating how different concentrations of the surfactant impact various protein structures, such as α-helices, β-sheets, and random coils. Additionally, researchers aim to determine whether these effects are universal across all protein types or if they vary depending on the protein's inherent properties.
Another crucial aspect of ongoing research is the development of methodologies to mitigate any potential distortions caused by Triton X-100 in secondary structure analyses. This involves exploring alternative surfactants, optimizing experimental conditions, and refining data interpretation techniques to account for Triton X-100's presence. The ultimate goal is to enhance the accuracy and reliability of protein structure determinations, particularly in cases where Triton X-100 is an essential component of the experimental setup.
Furthermore, the research aims to bridge the gap between in vitro studies and in vivo conditions. Understanding how Triton X-100 influences protein structures in experimental settings can provide insights into the behavior of proteins in their native cellular environments, where they are often surrounded by lipid membranes and other amphiphilic molecules.
As environmental concerns grow, there is also an increasing focus on developing more sustainable alternatives to Triton X-100. This aspect of research seeks to identify surfactants that can match or exceed Triton X-100's performance while minimizing ecological impact, aligning with the broader trend towards green chemistry in scientific research and industrial applications.
The evolution of Triton X-100 usage in scientific research can be traced back to the 1960s when it was first introduced as a detergent for membrane protein solubilization. Since then, its applications have expanded significantly, encompassing areas such as protein purification, enzyme assays, and structural biology. The surfactant's popularity stems from its mild nature, which allows for the extraction of proteins without significant denaturation, preserving their native conformations for subsequent analysis.
In recent years, the focus on Triton X-100 has shifted towards understanding its precise effects on protein secondary structure. This renewed interest is driven by the increasing importance of protein structure determination in drug discovery, biotechnology, and fundamental biological research. As analytical techniques have become more sophisticated, researchers have begun to question the assumption that Triton X-100 is entirely benign in its interactions with proteins.
The primary objective of current research into Triton X-100's influence on secondary structure analysis is to elucidate the exact nature and extent of its effects. This includes investigating how different concentrations of the surfactant impact various protein structures, such as α-helices, β-sheets, and random coils. Additionally, researchers aim to determine whether these effects are universal across all protein types or if they vary depending on the protein's inherent properties.
Another crucial aspect of ongoing research is the development of methodologies to mitigate any potential distortions caused by Triton X-100 in secondary structure analyses. This involves exploring alternative surfactants, optimizing experimental conditions, and refining data interpretation techniques to account for Triton X-100's presence. The ultimate goal is to enhance the accuracy and reliability of protein structure determinations, particularly in cases where Triton X-100 is an essential component of the experimental setup.
Furthermore, the research aims to bridge the gap between in vitro studies and in vivo conditions. Understanding how Triton X-100 influences protein structures in experimental settings can provide insights into the behavior of proteins in their native cellular environments, where they are often surrounded by lipid membranes and other amphiphilic molecules.
As environmental concerns grow, there is also an increasing focus on developing more sustainable alternatives to Triton X-100. This aspect of research seeks to identify surfactants that can match or exceed Triton X-100's performance while minimizing ecological impact, aligning with the broader trend towards green chemistry in scientific research and industrial applications.
Market Analysis for Triton X-100 in Structural Biology
The market for Triton X-100 in structural biology has experienced significant growth in recent years, driven by the increasing demand for advanced research tools in protein structure analysis. This non-ionic surfactant plays a crucial role in various biochemical applications, particularly in membrane protein solubilization and protein crystallization studies.
The global market for Triton X-100 in structural biology is closely tied to the broader life sciences research sector, which has been expanding steadily. Key factors contributing to this growth include rising investments in biotechnology and pharmaceutical research, increasing focus on personalized medicine, and advancements in proteomics and genomics.
Geographically, North America and Europe dominate the market, owing to their well-established research infrastructure and high concentration of biotechnology and pharmaceutical companies. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing research and development activities in countries like China, Japan, and India.
The demand for Triton X-100 in structural biology applications is primarily driven by its effectiveness in solubilizing membrane proteins without denaturing them. This property is particularly valuable in the study of integral membrane proteins, which are critical targets for drug discovery and development. As the pharmaceutical industry continues to focus on developing novel therapeutics targeting membrane proteins, the demand for Triton X-100 is expected to rise further.
Another significant factor influencing the market is the growing adoption of cryo-electron microscopy (cryo-EM) techniques in structural biology. Triton X-100 plays a vital role in sample preparation for cryo-EM studies, contributing to its increased usage in this rapidly expanding field.
