Laminar Flow in Biomedical Devices: Current Trends
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Laminar Flow Fundamentals and Objectives
Laminar flow, characterized by smooth and predictable fluid motion, plays a crucial role in the design and operation of biomedical devices. This fundamental concept in fluid dynamics has gained significant attention in recent years due to its wide-ranging applications in medical technology. The evolution of laminar flow understanding has been closely tied to advancements in microfluidics and lab-on-a-chip technologies, which have revolutionized diagnostic and therapeutic approaches in healthcare.
The primary objective of studying laminar flow in biomedical devices is to optimize fluid behavior for enhanced performance and reliability. By controlling fluid dynamics at the microscale, researchers aim to improve the efficiency of drug delivery systems, develop more accurate diagnostic tools, and create innovative therapeutic devices. Understanding laminar flow characteristics enables the design of devices that can manipulate small volumes of fluids with high precision, a critical factor in many biomedical applications.
Recent trends in laminar flow research for biomedical devices focus on exploiting its unique properties to achieve specific functional outcomes. For instance, the ability to maintain distinct fluid streams without mixing in laminar flow regimes has led to the development of novel separation and sorting techniques for cells and biomolecules. This has significant implications for personalized medicine and point-of-care diagnostics.
Another key trend is the integration of laminar flow principles with other physical phenomena, such as electrokinetics and acoustics, to create multifunctional biomedical devices. These hybrid systems leverage the predictable nature of laminar flow to enhance the control and manipulation of biological samples, leading to more sensitive and versatile diagnostic platforms.
The ongoing exploration of laminar flow in biomedical devices aims to address several challenges, including the need for increased throughput, improved sensitivity, and reduced sample volumes. Researchers are investigating ways to maintain laminar flow conditions in more complex geometries and at higher flow rates, which could expand the applicability of microfluidic devices in clinical settings.
As the field progresses, the objectives of laminar flow research in biomedical devices are expanding to encompass sustainability and cost-effectiveness. There is a growing emphasis on developing devices that not only perform well but also minimize resource consumption and are accessible to a wider range of healthcare providers. This holistic approach to device design is driving innovation in materials and fabrication techniques that support laminar flow while meeting these broader goals.
The primary objective of studying laminar flow in biomedical devices is to optimize fluid behavior for enhanced performance and reliability. By controlling fluid dynamics at the microscale, researchers aim to improve the efficiency of drug delivery systems, develop more accurate diagnostic tools, and create innovative therapeutic devices. Understanding laminar flow characteristics enables the design of devices that can manipulate small volumes of fluids with high precision, a critical factor in many biomedical applications.
Recent trends in laminar flow research for biomedical devices focus on exploiting its unique properties to achieve specific functional outcomes. For instance, the ability to maintain distinct fluid streams without mixing in laminar flow regimes has led to the development of novel separation and sorting techniques for cells and biomolecules. This has significant implications for personalized medicine and point-of-care diagnostics.
Another key trend is the integration of laminar flow principles with other physical phenomena, such as electrokinetics and acoustics, to create multifunctional biomedical devices. These hybrid systems leverage the predictable nature of laminar flow to enhance the control and manipulation of biological samples, leading to more sensitive and versatile diagnostic platforms.
The ongoing exploration of laminar flow in biomedical devices aims to address several challenges, including the need for increased throughput, improved sensitivity, and reduced sample volumes. Researchers are investigating ways to maintain laminar flow conditions in more complex geometries and at higher flow rates, which could expand the applicability of microfluidic devices in clinical settings.
As the field progresses, the objectives of laminar flow research in biomedical devices are expanding to encompass sustainability and cost-effectiveness. There is a growing emphasis on developing devices that not only perform well but also minimize resource consumption and are accessible to a wider range of healthcare providers. This holistic approach to device design is driving innovation in materials and fabrication techniques that support laminar flow while meeting these broader goals.
Biomedical Device Market Analysis
The biomedical device market has experienced significant growth in recent years, driven by technological advancements, an aging population, and increasing healthcare expenditure. The global market for biomedical devices is projected to reach substantial figures in the coming years, with a compound annual growth rate (CAGR) that outpaces many other industries. This growth is particularly evident in sectors such as cardiovascular devices, diagnostic imaging equipment, and minimally invasive surgical instruments.
