PEMF Therapy Applications: Revolutionizing Bone Healing
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Evolution
Pulsed Electromagnetic Field (PEMF) therapy has undergone significant evolution since its inception in the mid-20th century. Initially developed for bone healing applications, PEMF therapy has expanded its reach to various medical fields, revolutionizing treatment approaches for musculoskeletal disorders and beyond.
The journey of PEMF therapy began in the 1950s when scientists first observed the piezoelectric effect in bones. This discovery led to the understanding that electrical stimulation could potentially accelerate bone healing. By the 1970s, researchers had developed the first PEMF devices specifically designed for treating nonunion fractures, marking a significant milestone in orthopedic medicine.
Throughout the 1980s and 1990s, PEMF therapy gained traction as clinical studies demonstrated its efficacy in promoting bone healing. The U.S. Food and Drug Administration (FDA) approved PEMF devices for treating nonunion fractures in 1979, followed by approval for spinal fusion surgery in 1987. These regulatory milestones paved the way for wider adoption of PEMF therapy in clinical settings.
As technology advanced, PEMF devices became more sophisticated and user-friendly. The early 2000s saw the development of portable and wearable PEMF devices, making the therapy more accessible for home use. This shift allowed for more consistent and prolonged treatment, potentially improving outcomes for patients with chronic conditions.
In recent years, research has expanded the potential applications of PEMF therapy beyond bone healing. Studies have explored its effectiveness in treating osteoarthritis, chronic pain, wound healing, and even neurological disorders. The therapy's ability to modulate cellular activity and promote tissue regeneration has opened new avenues for its use in regenerative medicine.
The evolution of PEMF therapy has also been marked by advancements in treatment protocols and device design. Modern PEMF devices offer a range of frequencies and intensities, allowing for more targeted and personalized treatments. Additionally, the integration of PEMF therapy with other treatment modalities, such as physical therapy and pharmacological interventions, has led to more comprehensive approaches to patient care.
As PEMF therapy continues to evolve, researchers are exploring its potential in emerging fields such as stem cell therapy and tissue engineering. The therapy's non-invasive nature and minimal side effects make it an attractive option for enhancing traditional treatment methods and developing novel therapeutic approaches.
The journey of PEMF therapy began in the 1950s when scientists first observed the piezoelectric effect in bones. This discovery led to the understanding that electrical stimulation could potentially accelerate bone healing. By the 1970s, researchers had developed the first PEMF devices specifically designed for treating nonunion fractures, marking a significant milestone in orthopedic medicine.
Throughout the 1980s and 1990s, PEMF therapy gained traction as clinical studies demonstrated its efficacy in promoting bone healing. The U.S. Food and Drug Administration (FDA) approved PEMF devices for treating nonunion fractures in 1979, followed by approval for spinal fusion surgery in 1987. These regulatory milestones paved the way for wider adoption of PEMF therapy in clinical settings.
As technology advanced, PEMF devices became more sophisticated and user-friendly. The early 2000s saw the development of portable and wearable PEMF devices, making the therapy more accessible for home use. This shift allowed for more consistent and prolonged treatment, potentially improving outcomes for patients with chronic conditions.
In recent years, research has expanded the potential applications of PEMF therapy beyond bone healing. Studies have explored its effectiveness in treating osteoarthritis, chronic pain, wound healing, and even neurological disorders. The therapy's ability to modulate cellular activity and promote tissue regeneration has opened new avenues for its use in regenerative medicine.
The evolution of PEMF therapy has also been marked by advancements in treatment protocols and device design. Modern PEMF devices offer a range of frequencies and intensities, allowing for more targeted and personalized treatments. Additionally, the integration of PEMF therapy with other treatment modalities, such as physical therapy and pharmacological interventions, has led to more comprehensive approaches to patient care.
As PEMF therapy continues to evolve, researchers are exploring its potential in emerging fields such as stem cell therapy and tissue engineering. The therapy's non-invasive nature and minimal side effects make it an attractive option for enhancing traditional treatment methods and developing novel therapeutic approaches.
Bone Healing Market Needs
The global bone healing market is experiencing significant growth, driven by an aging population, increasing incidence of bone fractures, and a rising demand for advanced treatment options. As populations age worldwide, the prevalence of osteoporosis and related fractures is on the rise, creating a substantial need for effective bone healing therapies. Additionally, the growing participation in sports and recreational activities has led to an increase in sports-related injuries, further expanding the market for bone healing solutions.