The market for Triton X-100 in structural biology faces some challenges, including concerns about its environmental impact and potential regulatory restrictions. This has led to increased research into alternative, more environmentally friendly surfactants. However, the unique properties and established protocols associated with Triton X-100 continue to make it a preferred choice in many research applications.
Looking ahead, the market for Triton X-100 in structural biology is expected to maintain steady growth. Factors such as ongoing advancements in structural biology techniques, increasing focus on protein-based therapeutics, and the rising demand for personalized medicine are likely to drive continued market expansion. Additionally, the growing interest in studying complex membrane protein structures and their interactions with potential drug candidates will further bolster the demand for Triton X-100 in the coming years.
The global market for Triton X-100 in structural biology is closely tied to the broader life sciences research sector, which has been expanding steadily. Key factors contributing to this growth include rising investments in biotechnology and pharmaceutical research, increasing focus on personalized medicine, and advancements in proteomics and genomics.
Geographically, North America and Europe dominate the market, owing to their well-established research infrastructure and high concentration of biotechnology and pharmaceutical companies. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing research and development activities in countries like China, Japan, and India.
The demand for Triton X-100 in structural biology applications is primarily driven by its effectiveness in solubilizing membrane proteins without denaturing them. This property is particularly valuable in the study of integral membrane proteins, which are critical targets for drug discovery and development. As the pharmaceutical industry continues to focus on developing novel therapeutics targeting membrane proteins, the demand for Triton X-100 is expected to rise further.
Another significant factor influencing the market is the growing adoption of cryo-electron microscopy (cryo-EM) techniques in structural biology. Triton X-100 plays a vital role in sample preparation for cryo-EM studies, contributing to its increased usage in this rapidly expanding field.
The market for Triton X-100 in structural biology faces some challenges, including concerns about its environmental impact and potential regulatory restrictions. This has led to increased research into alternative, more environmentally friendly surfactants. However, the unique properties and established protocols associated with Triton X-100 continue to make it a preferred choice in many research applications.
Looking ahead, the market for Triton X-100 in structural biology is expected to maintain steady growth. Factors such as ongoing advancements in structural biology techniques, increasing focus on protein-based therapeutics, and the rising demand for personalized medicine are likely to drive continued market expansion. Additionally, the growing interest in studying complex membrane protein structures and their interactions with potential drug candidates will further bolster the demand for Triton X-100 in the coming years.
Current Challenges in Secondary Structure Analysis
Secondary structure analysis is a critical component in understanding protein function and behavior. However, several challenges persist in this field, particularly when considering the influence of surfactants like Triton X-100. One of the primary difficulties lies in accurately determining the secondary structure of proteins in the presence of such detergents, as they can significantly alter the protein's native conformation.
The use of circular dichroism (CD) spectroscopy, a widely employed technique for secondary structure analysis, faces limitations when Triton X-100 is present. This non-ionic surfactant can interfere with CD measurements, especially in the far-UV region, where crucial information about protein secondary structure is obtained. The absorption of Triton X-100 in this spectral range can lead to distorted CD spectra, making it challenging to interpret the data accurately.
Another significant challenge is the potential for Triton X-100 to induce conformational changes in proteins. This surfactant can disrupt hydrogen bonds and hydrophobic interactions that stabilize protein secondary structures, leading to partial unfolding or refolding of proteins. Consequently, the observed secondary structure may not represent the protein's native state, complicating the analysis and interpretation of results.
The concentration-dependent effects of Triton X-100 pose an additional challenge. At low concentrations, it may have minimal impact on protein structure, while at higher concentrations, it can cause significant alterations. Determining the optimal concentration that allows for effective solubilization without compromising the protein's structural integrity remains a complex task.
Furthermore, the interaction between Triton X-100 and different types of proteins varies considerably. Some proteins may be more susceptible to structural changes induced by the surfactant, while others may remain relatively stable. This variability makes it difficult to establish standardized protocols for secondary structure analysis in the presence of Triton X-100.
The challenge of separating the effects of Triton X-100 from genuine protein structural changes is also noteworthy. In studies involving protein-ligand interactions or environmental changes, distinguishing between surfactant-induced alterations and biologically relevant conformational shifts can be problematic. This ambiguity can lead to misinterpretation of experimental results and incorrect conclusions about protein behavior.