Laminar flow technology plays a crucial role in various biomedical devices, especially in applications related to fluid dynamics and precision control. The demand for devices incorporating laminar flow principles is on the rise, particularly in areas such as microfluidics, lab-on-a-chip devices, and advanced drug delivery systems. These applications leverage laminar flow to achieve precise control over fluid behavior, enabling more accurate diagnostics, targeted therapies, and improved patient outcomes.
The market for laminar flow-based biomedical devices is segmented by application, end-user, and geography. Key application areas include point-of-care diagnostics, drug discovery and development, and personalized medicine. End-users span across hospitals, research laboratories, pharmaceutical companies, and academic institutions. Geographically, North America and Europe currently dominate the market, but rapid growth is observed in Asia-Pacific regions, particularly in countries like China and India.
Several factors are driving the adoption of laminar flow technologies in biomedical devices. The increasing prevalence of chronic diseases and the need for more efficient diagnostic tools are primary drivers. Additionally, the push towards miniaturization and portability in medical devices aligns well with the capabilities of laminar flow systems. The growing focus on personalized medicine and the demand for high-throughput screening in drug discovery are also contributing to market expansion.
However, the market faces certain challenges. The high cost of advanced laminar flow devices and the complexity of integrating these technologies into existing healthcare systems pose barriers to widespread adoption. Regulatory hurdles and the need for extensive clinical validation of new devices also impact market growth. Despite these challenges, the potential benefits of laminar flow technologies in improving patient care and research outcomes continue to drive innovation and investment in this sector.
Looking ahead, the biomedical device market, particularly in the context of laminar flow applications, is poised for continued growth. Emerging trends such as the integration of artificial intelligence and machine learning with laminar flow devices, the development of organ-on-a-chip platforms, and advancements in 3D-printed microfluidic devices are expected to open new avenues for market expansion and technological innovation in the coming years.
Laminar flow technology plays a crucial role in various biomedical devices, especially in applications related to fluid dynamics and precision control. The demand for devices incorporating laminar flow principles is on the rise, particularly in areas such as microfluidics, lab-on-a-chip devices, and advanced drug delivery systems. These applications leverage laminar flow to achieve precise control over fluid behavior, enabling more accurate diagnostics, targeted therapies, and improved patient outcomes.
The market for laminar flow-based biomedical devices is segmented by application, end-user, and geography. Key application areas include point-of-care diagnostics, drug discovery and development, and personalized medicine. End-users span across hospitals, research laboratories, pharmaceutical companies, and academic institutions. Geographically, North America and Europe currently dominate the market, but rapid growth is observed in Asia-Pacific regions, particularly in countries like China and India.
Several factors are driving the adoption of laminar flow technologies in biomedical devices. The increasing prevalence of chronic diseases and the need for more efficient diagnostic tools are primary drivers. Additionally, the push towards miniaturization and portability in medical devices aligns well with the capabilities of laminar flow systems. The growing focus on personalized medicine and the demand for high-throughput screening in drug discovery are also contributing to market expansion.
However, the market faces certain challenges. The high cost of advanced laminar flow devices and the complexity of integrating these technologies into existing healthcare systems pose barriers to widespread adoption. Regulatory hurdles and the need for extensive clinical validation of new devices also impact market growth. Despite these challenges, the potential benefits of laminar flow technologies in improving patient care and research outcomes continue to drive innovation and investment in this sector.
Looking ahead, the biomedical device market, particularly in the context of laminar flow applications, is poised for continued growth. Emerging trends such as the integration of artificial intelligence and machine learning with laminar flow devices, the development of organ-on-a-chip platforms, and advancements in 3D-printed microfluidic devices are expected to open new avenues for market expansion and technological innovation in the coming years.
