Latest Research on PEMF Therapy's Role in Diabetes Management
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF in Diabetes: Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in various medical fields, including diabetes management. The technology's roots can be traced back to the mid-20th century, with significant advancements occurring in recent decades. PEMF therapy utilizes electromagnetic fields to stimulate cellular repair and regeneration, potentially offering a novel approach to addressing the complex challenges associated with diabetes.
The evolution of PEMF technology in diabetes management has been driven by a growing understanding of the cellular mechanisms underlying the disease. Initial research focused on the effects of electromagnetic fields on glucose metabolism and insulin sensitivity. As the field progressed, studies expanded to explore PEMF's potential in addressing diabetes-related complications, such as neuropathy, wound healing, and cardiovascular issues.
Current research aims to elucidate the precise mechanisms by which PEMF therapy influences diabetic pathophysiology. Key objectives include determining optimal treatment parameters, such as frequency, intensity, and duration of exposure, to maximize therapeutic benefits while ensuring patient safety. Additionally, researchers are investigating the potential synergistic effects of combining PEMF therapy with conventional diabetes treatments.
The global prevalence of diabetes, coupled with the limitations of existing management strategies, underscores the importance of exploring innovative approaches like PEMF therapy. According to the International Diabetes Federation, approximately 537 million adults were living with diabetes in 2021, a number projected to rise to 783 million by 2045. This staggering increase highlights the urgent need for more effective and accessible treatment options.
PEMF therapy's non-invasive nature and potential for home-based application make it an attractive avenue for research in diabetes management. The technology's ability to potentially address multiple aspects of the disease, from glucose regulation to complication prevention, aligns with the growing emphasis on holistic approaches to diabetes care.
As research in this field progresses, key objectives include establishing standardized protocols for PEMF application in diabetes, conducting large-scale clinical trials to validate efficacy and safety, and exploring the long-term effects of PEMF therapy on diabetes progression. Furthermore, there is a growing interest in understanding how PEMF therapy might be tailored to individual patient profiles, considering factors such as diabetes type, duration, and comorbidities.
The integration of PEMF therapy into diabetes management represents a convergence of electromagnetic science, cellular biology, and endocrinology. This interdisciplinary approach reflects the complex nature of diabetes and the need for innovative solutions that address the disease's multifaceted challenges. As research continues to unfold, PEMF therapy holds promise as a complementary tool in the comprehensive management of diabetes, potentially improving patient outcomes and quality of life.
The evolution of PEMF technology in diabetes management has been driven by a growing understanding of the cellular mechanisms underlying the disease. Initial research focused on the effects of electromagnetic fields on glucose metabolism and insulin sensitivity. As the field progressed, studies expanded to explore PEMF's potential in addressing diabetes-related complications, such as neuropathy, wound healing, and cardiovascular issues.
Current research aims to elucidate the precise mechanisms by which PEMF therapy influences diabetic pathophysiology. Key objectives include determining optimal treatment parameters, such as frequency, intensity, and duration of exposure, to maximize therapeutic benefits while ensuring patient safety. Additionally, researchers are investigating the potential synergistic effects of combining PEMF therapy with conventional diabetes treatments.
The global prevalence of diabetes, coupled with the limitations of existing management strategies, underscores the importance of exploring innovative approaches like PEMF therapy. According to the International Diabetes Federation, approximately 537 million adults were living with diabetes in 2021, a number projected to rise to 783 million by 2045. This staggering increase highlights the urgent need for more effective and accessible treatment options.
PEMF therapy's non-invasive nature and potential for home-based application make it an attractive avenue for research in diabetes management. The technology's ability to potentially address multiple aspects of the disease, from glucose regulation to complication prevention, aligns with the growing emphasis on holistic approaches to diabetes care.
As research in this field progresses, key objectives include establishing standardized protocols for PEMF application in diabetes, conducting large-scale clinical trials to validate efficacy and safety, and exploring the long-term effects of PEMF therapy on diabetes progression. Furthermore, there is a growing interest in understanding how PEMF therapy might be tailored to individual patient profiles, considering factors such as diabetes type, duration, and comorbidities.