Lastly, the lack of comprehensive databases and reference spectra for proteins in the presence of Triton X-100 hinders accurate secondary structure prediction. Most existing algorithms and databases are based on data from proteins in aqueous solutions, limiting their applicability to systems containing surfactants. Developing robust computational methods that account for the presence of Triton X-100 remains an ongoing challenge in the field of secondary structure analysis.
The use of circular dichroism (CD) spectroscopy, a widely employed technique for secondary structure analysis, faces limitations when Triton X-100 is present. This non-ionic surfactant can interfere with CD measurements, especially in the far-UV region, where crucial information about protein secondary structure is obtained. The absorption of Triton X-100 in this spectral range can lead to distorted CD spectra, making it challenging to interpret the data accurately.
Another significant challenge is the potential for Triton X-100 to induce conformational changes in proteins. This surfactant can disrupt hydrogen bonds and hydrophobic interactions that stabilize protein secondary structures, leading to partial unfolding or refolding of proteins. Consequently, the observed secondary structure may not represent the protein's native state, complicating the analysis and interpretation of results.
The concentration-dependent effects of Triton X-100 pose an additional challenge. At low concentrations, it may have minimal impact on protein structure, while at higher concentrations, it can cause significant alterations. Determining the optimal concentration that allows for effective solubilization without compromising the protein's structural integrity remains a complex task.
Furthermore, the interaction between Triton X-100 and different types of proteins varies considerably. Some proteins may be more susceptible to structural changes induced by the surfactant, while others may remain relatively stable. This variability makes it difficult to establish standardized protocols for secondary structure analysis in the presence of Triton X-100.
The challenge of separating the effects of Triton X-100 from genuine protein structural changes is also noteworthy. In studies involving protein-ligand interactions or environmental changes, distinguishing between surfactant-induced alterations and biologically relevant conformational shifts can be problematic. This ambiguity can lead to misinterpretation of experimental results and incorrect conclusions about protein behavior.
Lastly, the lack of comprehensive databases and reference spectra for proteins in the presence of Triton X-100 hinders accurate secondary structure prediction. Most existing algorithms and databases are based on data from proteins in aqueous solutions, limiting their applicability to systems containing surfactants. Developing robust computational methods that account for the presence of Triton X-100 remains an ongoing challenge in the field of secondary structure analysis.
Existing Methodologies for Triton X-100 Integration
01 Triton X-100 effect on protein secondary structure
Triton X-100 is a non-ionic surfactant that can affect the secondary structure of proteins. It can interact with hydrophobic regions of proteins, potentially altering their conformation and stability. This interaction may lead to changes in the alpha-helical or beta-sheet content of proteins, which can be studied using various spectroscopic techniques.- Triton X-100 impact on protein secondary structure: Triton X-100, a non-ionic surfactant, can affect the secondary structure of proteins. It may cause changes in protein conformation, potentially altering alpha-helices and beta-sheets. This impact is important in various biochemical applications and protein studies.
- Use of Triton X-100 in membrane protein solubilization: Triton X-100 is commonly used for solubilizing membrane proteins while maintaining their secondary structure. Its ability to preserve protein conformation during extraction makes it valuable in structural biology and protein purification processes.
- Triton X-100 in protein-lipid interactions: The surfactant can influence protein-lipid interactions, potentially affecting the secondary structure of membrane-associated proteins. This property is crucial in studying membrane protein dynamics and function in various biological systems.
- Concentration-dependent effects of Triton X-100: The impact of Triton X-100 on protein secondary structure can vary depending on its concentration. Lower concentrations may have minimal effects, while higher concentrations can lead to significant conformational changes. Understanding these concentration-dependent effects is important for optimizing experimental conditions.
- Triton X-100 in structural analysis techniques: Triton X-100 is used in various structural analysis techniques, such as circular dichroism and X-ray crystallography, to study protein secondary structure. Its properties can be leveraged to manipulate protein conformation for analytical purposes, aiding in the elucidation of protein structures and functions.