Laminar Flow Challenges in Biomedical Applications
Laminar flow in biomedical devices presents several significant challenges that researchers and engineers must address to optimize device performance and ensure patient safety. One of the primary obstacles is maintaining consistent laminar flow across various geometries and scales within biomedical devices. The complex internal structures of these devices, such as microfluidic channels, often create regions where flow transitions from laminar to turbulent, compromising the intended functionality.
Another critical challenge is the interaction between laminar flow and biological materials. In many biomedical applications, the flow must transport cells, proteins, or other biomolecules without causing damage or altering their properties. Achieving this delicate balance requires precise control over shear stress and flow velocities, which can be difficult to maintain in intricate device designs.
The presence of surface irregularities and manufacturing imperfections in biomedical devices further complicates laminar flow control. Even minor surface defects can disrupt the flow pattern, leading to unexpected turbulence or flow separation. This issue is particularly pronounced in micro- and nano-scale devices, where surface effects become increasingly dominant.
Temperature gradients pose another significant challenge in maintaining laminar flow. Many biomedical applications involve temperature-sensitive processes or materials, and local temperature variations can induce convection currents that disrupt laminar flow. Controlling these thermal effects while preserving the desired flow characteristics requires sophisticated thermal management strategies.
The need for biocompatibility adds an extra layer of complexity to laminar flow challenges. Materials used in biomedical devices must not only support laminar flow but also be non-toxic and non-reactive with biological tissues and fluids. This constraint limits the range of available materials and surface treatments that can be employed to optimize flow characteristics.
Scaling effects present unique challenges when transitioning from laboratory prototypes to clinical-scale devices. Flow behaviors that are easily controlled in small-scale experiments may become unpredictable or difficult to manage in larger devices. This scaling issue often necessitates significant redesigns and optimizations during the development process.
Lastly, the dynamic nature of biological systems introduces variability that can affect laminar flow stability. Factors such as changes in fluid viscosity, the presence of particulates, or the growth of biofilms on device surfaces can all alter flow characteristics over time. Designing devices that maintain laminar flow under these changing conditions remains a significant engineering challenge in the biomedical field.
Another critical challenge is the interaction between laminar flow and biological materials. In many biomedical applications, the flow must transport cells, proteins, or other biomolecules without causing damage or altering their properties. Achieving this delicate balance requires precise control over shear stress and flow velocities, which can be difficult to maintain in intricate device designs.
The presence of surface irregularities and manufacturing imperfections in biomedical devices further complicates laminar flow control. Even minor surface defects can disrupt the flow pattern, leading to unexpected turbulence or flow separation. This issue is particularly pronounced in micro- and nano-scale devices, where surface effects become increasingly dominant.
Temperature gradients pose another significant challenge in maintaining laminar flow. Many biomedical applications involve temperature-sensitive processes or materials, and local temperature variations can induce convection currents that disrupt laminar flow. Controlling these thermal effects while preserving the desired flow characteristics requires sophisticated thermal management strategies.
The need for biocompatibility adds an extra layer of complexity to laminar flow challenges. Materials used in biomedical devices must not only support laminar flow but also be non-toxic and non-reactive with biological tissues and fluids. This constraint limits the range of available materials and surface treatments that can be employed to optimize flow characteristics.
Scaling effects present unique challenges when transitioning from laboratory prototypes to clinical-scale devices. Flow behaviors that are easily controlled in small-scale experiments may become unpredictable or difficult to manage in larger devices. This scaling issue often necessitates significant redesigns and optimizations during the development process.
Lastly, the dynamic nature of biological systems introduces variability that can affect laminar flow stability. Factors such as changes in fluid viscosity, the presence of particulates, or the growth of biofilms on device surfaces can all alter flow characteristics over time. Designing devices that maintain laminar flow under these changing conditions remains a significant engineering challenge in the biomedical field.
Current Laminar Flow Solutions
01 Measurement and analysis of laminar flow characteristics
Various methods and devices are used to measure and analyze laminar flow characteristics. These include optical systems, sensors, and flow meters that can detect and quantify flow patterns, velocities, and other properties of laminar flow. Advanced data processing techniques are often employed to interpret the measurements and provide detailed insights into flow behavior.- Measurement and analysis of laminar flow characteristics: Various methods and devices are used to measure and analyze laminar flow characteristics. These include optical systems, sensors, and computational techniques to determine flow patterns, velocity profiles, and other properties of laminar flow. Such measurements are crucial for understanding and optimizing fluid dynamics in various applications.