The integration of PEMF therapy into diabetes management represents a convergence of electromagnetic science, cellular biology, and endocrinology. This interdisciplinary approach reflects the complex nature of diabetes and the need for innovative solutions that address the disease's multifaceted challenges. As research continues to unfold, PEMF therapy holds promise as a complementary tool in the comprehensive management of diabetes, potentially improving patient outcomes and quality of life.
Market Analysis for PEMF Diabetes Therapies
The market for PEMF (Pulsed Electromagnetic Field) therapies in diabetes management is experiencing significant growth, driven by the increasing prevalence of diabetes worldwide and the growing interest in non-invasive treatment options. The global diabetes market is projected to reach $78.3 billion by 2026, with a compound annual growth rate (CAGR) of 7.5% from 2021 to 2026. Within this broader market, PEMF therapies are carving out a niche as a complementary treatment approach.
The demand for PEMF diabetes therapies is primarily fueled by the rising number of diabetic patients seeking alternative or adjunct treatments to traditional medication-based approaches. As of 2021, an estimated 537 million adults worldwide were living with diabetes, and this number is expected to rise to 783 million by 2045. This growing patient population represents a substantial market opportunity for PEMF therapy devices and services.
Market segmentation for PEMF diabetes therapies can be categorized into home-use devices and clinical-grade equipment. The home-use segment is witnessing rapid growth due to the increasing preference for self-management tools among diabetic patients. Clinical-grade PEMF devices, while representing a smaller market share, are gaining traction in specialized diabetes treatment centers and hospitals.
Geographically, North America currently dominates the PEMF diabetes therapy market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years, driven by their large diabetic populations and improving healthcare access.
Key market drivers include the growing body of clinical evidence supporting the efficacy of PEMF therapy in managing diabetes-related complications, such as neuropathy and wound healing. Additionally, the non-invasive nature of PEMF therapy and its potential to reduce reliance on pharmaceutical interventions are attracting both patients and healthcare providers.
However, the market faces challenges, including the need for more extensive clinical trials to establish long-term efficacy and safety, regulatory hurdles in some regions, and the relatively high cost of advanced PEMF devices. These factors may slow market penetration, particularly in price-sensitive regions.
Looking ahead, the PEMF diabetes therapy market is poised for continued growth. Technological advancements, such as the development of more portable and user-friendly devices, are expected to drive adoption rates. Moreover, the increasing focus on personalized medicine and the integration of PEMF therapies into comprehensive diabetes management programs are likely to create new market opportunities in the coming years.
The demand for PEMF diabetes therapies is primarily fueled by the rising number of diabetic patients seeking alternative or adjunct treatments to traditional medication-based approaches. As of 2021, an estimated 537 million adults worldwide were living with diabetes, and this number is expected to rise to 783 million by 2045. This growing patient population represents a substantial market opportunity for PEMF therapy devices and services.
Market segmentation for PEMF diabetes therapies can be categorized into home-use devices and clinical-grade equipment. The home-use segment is witnessing rapid growth due to the increasing preference for self-management tools among diabetic patients. Clinical-grade PEMF devices, while representing a smaller market share, are gaining traction in specialized diabetes treatment centers and hospitals.
Geographically, North America currently dominates the PEMF diabetes therapy market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years, driven by their large diabetic populations and improving healthcare access.
Key market drivers include the growing body of clinical evidence supporting the efficacy of PEMF therapy in managing diabetes-related complications, such as neuropathy and wound healing. Additionally, the non-invasive nature of PEMF therapy and its potential to reduce reliance on pharmaceutical interventions are attracting both patients and healthcare providers.
However, the market faces challenges, including the need for more extensive clinical trials to establish long-term efficacy and safety, regulatory hurdles in some regions, and the relatively high cost of advanced PEMF devices. These factors may slow market penetration, particularly in price-sensitive regions.