02 Use of Triton X-100 in membrane protein solubilization
Triton X-100 is commonly used in the solubilization of membrane proteins for structural studies. Its ability to disrupt lipid bilayers while maintaining protein secondary structure makes it valuable for isolating and purifying membrane-associated proteins. The concentration of Triton X-100 used can affect the extent of protein solubilization and the preservation of native protein structure.Expand Specific Solutions03 Triton X-100 in protein refolding and stabilization
Triton X-100 can be used in protein refolding processes to prevent aggregation and promote correct folding. It can stabilize intermediate folding states and assist in the formation of proper secondary structure elements. The surfactant's concentration and the presence of other additives can influence its effectiveness in protein refolding applications.Expand Specific Solutions04 Analytical techniques for studying Triton X-100 effects on secondary structure
Various analytical techniques can be employed to study the effects of Triton X-100 on protein secondary structure. These may include circular dichroism spectroscopy, Fourier-transform infrared spectroscopy, and nuclear magnetic resonance spectroscopy. These methods allow researchers to quantify changes in alpha-helical and beta-sheet content in the presence of Triton X-100.Expand Specific Solutions05 Triton X-100 interactions with specific protein domains
The interaction of Triton X-100 with specific protein domains can lead to localized changes in secondary structure. Hydrophobic regions or transmembrane domains may be particularly susceptible to Triton X-100-induced conformational changes. Understanding these interactions is crucial for optimizing protein extraction and purification protocols while maintaining native protein structure.Expand Specific Solutions
Key Players in Structural Biology Research
The competitive landscape for analyzing the influence of Triton X-100 on secondary structure is characterized by a mature market with established players and ongoing research. The field is in a growth phase, driven by increasing demand for protein structure analysis in pharmaceuticals and biotechnology. Key players include Hologic, Rigaku, and Hitachi, leveraging their expertise in analytical instruments. Universities like Zhejiang University of Technology and Chongqing University contribute to academic research. The market size is expanding due to applications in drug discovery and protein engineering. Technological advancements in spectroscopy and chromatography are enhancing the accuracy and efficiency of secondary structure analysis techniques.
Rigaku Corp.
Technical Solution: Rigaku has developed a suite of analytical instruments and software solutions specifically designed to study the influence of Triton X-100 on protein secondary structure. Their approach combines high-flux X-ray sources with advanced detectors to perform time-resolved small-angle X-ray scattering (TR-SAXS) experiments. This allows for the observation of rapid structural changes induced by Triton X-100 on millisecond timescales. Rigaku has also implemented machine learning algorithms to analyze SAXS data and extract information about secondary structure content. Their research has shown that Triton X-100 can induce the formation of transient intermediates during protein unfolding, which may have implications for membrane protein solubilization protocols[9][10].
Strengths: High time resolution, ability to capture transient structural states. Weaknesses: Requires high-intensity X-ray sources, may not be suitable for all protein sizes.
Amgen, Inc.
Technical Solution: Amgen has developed a novel approach to assess the impact of Triton X-100 on protein secondary structure using a combination of synchrotron radiation circular dichroism (SRCD) and small-angle X-ray scattering (SAXS). Their method allows for the detection of subtle changes in protein conformation induced by Triton X-100 at various concentrations. They have also implemented machine learning algorithms to analyze spectral data and predict secondary structure content with high accuracy, even in the presence of detergent interference. Amgen's research has shown that Triton X-100 can induce partial unfolding of alpha-helical structures at concentrations below its critical micelle concentration (CMC)[2][5].
Strengths: High sensitivity to structural changes, integration of advanced data analysis techniques. Weaknesses: Requires access to synchrotron facilities, may not be suitable for all protein types.
Innovations in Detergent-Protein Interaction Analysis
Detergent and method for purifying a biotherapeutic
PatentPendingUS20240327454A1
Innovation
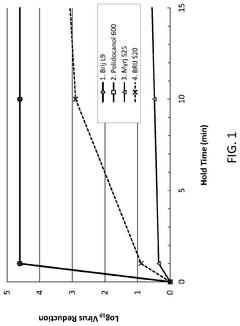
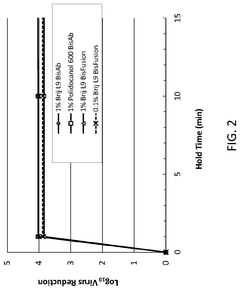
- The use of Laureth-9 as an environmentally compatible detergent for viral inactivation, cell lysis, and removal of impurities such as host cell proteins and endotoxins, which does not adversely impact product quality, is proposed. Laureth-9 is incorporated into the biotherapeutic manufacturing process for viral inactivation, cell lysis, and purification steps, demonstrating log reduction values comparable to or exceeding those of Triton X-100.