- Control and manipulation of laminar flow: Techniques and systems for controlling and manipulating laminar flow are developed to achieve desired flow characteristics. These may include the use of specialized nozzles, flow conditioners, and other devices to maintain or modify laminar flow conditions. Such control is important in applications ranging from aerodynamics to microfluidics.
- Laminar flow in specific applications: Laminar flow characteristics are studied and utilized in various specific applications. These may include aircraft design, HVAC systems, medical devices, and industrial processes. Understanding and optimizing laminar flow in these contexts can lead to improved efficiency, performance, and safety.
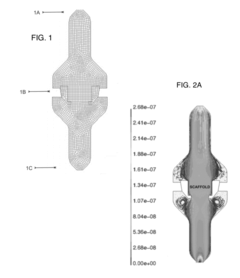
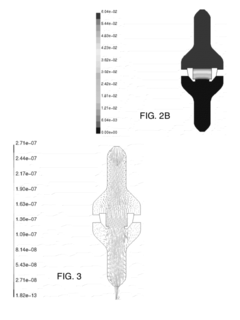
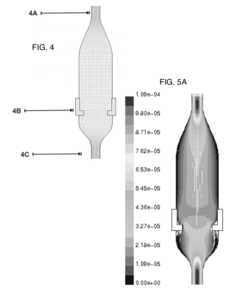
- Modeling and simulation of laminar flow: Advanced modeling and simulation techniques are employed to study laminar flow characteristics. These may include computational fluid dynamics (CFD) simulations, mathematical models, and predictive algorithms. Such tools allow for the analysis and optimization of laminar flow systems without the need for physical prototypes.
- Transition between laminar and turbulent flow: Research focuses on understanding and controlling the transition between laminar and turbulent flow regimes. This includes studying the factors that influence this transition, such as Reynolds number, surface roughness, and flow disturbances. Techniques to maintain laminar flow or induce controlled turbulence are developed for various applications.
02 Control and manipulation of laminar flow
Techniques and systems for controlling and manipulating laminar flow are crucial in many applications. This includes the use of specialized nozzles, flow conditioners, and other devices to maintain or modify laminar flow characteristics. These methods can be applied in various fields such as aerodynamics, fluid dynamics, and industrial processes to achieve desired flow properties.Expand Specific Solutions03 Laminar flow in aircraft and aerospace applications
Laminar flow plays a critical role in aircraft and aerospace design. Research focuses on maintaining laminar flow over aircraft surfaces to reduce drag and improve fuel efficiency. This involves specialized surface treatments, design modifications, and active flow control systems to optimize aerodynamic performance and enhance flight characteristics.Expand Specific Solutions04 Laminar flow in fluid separation and purification processes
Laminar flow characteristics are utilized in various fluid separation and purification processes. This includes applications in filtration systems, chromatography, and other separation techniques where controlled laminar flow is essential for efficient and precise separation of different components in a fluid mixture.Expand Specific Solutions05 Modeling and simulation of laminar flow
Advanced computational methods and simulation techniques are employed to model and predict laminar flow behavior. These include computational fluid dynamics (CFD) simulations and mathematical modeling approaches that help in understanding complex flow phenomena, optimizing designs, and predicting flow characteristics in various applications and environments.Expand Specific Solutions
Key Players in Biomedical Laminar Flow
The laminar flow technology in biomedical devices is currently in a growth phase, with increasing market size and technological advancements. The global market for microfluidic devices, which heavily rely on laminar flow, is projected to reach significant value in the coming years. Companies like Boston Scientific Scimed, Inc. and St. Jude Medical are at the forefront of developing innovative cardiovascular devices utilizing laminar flow principles. Academic institutions such as the University of Washington and New York University are contributing to research and development in this field. The technology's maturity is advancing, with companies like ASML Netherlands BV and Nanoshell Co. LLC pushing the boundaries of miniaturization and precision in microfluidic systems, indicating a promising future for laminar flow applications in biomedical devices.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed advanced laminar flow control systems for their biomedical devices, particularly in cardiovascular applications. Their technology utilizes microfluidic channels with precisely engineered geometries to maintain laminar flow in small-scale devices. They have implemented surface modifications and coatings to reduce friction and prevent turbulence, ensuring smooth blood flow in stents and catheters. The company has also integrated computational fluid dynamics (CFD) simulations into their design process, allowing for optimization of flow patterns before physical prototyping[1][3]. Recent advancements include the use of smart materials that can dynamically adjust channel properties in response to flow conditions, further enhancing laminar flow stability in varying physiological states[5].