Looking ahead, the PEMF diabetes therapy market is poised for continued growth. Technological advancements, such as the development of more portable and user-friendly devices, are expected to drive adoption rates. Moreover, the increasing focus on personalized medicine and the integration of PEMF therapies into comprehensive diabetes management programs are likely to create new market opportunities in the coming years.
Current PEMF Technology in Diabetes Management
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in diabetes management. Current PEMF technology for diabetes focuses on enhancing cellular function and promoting tissue repair through the application of low-frequency electromagnetic fields. These devices typically generate electromagnetic pulses in the range of 1-100 Hz, with field strengths varying from a few microtesla to several millitesla.
The most common PEMF devices used in diabetes management are portable, battery-operated units that can be applied directly to affected areas. These devices often feature adjustable intensity settings and pre-programmed treatment protocols tailored for different aspects of diabetes care. Some advanced systems incorporate real-time biofeedback mechanisms to optimize the electromagnetic field based on the patient's physiological responses.
In terms of application methods, PEMF therapy for diabetes management generally employs two main approaches: localized treatment and whole-body exposure. Localized treatment involves the use of small applicators or pads placed directly on specific areas of concern, such as diabetic foot ulcers or areas of neuropathic pain. Whole-body PEMF systems, on the other hand, utilize larger mats or chambers that envelop the entire body, aiming to provide systemic benefits.
Recent technological advancements have led to the development of more sophisticated PEMF devices specifically designed for diabetes management. These include wearable PEMF units that can be integrated into clothing or accessories, allowing for continuous treatment throughout the day. Some innovative systems combine PEMF therapy with other modalities, such as infrared light therapy or electrical stimulation, to enhance therapeutic outcomes.
The underlying mechanisms of PEMF therapy in diabetes management are believed to involve multiple pathways. At the cellular level, PEMF is thought to modulate ion channels, enhance mitochondrial function, and stimulate the production of growth factors and anti-inflammatory cytokines. These effects may contribute to improved insulin sensitivity, enhanced wound healing, and reduced diabetic complications.
Current PEMF technology also addresses the challenge of treatment adherence through user-friendly interfaces and mobile app integration. Many modern devices offer smartphone connectivity, allowing patients to track their treatment sessions, set reminders, and share data with healthcare providers. This integration of digital health technologies with PEMF therapy aims to improve patient engagement and treatment outcomes in diabetes management.
While PEMF therapy shows promise in diabetes care, it is important to note that the technology is still evolving, and more research is needed to establish standardized protocols and optimize treatment parameters for different aspects of diabetes management. As the field progresses, we can expect to see further refinements in PEMF technology, potentially leading to more targeted and personalized approaches in diabetes care.
The most common PEMF devices used in diabetes management are portable, battery-operated units that can be applied directly to affected areas. These devices often feature adjustable intensity settings and pre-programmed treatment protocols tailored for different aspects of diabetes care. Some advanced systems incorporate real-time biofeedback mechanisms to optimize the electromagnetic field based on the patient's physiological responses.
In terms of application methods, PEMF therapy for diabetes management generally employs two main approaches: localized treatment and whole-body exposure. Localized treatment involves the use of small applicators or pads placed directly on specific areas of concern, such as diabetic foot ulcers or areas of neuropathic pain. Whole-body PEMF systems, on the other hand, utilize larger mats or chambers that envelop the entire body, aiming to provide systemic benefits.
Recent technological advancements have led to the development of more sophisticated PEMF devices specifically designed for diabetes management. These include wearable PEMF units that can be integrated into clothing or accessories, allowing for continuous treatment throughout the day. Some innovative systems combine PEMF therapy with other modalities, such as infrared light therapy or electrical stimulation, to enhance therapeutic outcomes.
The underlying mechanisms of PEMF therapy in diabetes management are believed to involve multiple pathways. At the cellular level, PEMF is thought to modulate ion channels, enhance mitochondrial function, and stimulate the production of growth factors and anti-inflammatory cytokines. These effects may contribute to improved insulin sensitivity, enhanced wound healing, and reduced diabetic complications.