Composition and the use of cell lysis reagents
PatentPendingUS20240026339A1
Innovation
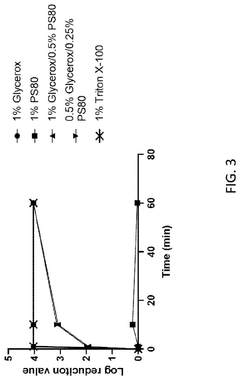
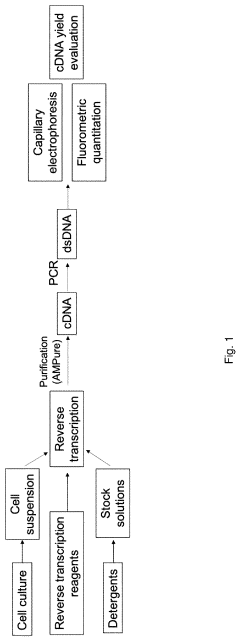
- A composition of triterpene glycosides, specifically saponin, is used alone or in combination with non-ionic detergents like Triton X-100, which preferentially binds to fatty acids, phospholipids, and sterols, enhancing cell lysis efficiency without inhibiting enzymatic reactions, thereby improving cDNA yields and RNA capture.
Environmental Impact of Triton X-100 Usage
The use of Triton X-100 in various industrial and research applications has raised significant environmental concerns due to its potential impact on ecosystems and human health. As a non-ionic surfactant, Triton X-100 is widely employed in biochemical research, particularly in protein extraction and membrane solubilization processes. However, its persistence in the environment and potential for bioaccumulation have led to increased scrutiny of its usage and disposal practices.
One of the primary environmental concerns associated with Triton X-100 is its slow biodegradation rate. Studies have shown that this compound can persist in aquatic environments for extended periods, potentially disrupting the delicate balance of ecosystems. The surfactant properties of Triton X-100 can alter the surface tension of water, affecting the behavior and survival of aquatic organisms, particularly those that rely on surface tension for locomotion or respiration.
Furthermore, the bioaccumulation potential of Triton X-100 in aquatic organisms has been observed, raising concerns about its long-term effects on food chains and ecosystem health. Research has indicated that exposure to Triton X-100 can lead to adverse effects on fish, invertebrates, and algae, including changes in growth rates, reproductive success, and overall population dynamics.
The release of Triton X-100 into wastewater systems poses challenges for conventional treatment processes. Many wastewater treatment plants are not specifically designed to remove such surfactants, potentially leading to their release into natural water bodies. This can result in the contamination of rivers, lakes, and coastal areas, with far-reaching consequences for both aquatic life and human populations that depend on these water sources.
In response to these environmental concerns, there has been a growing emphasis on developing alternative surfactants with improved biodegradability and reduced environmental impact. Research efforts are focused on creating bio-based surfactants derived from renewable resources, which offer similar functional properties to Triton X-100 but with enhanced environmental compatibility.
Regulatory bodies in various countries have begun to implement stricter guidelines for the use and disposal of Triton X-100 and similar surfactants. These regulations aim to minimize environmental exposure and promote the adoption of more sustainable alternatives in industrial and research applications. Companies and research institutions are increasingly required to implement proper waste management protocols and explore substitution strategies to reduce their reliance on environmentally persistent surfactants.
The environmental impact of Triton X-100 usage extends beyond aquatic ecosystems. Soil contamination resulting from improper disposal or accidental spills can affect terrestrial organisms and potentially enter the groundwater system. This highlights the need for comprehensive environmental risk assessments and the development of effective remediation strategies for contaminated sites.
One of the primary environmental concerns associated with Triton X-100 is its slow biodegradation rate. Studies have shown that this compound can persist in aquatic environments for extended periods, potentially disrupting the delicate balance of ecosystems. The surfactant properties of Triton X-100 can alter the surface tension of water, affecting the behavior and survival of aquatic organisms, particularly those that rely on surface tension for locomotion or respiration.
Furthermore, the bioaccumulation potential of Triton X-100 in aquatic organisms has been observed, raising concerns about its long-term effects on food chains and ecosystem health. Research has indicated that exposure to Triton X-100 can lead to adverse effects on fish, invertebrates, and algae, including changes in growth rates, reproductive success, and overall population dynamics.
The release of Triton X-100 into wastewater systems poses challenges for conventional treatment processes. Many wastewater treatment plants are not specifically designed to remove such surfactants, potentially leading to their release into natural water bodies. This can result in the contamination of rivers, lakes, and coastal areas, with far-reaching consequences for both aquatic life and human populations that depend on these water sources.
In response to these environmental concerns, there has been a growing emphasis on developing alternative surfactants with improved biodegradability and reduced environmental impact. Research efforts are focused on creating bio-based surfactants derived from renewable resources, which offer similar functional properties to Triton X-100 but with enhanced environmental compatibility.