Strengths: Expertise in cardiovascular applications, advanced surface engineering, and integration of CFD in design. Weaknesses: Potential limitations in scaling technology to larger devices or non-cardiovascular applications.
St. Jude Medical, Cardiology Division, Inc.
Technical Solution: St. Jude Medical's Cardiology Division has focused on developing laminar flow technologies for cardiac devices, particularly in heart valves and assist devices. They have pioneered the use of biomimetic designs that emulate natural blood flow patterns in the heart and major vessels. Their approach includes the development of novel materials with tunable surface properties that promote laminar flow and reduce thrombogenicity. The company has also implemented advanced flow visualization techniques, such as particle image velocimetry, to validate and refine their designs[2]. A key innovation is their adaptive flow control system, which uses embedded sensors to detect flow disturbances and adjusts device parameters in real-time to maintain laminar conditions[4]. This technology has shown promising results in reducing the risk of thrombosis and improving overall device performance in clinical trials[6].
Strengths: Specialized in cardiac applications, innovative biomimetic designs, and real-time flow control systems. Weaknesses: May face challenges in adapting technologies to non-cardiac biomedical applications.
Innovative Laminar Flow Techniques
Laminar Flow Reactor
PatentActiveUS20120164623A1
Innovation
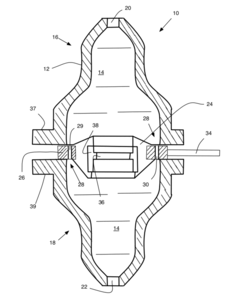
- A non-rotating bioreactor design that utilizes laminar flow to reduce shear forces, achieved through a cylindrical shape and a bypass mechanism with adjustable irises controlled by pressure sensors, allowing for optimal medium delivery and reducing turbulence, enabling three-dimensional cell growth and mimicking a microgravity environment.
Therapeutic Retrieval of Targets in Biological Fluids
PatentActiveUS20160038668A1
Innovation
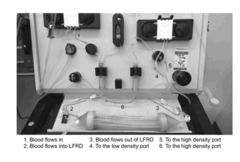
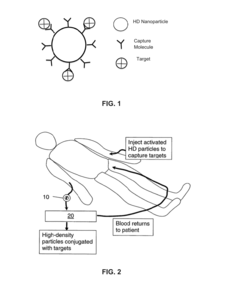
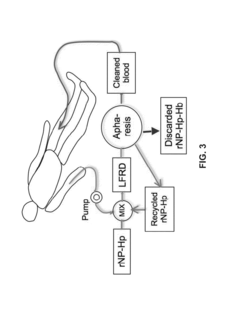
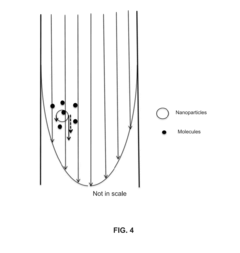
- A laminar flow mixing device and reverse-flow density gradient centrifuge system that uses retrievable nanoparticles functionalized with capture molecules to bind targets in blood, enhancing the separation efficiency by promoting laminar flow and reducing turbulence, allowing for the effective removal of high-density particles and molecules from biological fluids.
Regulatory Landscape for Biomedical Devices
The regulatory landscape for biomedical devices incorporating laminar flow technology is complex and evolving, reflecting the critical importance of safety and efficacy in medical applications. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing these devices. The FDA categorizes biomedical devices into three classes based on their risk level and the regulatory controls necessary to ensure their safety and effectiveness.