The market demand for bone healing treatments is also influenced by the rising prevalence of chronic diseases such as diabetes and obesity, which can impair natural bone healing processes. This has created a need for more advanced and targeted therapies to address complex bone healing challenges. Furthermore, there is a growing preference for minimally invasive procedures and outpatient treatments, driving the demand for innovative bone healing technologies that can be administered with minimal disruption to patients' lives.
In recent years, there has been a shift towards personalized medicine in bone healing, with patients and healthcare providers seeking tailored treatment options that consider individual patient factors such as age, overall health, and specific fracture characteristics. This trend has led to an increased demand for advanced diagnostic tools and customized treatment plans, including the use of biocompatible materials and growth factors to enhance bone regeneration.
The market is also witnessing a growing interest in non-invasive and drug-free treatment options, particularly for patients who may be at risk of complications from traditional surgical interventions. This has created an opportunity for technologies like Pulsed Electromagnetic Field (PEMF) therapy, which offers a non-invasive approach to stimulating bone healing and regeneration.
Economic factors play a significant role in shaping market demand, with healthcare systems and insurers increasingly focusing on cost-effective treatments that can reduce hospitalization times and improve patient outcomes. This has led to a growing interest in therapies that can accelerate the bone healing process and reduce the risk of complications, potentially lowering overall healthcare costs.
Geographically, the bone healing market is experiencing rapid growth in emerging economies, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical treatments. In developed markets, the demand is being fueled by technological advancements and a growing emphasis on improving quality of life for patients with bone-related conditions.
The market demand for bone healing treatments is also influenced by the rising prevalence of chronic diseases such as diabetes and obesity, which can impair natural bone healing processes. This has created a need for more advanced and targeted therapies to address complex bone healing challenges. Furthermore, there is a growing preference for minimally invasive procedures and outpatient treatments, driving the demand for innovative bone healing technologies that can be administered with minimal disruption to patients' lives.
In recent years, there has been a shift towards personalized medicine in bone healing, with patients and healthcare providers seeking tailored treatment options that consider individual patient factors such as age, overall health, and specific fracture characteristics. This trend has led to an increased demand for advanced diagnostic tools and customized treatment plans, including the use of biocompatible materials and growth factors to enhance bone regeneration.
The market is also witnessing a growing interest in non-invasive and drug-free treatment options, particularly for patients who may be at risk of complications from traditional surgical interventions. This has created an opportunity for technologies like Pulsed Electromagnetic Field (PEMF) therapy, which offers a non-invasive approach to stimulating bone healing and regeneration.
Economic factors play a significant role in shaping market demand, with healthcare systems and insurers increasingly focusing on cost-effective treatments that can reduce hospitalization times and improve patient outcomes. This has led to a growing interest in therapies that can accelerate the bone healing process and reduce the risk of complications, potentially lowering overall healthcare costs.
Geographically, the bone healing market is experiencing rapid growth in emerging economies, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical treatments. In developed markets, the demand is being fueled by technological advancements and a growing emphasis on improving quality of life for patients with bone-related conditions.
PEMF Tech Challenges
Despite the promising potential of Pulsed Electromagnetic Field (PEMF) therapy in bone healing, several technical challenges persist in its widespread adoption and optimization. One of the primary hurdles is the lack of standardization in PEMF devices and treatment protocols. The variability in frequency, intensity, and duration of electromagnetic pulses across different devices makes it difficult to establish consistent treatment guidelines and compare efficacy across studies.
Another significant challenge lies in the precise targeting of PEMF therapy to specific bone regions. Current technologies often struggle to focus the electromagnetic field accurately on the desired area without affecting surrounding tissues. This limitation can lead to reduced treatment effectiveness and potential side effects in non-targeted areas.
The optimization of PEMF parameters for different types of bone injuries and patient conditions remains a complex issue. Factors such as bone density, injury severity, and individual patient characteristics can significantly influence the optimal PEMF settings. Developing adaptive PEMF systems that can adjust parameters based on real-time feedback from the healing process is a major technical hurdle.
The integration of PEMF devices with existing medical imaging technologies presents another challenge. Enhancing the compatibility of PEMF devices with MRI, CT scans, and other diagnostic tools would greatly improve treatment monitoring and personalization. However, achieving this integration without compromising the functionality of either the PEMF device or the imaging equipment is technically demanding.
Long-term effects and safety concerns of PEMF therapy, especially in prolonged or repeated treatments, require further investigation. While short-term studies have shown promising results, the potential long-term impacts on bone structure, surrounding tissues, and overall patient health need more comprehensive research and advanced monitoring techniques.