Current PEMF technology also addresses the challenge of treatment adherence through user-friendly interfaces and mobile app integration. Many modern devices offer smartphone connectivity, allowing patients to track their treatment sessions, set reminders, and share data with healthcare providers. This integration of digital health technologies with PEMF therapy aims to improve patient engagement and treatment outcomes in diabetes management.
While PEMF therapy shows promise in diabetes care, it is important to note that the technology is still evolving, and more research is needed to establish standardized protocols and optimize treatment parameters for different aspects of diabetes management. As the field progresses, we can expect to see further refinements in PEMF technology, potentially leading to more targeted and personalized approaches in diabetes care.
Existing PEMF Protocols for Diabetes
01 PEMF devices for therapeutic applications
Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular repair and regeneration, potentially treating conditions such as pain, inflammation, and bone healing. The devices can be configured for different body parts and treatment protocols.- PEMF devices for therapeutic applications: Pulsed Electromagnetic Field (PEMF) therapy devices are designed for various therapeutic applications. These devices generate electromagnetic fields to stimulate cellular repair and improve overall health. They can be used for pain management, tissue healing, and treating various medical conditions.
- PEMF therapy for specific medical conditions: PEMF therapy is utilized to treat specific medical conditions such as osteoarthritis, bone fractures, and neurological disorders. The therapy aims to reduce inflammation, promote tissue regeneration, and alleviate symptoms associated with these conditions. Different frequencies and intensities of electromagnetic fields are applied depending on the targeted condition.
- Portable and wearable PEMF devices: Advancements in PEMF technology have led to the development of portable and wearable devices. These compact devices allow for convenient at-home use or on-the-go treatments. They are designed to be user-friendly and can be applied to specific body parts for targeted therapy.
- Combination of PEMF with other therapies: PEMF therapy is often combined with other treatment modalities to enhance therapeutic outcomes. This may include integration with light therapy, heat therapy, or other forms of electromagnetic stimulation. The synergistic effects of these combined therapies aim to provide more comprehensive and effective treatment options.
- PEMF technology advancements and applications: Ongoing research and development in PEMF technology have led to new applications and improved treatment protocols. This includes the use of PEMF in veterinary medicine, agriculture, and even in space medicine. Advanced PEMF devices may incorporate features such as programmable treatment schedules, real-time monitoring, and integration with mobile applications for personalized therapy management.
02 PEMF therapy for pain management and tissue healing
PEMF therapy is utilized for pain management and tissue healing. The electromagnetic fields generated by PEMF devices can help reduce pain, decrease inflammation, and promote faster healing of injuries. This non-invasive treatment method is applied to various conditions, including musculoskeletal disorders and chronic pain syndromes.Expand Specific Solutions03 Combination of PEMF with other therapies
PEMF therapy is often combined with other treatment modalities to enhance therapeutic outcomes. This may include integration with light therapy, heat therapy, or other forms of electromagnetic stimulation. The combination approach aims to provide synergistic effects and improve overall treatment efficacy for various health conditions.Expand Specific Solutions04 Portable and wearable PEMF devices
Advancements in PEMF technology have led to the development of portable and wearable devices. These compact units allow for convenient, at-home use and continuous treatment. Wearable PEMF devices can be designed for specific body parts, such as knee braces or back supports, enabling targeted therapy during daily activities.Expand Specific Solutions05 PEMF therapy in veterinary and agricultural applications
PEMF therapy is not limited to human applications but also extends to veterinary medicine and agriculture. In veterinary care, PEMF devices are used to treat animals for pain, injuries, and various health conditions. In agriculture, PEMF technology is explored for potential benefits in plant growth, seed germination, and crop yield improvement.Expand Specific Solutions
Key Players in PEMF Diabetes Research
The research on PEMF therapy's role in diabetes management is in its early stages, with the market still developing. While the global diabetes management market is substantial, PEMF therapy's specific application in this area remains niche. The technology's maturity varies among key players. Established companies like Novo Nordisk A/S and Eli Lilly & Co. are exploring PEMF's potential in diabetes care, leveraging their extensive pharmaceutical expertise. Emerging players such as Bigfoot Biomedical, Inc. and LeviCure Ltd. are focusing on innovative approaches, integrating PEMF technology with digital health solutions. Research institutions like Sun Yat-Sen University and the Korea Advanced Institute of Science & Technology are contributing to the scientific understanding of PEMF's effects on diabetes, potentially accelerating its clinical adoption.