Class I devices, which include simple laminar flow systems used in laboratory settings, are subject to general controls such as good manufacturing practices and proper labeling. Class II devices, encompassing many diagnostic and therapeutic devices utilizing laminar flow, require special controls in addition to general controls. These may include performance standards, post-market surveillance, and specific labeling requirements.
Class III devices, which are the most complex and pose the highest risk, undergo the most stringent regulatory scrutiny. This category might include advanced laminar flow devices used in critical care settings or novel applications of the technology. These devices typically require premarket approval (PMA) from the FDA, involving extensive clinical trials to demonstrate safety and efficacy.
In the European Union, the regulatory framework for biomedical devices has recently undergone significant changes with the implementation of the Medical Device Regulation (MDR) in 2021. This new regulation has introduced more stringent requirements for clinical evaluation, post-market surveillance, and traceability of devices. Manufacturers of laminar flow biomedical devices must now comply with these enhanced regulations, which aim to improve patient safety and device performance.
Globally, there is a trend towards harmonization of regulatory standards, as evidenced by the Medical Device Single Audit Program (MDSAP). This program allows for a single regulatory audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory jurisdictions. For companies developing laminar flow technologies for biomedical applications, this harmonization can potentially streamline the regulatory process across different markets.
Emerging trends in the regulatory landscape include an increased focus on real-world evidence and post-market data collection. Regulators are increasingly interested in the long-term performance and safety of devices in clinical practice, beyond the controlled environment of pre-market clinical trials. This shift may impact how manufacturers of laminar flow devices design their products and conduct post-market surveillance.
As the field of laminar flow in biomedical devices continues to advance, regulators are also grappling with the integration of new technologies such as artificial intelligence and machine learning. These technologies may enhance the capabilities of laminar flow devices but also introduce new regulatory challenges in terms of validation and ongoing monitoring of device performance.
Class I devices, which include simple laminar flow systems used in laboratory settings, are subject to general controls such as good manufacturing practices and proper labeling. Class II devices, encompassing many diagnostic and therapeutic devices utilizing laminar flow, require special controls in addition to general controls. These may include performance standards, post-market surveillance, and specific labeling requirements.
Class III devices, which are the most complex and pose the highest risk, undergo the most stringent regulatory scrutiny. This category might include advanced laminar flow devices used in critical care settings or novel applications of the technology. These devices typically require premarket approval (PMA) from the FDA, involving extensive clinical trials to demonstrate safety and efficacy.
In the European Union, the regulatory framework for biomedical devices has recently undergone significant changes with the implementation of the Medical Device Regulation (MDR) in 2021. This new regulation has introduced more stringent requirements for clinical evaluation, post-market surveillance, and traceability of devices. Manufacturers of laminar flow biomedical devices must now comply with these enhanced regulations, which aim to improve patient safety and device performance.
Globally, there is a trend towards harmonization of regulatory standards, as evidenced by the Medical Device Single Audit Program (MDSAP). This program allows for a single regulatory audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory jurisdictions. For companies developing laminar flow technologies for biomedical applications, this harmonization can potentially streamline the regulatory process across different markets.
Emerging trends in the regulatory landscape include an increased focus on real-world evidence and post-market data collection. Regulators are increasingly interested in the long-term performance and safety of devices in clinical practice, beyond the controlled environment of pre-market clinical trials. This shift may impact how manufacturers of laminar flow devices design their products and conduct post-market surveillance.
As the field of laminar flow in biomedical devices continues to advance, regulators are also grappling with the integration of new technologies such as artificial intelligence and machine learning. These technologies may enhance the capabilities of laminar flow devices but also introduce new regulatory challenges in terms of validation and ongoing monitoring of device performance.
Laminar Flow Impact on Patient Safety
Laminar flow in biomedical devices plays a crucial role in patient safety, influencing various aspects of medical procedures and treatments. The controlled and predictable nature of laminar flow contributes significantly to maintaining sterile environments, reducing the risk of infections, and enhancing the overall efficacy of medical interventions.