The miniaturization and portability of PEMF devices for home use present additional technical challenges. Developing compact, user-friendly devices that maintain therapeutic efficacy while ensuring patient safety and compliance is a complex engineering task. This includes addressing issues of power supply, durability, and ease of use for non-medical professionals.
Lastly, the development of more sophisticated computational models for predicting PEMF therapy outcomes is crucial. Current models often struggle to accurately simulate the complex interactions between electromagnetic fields and biological tissues. Improving these models would greatly enhance treatment planning and device optimization, but requires advancements in both computational power and our understanding of bioelectromagnetic interactions.
Another significant challenge lies in the precise targeting of PEMF therapy to specific bone regions. Current technologies often struggle to focus the electromagnetic field accurately on the desired area without affecting surrounding tissues. This limitation can lead to reduced treatment effectiveness and potential side effects in non-targeted areas.
The optimization of PEMF parameters for different types of bone injuries and patient conditions remains a complex issue. Factors such as bone density, injury severity, and individual patient characteristics can significantly influence the optimal PEMF settings. Developing adaptive PEMF systems that can adjust parameters based on real-time feedback from the healing process is a major technical hurdle.
The integration of PEMF devices with existing medical imaging technologies presents another challenge. Enhancing the compatibility of PEMF devices with MRI, CT scans, and other diagnostic tools would greatly improve treatment monitoring and personalization. However, achieving this integration without compromising the functionality of either the PEMF device or the imaging equipment is technically demanding.
Long-term effects and safety concerns of PEMF therapy, especially in prolonged or repeated treatments, require further investigation. While short-term studies have shown promising results, the potential long-term impacts on bone structure, surrounding tissues, and overall patient health need more comprehensive research and advanced monitoring techniques.
The miniaturization and portability of PEMF devices for home use present additional technical challenges. Developing compact, user-friendly devices that maintain therapeutic efficacy while ensuring patient safety and compliance is a complex engineering task. This includes addressing issues of power supply, durability, and ease of use for non-medical professionals.
Lastly, the development of more sophisticated computational models for predicting PEMF therapy outcomes is crucial. Current models often struggle to accurately simulate the complex interactions between electromagnetic fields and biological tissues. Improving these models would greatly enhance treatment planning and device optimization, but requires advancements in both computational power and our understanding of bioelectromagnetic interactions.
Current PEMF Solutions
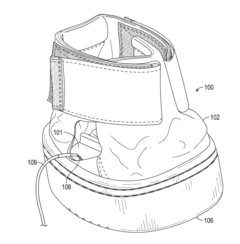
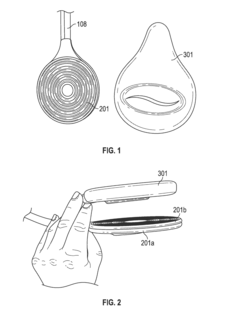
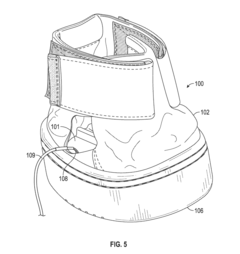
01 PEMF devices for bone healing
Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for bone healing. These devices generate electromagnetic fields that stimulate cellular activity and promote bone regeneration. They can be used to treat various bone-related conditions, including fractures, osteoporosis, and non-union fractures.- PEMF devices for bone healing: Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for bone healing. These devices generate electromagnetic fields that stimulate cellular activity and promote bone regeneration. They can be used to treat various bone-related conditions, including fractures, osteoporosis, and non-union fractures.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other treatment modalities to enhance bone healing. This may include combining PEMF with ultrasound therapy, electrical stimulation, or specific medications. The synergistic effect of these combined therapies can potentially accelerate the bone healing process and improve overall outcomes.
- Wearable PEMF devices for continuous treatment: Wearable PEMF devices allow for continuous or extended treatment periods, which can be beneficial for bone healing. These devices are designed to be portable and comfortable, enabling patients to receive therapy while going about their daily activities. This approach may lead to improved compliance and potentially better healing outcomes.
- PEMF therapy for specific bone conditions: PEMF therapy can be tailored for specific bone conditions or anatomical locations. This includes specialized treatments for spinal fusion, dental implants, or joint-specific applications. By optimizing the PEMF parameters for these specific conditions, the therapy can be more effective in promoting bone healing in targeted areas.