Novo Nordisk A/S
Technical Solution: Novo Nordisk, a global leader in diabetes care, has been investing in research on PEMF therapy as a potential complementary treatment for diabetes. Their approach focuses on using PEMF to enhance the effectiveness of insulin therapy and improve overall glucose metabolism. The company is developing a novel PEMF device designed to be used in conjunction with their insulin products, aiming to improve insulin sensitivity and glucose uptake in tissues[8]. Preliminary studies have shown that PEMF therapy may help reduce insulin resistance and improve glycemic control in type 2 diabetes patients when used alongside standard insulin regimens[9]. Novo Nordisk is also exploring the potential of PEMF to stimulate pancreatic beta cell regeneration, which could have significant implications for both type 1 and type 2 diabetes treatment[10].
Strengths: Extensive experience in diabetes research and treatment, potential for synergistic effects with existing insulin therapies. Weaknesses: PEMF technology is a departure from their core expertise, requiring significant investment in new research and development.
Bigfoot Biomedical, Inc.
Technical Solution: Bigfoot Biomedical, while primarily known for its diabetes management systems, has been exploring the integration of PEMF therapy into their existing technologies. Their approach combines continuous glucose monitoring (CGM) with a smart insulin delivery system, and they are investigating the potential of PEMF to enhance insulin sensitivity and glucose uptake. The company is developing a wearable PEMF device that can be synchronized with their insulin pump and CGM system, potentially offering a comprehensive diabetes management solution[4]. Preliminary research suggests that the addition of PEMF therapy to their system could lead to more stable blood glucose levels and reduced insulin requirements in some patients[5].
Strengths: Integrated approach combining established diabetes management technology with PEMF therapy. Weaknesses: Still in early stages of development and clinical validation for PEMF integration.
Innovative PEMF Mechanisms in Glucose Regulation
Pulsed Electromagnetic Field Devices Integrated into Adjustable Clothing
PatentPendingUS20230104434A1
Innovation
- A pulsed electromagnetic field device integrated into wearable clothing, using arrays of planar microcoils that generate controlled, homogenous magnetic fields, allowing for comfortable, long-term use and targeted treatment of various brain-related disorders and conditions.
Pulsed Electromagnetic Field (PEMF) Therapy Whole Body Wellness Device to increase cells energy, strengthen immune system and promote cell regeneration
PatentInactiveUS20190054308A1
Innovation
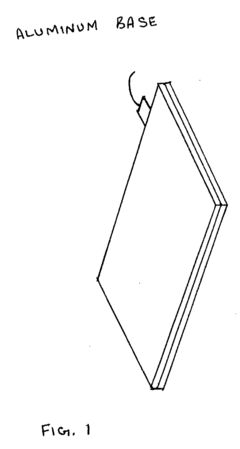
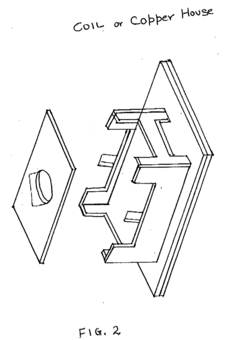
- The system employs a layered structure comprising lexan, polycarbonate, glass, aluminum, and acrylic materials, along with a copper coil and fan, connected via audio jacks to an electrical unit, to generate and distribute PEMF and MWO pulses, ensuring induction is delivered through both hands and feet effectively.