In surgical settings, laminar flow systems are instrumental in creating ultra-clean environments. These systems generate a unidirectional airflow that sweeps contaminants away from the surgical site, minimizing the risk of airborne pathogens reaching the patient. Studies have shown that the implementation of laminar flow in operating rooms can lead to a substantial reduction in post-operative infection rates, particularly in orthopedic and implant surgeries where the risk of infection is notably high.
The application of laminar flow principles extends beyond surgical environments to various medical devices. In dialysis machines, for instance, laminar flow ensures the efficient and controlled filtration of blood, reducing the risk of clotting and improving the overall effectiveness of the treatment. Similarly, in intravenous drug delivery systems, laminar flow helps maintain precise dosage control, preventing sudden surges or inconsistencies that could potentially harm the patient.
Laminar flow also plays a vital role in diagnostic devices. In flow cytometry, a technique widely used for cell analysis and disease diagnosis, laminar flow is essential for aligning cells in a single file, enabling accurate measurements and analysis. This precision is critical for early detection and monitoring of various diseases, including cancer and blood disorders.
In the realm of respiratory care, laminar flow contributes to the design of more efficient ventilators and oxygen delivery systems. By optimizing gas flow patterns, these devices can provide more consistent and controlled oxygen delivery, crucial for patients with respiratory distress or those undergoing mechanical ventilation.
The impact of laminar flow on patient safety extends to the field of tissue engineering and regenerative medicine. In bioreactors used for tissue culture, laminar flow conditions help maintain a uniform distribution of nutrients and oxygen, promoting consistent cell growth and reducing the risk of contamination. This is particularly important in the development of engineered tissues and organs for transplantation.
As medical technologies continue to advance, the role of laminar flow in ensuring patient safety is likely to expand. Emerging applications in microfluidic devices for point-of-care diagnostics and personalized medicine rely heavily on laminar flow principles to achieve precise sample handling and analysis. These innovations promise faster, more accurate diagnoses and tailored treatments, ultimately enhancing patient outcomes and safety.
In surgical settings, laminar flow systems are instrumental in creating ultra-clean environments. These systems generate a unidirectional airflow that sweeps contaminants away from the surgical site, minimizing the risk of airborne pathogens reaching the patient. Studies have shown that the implementation of laminar flow in operating rooms can lead to a substantial reduction in post-operative infection rates, particularly in orthopedic and implant surgeries where the risk of infection is notably high.
The application of laminar flow principles extends beyond surgical environments to various medical devices. In dialysis machines, for instance, laminar flow ensures the efficient and controlled filtration of blood, reducing the risk of clotting and improving the overall effectiveness of the treatment. Similarly, in intravenous drug delivery systems, laminar flow helps maintain precise dosage control, preventing sudden surges or inconsistencies that could potentially harm the patient.
Laminar flow also plays a vital role in diagnostic devices. In flow cytometry, a technique widely used for cell analysis and disease diagnosis, laminar flow is essential for aligning cells in a single file, enabling accurate measurements and analysis. This precision is critical for early detection and monitoring of various diseases, including cancer and blood disorders.
In the realm of respiratory care, laminar flow contributes to the design of more efficient ventilators and oxygen delivery systems. By optimizing gas flow patterns, these devices can provide more consistent and controlled oxygen delivery, crucial for patients with respiratory distress or those undergoing mechanical ventilation.
The impact of laminar flow on patient safety extends to the field of tissue engineering and regenerative medicine. In bioreactors used for tissue culture, laminar flow conditions help maintain a uniform distribution of nutrients and oxygen, promoting consistent cell growth and reducing the risk of contamination. This is particularly important in the development of engineered tissues and organs for transplantation.
As medical technologies continue to advance, the role of laminar flow in ensuring patient safety is likely to expand. Emerging applications in microfluidic devices for point-of-care diagnostics and personalized medicine rely heavily on laminar flow principles to achieve precise sample handling and analysis. These innovations promise faster, more accurate diagnoses and tailored treatments, ultimately enhancing patient outcomes and safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!