- Advanced PEMF signal patterns and control systems: Innovations in PEMF therapy for bone healing include advanced signal patterns and control systems. These developments focus on optimizing the electromagnetic field characteristics, such as frequency, intensity, and waveform, to maximize the therapeutic effect on bone cells. Smart control systems may also adjust treatment parameters based on patient feedback or healing progress.
02 Combination of PEMF with other therapies
PEMF therapy can be combined with other treatment modalities to enhance bone healing. This may include combining PEMF with ultrasound therapy, electrical stimulation, or specific medications. The synergistic effect of these combinations can potentially accelerate the healing process and improve overall outcomes in bone-related injuries and conditions.Expand Specific Solutions03 Wearable PEMF devices for continuous treatment
Wearable PEMF devices allow for continuous or extended treatment periods, which can be beneficial for bone healing. These devices are designed to be portable and comfortable, enabling patients to receive therapy while going about their daily activities. This approach may lead to improved patient compliance and potentially faster healing times.Expand Specific Solutions04 PEMF therapy for specific bone conditions
PEMF therapy can be tailored to address specific bone conditions. This includes treatments for osteoporosis, spinal fusion, dental implants, and joint replacements. The therapy parameters, such as frequency, intensity, and duration, can be adjusted to target the specific needs of different bone-related issues, potentially improving the efficacy of the treatment.Expand Specific Solutions05 PEMF therapy in veterinary applications
PEMF therapy is not limited to human applications but can also be used in veterinary medicine for bone healing in animals. This includes treatments for fractures, arthritis, and other bone-related conditions in pets and livestock. The therapy can be adapted to suit different animal sizes and species, providing a non-invasive treatment option for veterinary care.Expand Specific Solutions
PEMF Industry Leaders
The PEMF therapy market for bone healing is in a growth phase, with increasing adoption and expanding applications. The market size is projected to grow significantly due to rising awareness of non-invasive treatments and an aging population prone to bone-related issues. Technologically, PEMF therapy is advancing rapidly, with companies like Venus Concept Ltd., SofPulse, Inc., and Regenesis Biomedical, Inc. leading innovation. These firms are developing more sophisticated, targeted devices, improving efficacy and patient outcomes. Academic institutions such as the National University of Singapore and Swiss Federal Institute of Technology are contributing to the research base, enhancing the technology's credibility and potential applications. The competitive landscape is diverse, with both established medical device companies and specialized PEMF therapy providers vying for market share.
Venus Concept Ltd.
Technical Solution: Venus Concept has developed the Venus Heal system, which incorporates PEMF technology for musculoskeletal conditions, including bone healing. Their approach combines PEMF with other modalities such as massage therapy and heat for a comprehensive treatment solution[6]. The Venus Heal system uses adjustable PEMF frequencies and intensities to target different tissue types and conditions. The technology is designed to increase local blood circulation, reduce inflammation, and promote cellular regeneration. Venus Concept's PEMF devices are used in clinical settings and have shown promising results in accelerating healing processes for various musculoskeletal injuries[7]. The company focuses on creating versatile, multi-modality systems that can address a range of therapeutic needs.
Strengths: Multi-modality approach, versatile applications beyond bone healing. Weaknesses: Primarily designed for clinical use, may be less accessible for home treatment.
SofPulse, Inc.
Technical Solution: SofPulse has developed a unique PEMF technology focused on reducing post-surgical pain and edema, which can indirectly benefit bone healing processes. Their devices use targeted electromagnetic field therapy (tEMF) to deliver low-level, pulsed electromagnetic fields to the treatment area[10]. The SofPulse system is designed to be ultra-portable and easy to use, allowing for continuous treatment immediately after surgery. While not specifically marketed for bone healing, the technology's ability to reduce inflammation and promote tissue repair can support the overall healing process in orthopedic surgeries. SofPulse devices have been used in various clinical settings, with studies showing significant reductions in pain medication use and faster recovery times[11]. The company's focus on creating wearable, user-friendly PEMF devices sets them apart in the field of post-operative care.
Strengths: Ultra-portable design, focused on post-operative care which can indirectly benefit bone healing. Weaknesses: Not primarily designed for bone healing, may have limited direct impact on bone regeneration.