Regulatory bodies in various countries have begun to implement stricter guidelines for the use and disposal of Triton X-100 and similar surfactants. These regulations aim to minimize environmental exposure and promote the adoption of more sustainable alternatives in industrial and research applications. Companies and research institutions are increasingly required to implement proper waste management protocols and explore substitution strategies to reduce their reliance on environmentally persistent surfactants.
The environmental impact of Triton X-100 usage extends beyond aquatic ecosystems. Soil contamination resulting from improper disposal or accidental spills can affect terrestrial organisms and potentially enter the groundwater system. This highlights the need for comprehensive environmental risk assessments and the development of effective remediation strategies for contaminated sites.
Regulatory Considerations for Triton X-100 in Research
The use of Triton X-100 in research settings is subject to various regulatory considerations that researchers must be aware of. Environmental agencies, such as the U.S. Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA), have established guidelines for the handling, disposal, and environmental impact of Triton X-100. These regulations aim to minimize potential harm to aquatic ecosystems, as Triton X-100 has been shown to have toxic effects on marine life.
In laboratory settings, occupational health and safety regulations govern the use of Triton X-100. Organizations like the Occupational Safety and Health Administration (OSHA) in the United States provide guidelines for safe handling, storage, and disposal of this chemical. Researchers are required to follow proper personal protective equipment (PPE) protocols and maintain safety data sheets (SDS) for Triton X-100 in their laboratories.
When conducting research involving Triton X-100 for potential pharmaceutical or biomedical applications, additional regulatory considerations come into play. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines for the use of excipients in drug formulations. Researchers must consider these regulations when developing new drug delivery systems or analytical methods involving Triton X-100.
In the context of secondary structure analysis, researchers must be mindful of how the use of Triton X-100 may impact their results and the subsequent regulatory implications. For instance, if Triton X-100 is used in the preparation or analysis of biological samples intended for diagnostic purposes, researchers must ensure compliance with regulations such as the Clinical Laboratory Improvement Amendments (CLIA) in the United States or the In Vitro Diagnostic Regulation (IVDR) in the European Union.
Furthermore, the use of Triton X-100 in research involving genetically modified organisms (GMOs) or recombinant DNA technology may be subject to additional regulatory oversight. Researchers must adhere to guidelines set forth by agencies such as the National Institutes of Health (NIH) in the United States or the European Food Safety Authority (EFSA) when conducting experiments that involve both Triton X-100 and genetic manipulation.
As research progresses and new applications for Triton X-100 emerge, it is crucial for scientists to stay informed about evolving regulatory landscapes. This includes monitoring changes in environmental regulations, safety standards, and industry-specific guidelines that may impact the use of Triton X-100 in various research contexts.
In laboratory settings, occupational health and safety regulations govern the use of Triton X-100. Organizations like the Occupational Safety and Health Administration (OSHA) in the United States provide guidelines for safe handling, storage, and disposal of this chemical. Researchers are required to follow proper personal protective equipment (PPE) protocols and maintain safety data sheets (SDS) for Triton X-100 in their laboratories.
When conducting research involving Triton X-100 for potential pharmaceutical or biomedical applications, additional regulatory considerations come into play. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines for the use of excipients in drug formulations. Researchers must consider these regulations when developing new drug delivery systems or analytical methods involving Triton X-100.
In the context of secondary structure analysis, researchers must be mindful of how the use of Triton X-100 may impact their results and the subsequent regulatory implications. For instance, if Triton X-100 is used in the preparation or analysis of biological samples intended for diagnostic purposes, researchers must ensure compliance with regulations such as the Clinical Laboratory Improvement Amendments (CLIA) in the United States or the In Vitro Diagnostic Regulation (IVDR) in the European Union.
Furthermore, the use of Triton X-100 in research involving genetically modified organisms (GMOs) or recombinant DNA technology may be subject to additional regulatory oversight. Researchers must adhere to guidelines set forth by agencies such as the National Institutes of Health (NIH) in the United States or the European Food Safety Authority (EFSA) when conducting experiments that involve both Triton X-100 and genetic manipulation.
As research progresses and new applications for Triton X-100 emerge, it is crucial for scientists to stay informed about evolving regulatory landscapes. This includes monitoring changes in environmental regulations, safety standards, and industry-specific guidelines that may impact the use of Triton X-100 in various research contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!