Clinical Trial Landscape for PEMF in Diabetes
The clinical trial landscape for PEMF in diabetes management has been evolving rapidly in recent years, reflecting the growing interest in this non-invasive therapeutic approach. Several key trends have emerged in the design and execution of these trials, providing valuable insights into the potential efficacy of PEMF therapy for diabetes patients.
One notable trend is the increasing focus on long-term studies to assess the sustained effects of PEMF therapy. While earlier trials often focused on short-term outcomes, recent studies have extended their duration to six months or longer. This shift allows researchers to evaluate the therapy's impact on glycemic control, insulin sensitivity, and other diabetes-related parameters over extended periods.
Another significant development in the clinical trial landscape is the diversification of study populations. Recent trials have included participants with various stages of diabetes, from pre-diabetes to advanced complications. This broader inclusion criteria enables a more comprehensive understanding of PEMF therapy's potential across the diabetes spectrum.
The use of advanced outcome measures has also become more prevalent in recent clinical trials. Beyond traditional markers like HbA1c levels, researchers are increasingly incorporating measures of oxidative stress, inflammatory markers, and microvascular function. This multifaceted approach provides a more nuanced understanding of PEMF therapy's physiological effects on diabetes management.
Interestingly, there has been a trend towards combining PEMF therapy with other interventions in clinical trials. Some studies have explored the synergistic effects of PEMF with lifestyle modifications, dietary interventions, or conventional diabetes medications. This combinatorial approach aims to identify optimal treatment strategies that leverage the potential benefits of PEMF therapy.
The design of PEMF devices used in clinical trials has also evolved. Researchers are now experimenting with different field strengths, frequencies, and exposure durations to optimize therapeutic outcomes. Some trials are utilizing portable PEMF devices, allowing for home-based treatments and potentially improving patient compliance.
Despite these advancements, challenges remain in the clinical trial landscape for PEMF in diabetes. One significant issue is the lack of standardization in treatment protocols across studies, making direct comparisons challenging. Additionally, many trials have relatively small sample sizes, limiting the generalizability of their findings.
Looking ahead, the future of clinical trials in this field is likely to focus on addressing these limitations. Larger, multi-center trials are being planned to provide more robust evidence. There is also a growing emphasis on personalized medicine approaches, with trials exploring how individual patient characteristics may influence responses to PEMF therapy in diabetes management.
One notable trend is the increasing focus on long-term studies to assess the sustained effects of PEMF therapy. While earlier trials often focused on short-term outcomes, recent studies have extended their duration to six months or longer. This shift allows researchers to evaluate the therapy's impact on glycemic control, insulin sensitivity, and other diabetes-related parameters over extended periods.
Another significant development in the clinical trial landscape is the diversification of study populations. Recent trials have included participants with various stages of diabetes, from pre-diabetes to advanced complications. This broader inclusion criteria enables a more comprehensive understanding of PEMF therapy's potential across the diabetes spectrum.
The use of advanced outcome measures has also become more prevalent in recent clinical trials. Beyond traditional markers like HbA1c levels, researchers are increasingly incorporating measures of oxidative stress, inflammatory markers, and microvascular function. This multifaceted approach provides a more nuanced understanding of PEMF therapy's physiological effects on diabetes management.
Interestingly, there has been a trend towards combining PEMF therapy with other interventions in clinical trials. Some studies have explored the synergistic effects of PEMF with lifestyle modifications, dietary interventions, or conventional diabetes medications. This combinatorial approach aims to identify optimal treatment strategies that leverage the potential benefits of PEMF therapy.
The design of PEMF devices used in clinical trials has also evolved. Researchers are now experimenting with different field strengths, frequencies, and exposure durations to optimize therapeutic outcomes. Some trials are utilizing portable PEMF devices, allowing for home-based treatments and potentially improving patient compliance.
Despite these advancements, challenges remain in the clinical trial landscape for PEMF in diabetes. One significant issue is the lack of standardization in treatment protocols across studies, making direct comparisons challenging. Additionally, many trials have relatively small sample sizes, limiting the generalizability of their findings.
Looking ahead, the future of clinical trials in this field is likely to focus on addressing these limitations. Larger, multi-center trials are being planned to provide more robust evidence. There is also a growing emphasis on personalized medicine approaches, with trials exploring how individual patient characteristics may influence responses to PEMF therapy in diabetes management.
Regulatory Considerations for PEMF Medical Devices
The regulatory landscape for Pulsed Electromagnetic Field (PEMF) medical devices in diabetes management is complex and evolving. In the United States, the Food and Drug Administration (FDA) classifies PEMF devices as Class II medical devices, requiring premarket notification (510(k)) clearance before they can be legally marketed for therapeutic use. This classification reflects the moderate risk associated with these devices and the need for special controls to ensure their safety and effectiveness.
For PEMF devices targeting diabetes management, manufacturers must demonstrate substantial equivalence to a legally marketed predicate device. This process involves providing comprehensive data on the device's safety, effectiveness, and technological characteristics. Clinical studies may be necessary to support claims related to diabetes management, particularly if the intended use differs significantly from existing cleared devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must comply with the MDR's stringent requirements, including clinical evaluation, risk management, and post-market surveillance. The CE marking process for these devices typically involves a notified body assessment, especially for higher-risk classifications.
Regulatory bodies worldwide are increasingly focusing on the quality of clinical evidence supporting medical device claims. For PEMF devices in diabetes management, this translates to a growing need for well-designed clinical trials that specifically address diabetic outcomes. Manufacturers should be prepared to provide robust data on the device's impact on blood glucose control, insulin sensitivity, and other relevant diabetes markers.
Safety considerations are paramount in the regulatory process. PEMF devices must meet electromagnetic compatibility (EMC) standards to ensure they do not interfere with other medical devices or implants. Additionally, regulators may require specific labeling and user instructions to address potential risks associated with electromagnetic field exposure, particularly for long-term use in diabetes management.
As the field of PEMF therapy in diabetes management is relatively new, regulatory agencies may require post-market studies to gather long-term safety and efficacy data. This ongoing surveillance helps to identify any unforeseen risks or benefits that may emerge with widespread use in the diabetic population.
Manufacturers should also be aware of the potential for regulatory changes as more research emerges on PEMF therapy for diabetes. Staying informed about evolving guidelines and maintaining open communication with regulatory bodies is crucial for successful market entry and continued compliance.
For PEMF devices targeting diabetes management, manufacturers must demonstrate substantial equivalence to a legally marketed predicate device. This process involves providing comprehensive data on the device's safety, effectiveness, and technological characteristics. Clinical studies may be necessary to support claims related to diabetes management, particularly if the intended use differs significantly from existing cleared devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must comply with the MDR's stringent requirements, including clinical evaluation, risk management, and post-market surveillance. The CE marking process for these devices typically involves a notified body assessment, especially for higher-risk classifications.
Regulatory bodies worldwide are increasingly focusing on the quality of clinical evidence supporting medical device claims. For PEMF devices in diabetes management, this translates to a growing need for well-designed clinical trials that specifically address diabetic outcomes. Manufacturers should be prepared to provide robust data on the device's impact on blood glucose control, insulin sensitivity, and other relevant diabetes markers.
Safety considerations are paramount in the regulatory process. PEMF devices must meet electromagnetic compatibility (EMC) standards to ensure they do not interfere with other medical devices or implants. Additionally, regulators may require specific labeling and user instructions to address potential risks associated with electromagnetic field exposure, particularly for long-term use in diabetes management.
As the field of PEMF therapy in diabetes management is relatively new, regulatory agencies may require post-market studies to gather long-term safety and efficacy data. This ongoing surveillance helps to identify any unforeseen risks or benefits that may emerge with widespread use in the diabetic population.
Manufacturers should also be aware of the potential for regulatory changes as more research emerges on PEMF therapy for diabetes. Staying informed about evolving guidelines and maintaining open communication with regulatory bodies is crucial for successful market entry and continued compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!