Key PEMF Innovations
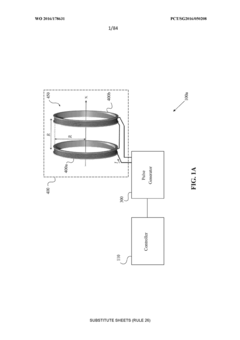
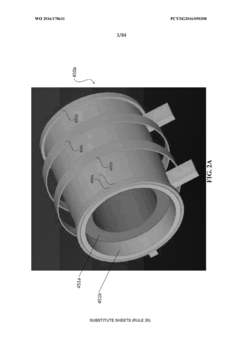
Equine Hoof Pulsed Electromagnetic Field Therapy System and Apparatus
PatentActiveUS20150151136A1
Innovation
- A system comprising PEMF coils encased in an elastomer structure, forming a 'puck', is integrated into an equine boot assembly with an elastomeric orthotic pad to apply PEMF directly to the underside of the hoof, providing protection and stability.
System and method for applying pulsed electromagnetic fields
PatentWO2016178631A1
Innovation
- A system comprising a sensor to detect tissue characteristics, a memory module with EMF efficacy window data, and a controller to adjust PEMF signal parameters such as amplitude, frequency, and duration based on the detected characteristics, ensuring optimal PEMF application.
Clinical Trial Landscape
The clinical trial landscape for PEMF (Pulsed Electromagnetic Field) therapy in bone healing has been rapidly evolving, with an increasing number of studies demonstrating its efficacy and safety. Recent years have seen a surge in well-designed, randomized controlled trials that aim to establish the optimal parameters and protocols for PEMF application in various bone healing scenarios.
One of the most significant trends in PEMF clinical trials is the focus on specific bone healing conditions. Studies have been conducted on fracture healing, spinal fusion, osteoporosis, and non-union fractures. These trials have shown promising results, with many reporting accelerated healing times and improved bone density compared to conventional treatments alone.
The design of PEMF clinical trials has also become more sophisticated. Many recent studies have employed double-blind, placebo-controlled methodologies to minimize bias and enhance the reliability of results. Additionally, there has been a shift towards longer follow-up periods, allowing researchers to assess the long-term effects of PEMF therapy on bone health and patient outcomes.
Another notable aspect of the clinical trial landscape is the exploration of different PEMF parameters. Researchers have been investigating the impact of varying frequencies, intensities, and treatment durations on bone healing efficacy. This has led to a better understanding of the optimal PEMF settings for different types of bone injuries and conditions.
Multicenter trials have become more common, involving collaboration between multiple hospitals and research institutions. These larger-scale studies have provided more robust data sets and increased the generalizability of findings across diverse patient populations.
The integration of advanced imaging techniques in PEMF clinical trials has significantly enhanced the ability to quantify and visualize bone healing progress. High-resolution CT scans, MRI, and bone densitometry are now routinely used to assess outcomes, providing more objective and detailed measures of bone regeneration and structural integrity.
Recent clinical trials have also begun to explore the combination of PEMF therapy with other bone healing modalities, such as growth factors, stem cell treatments, and bioengineered scaffolds. These combination therapies show promise in addressing complex bone healing challenges and may represent the future direction of orthopedic treatments.
As the body of evidence grows, meta-analyses and systematic reviews of PEMF clinical trials are becoming more prevalent. These comprehensive studies are crucial in synthesizing data from multiple trials and providing a more holistic view of PEMF therapy's effectiveness across different bone healing applications.
One of the most significant trends in PEMF clinical trials is the focus on specific bone healing conditions. Studies have been conducted on fracture healing, spinal fusion, osteoporosis, and non-union fractures. These trials have shown promising results, with many reporting accelerated healing times and improved bone density compared to conventional treatments alone.
The design of PEMF clinical trials has also become more sophisticated. Many recent studies have employed double-blind, placebo-controlled methodologies to minimize bias and enhance the reliability of results. Additionally, there has been a shift towards longer follow-up periods, allowing researchers to assess the long-term effects of PEMF therapy on bone health and patient outcomes.
Another notable aspect of the clinical trial landscape is the exploration of different PEMF parameters. Researchers have been investigating the impact of varying frequencies, intensities, and treatment durations on bone healing efficacy. This has led to a better understanding of the optimal PEMF settings for different types of bone injuries and conditions.
Multicenter trials have become more common, involving collaboration between multiple hospitals and research institutions. These larger-scale studies have provided more robust data sets and increased the generalizability of findings across diverse patient populations.
The integration of advanced imaging techniques in PEMF clinical trials has significantly enhanced the ability to quantify and visualize bone healing progress. High-resolution CT scans, MRI, and bone densitometry are now routinely used to assess outcomes, providing more objective and detailed measures of bone regeneration and structural integrity.
Recent clinical trials have also begun to explore the combination of PEMF therapy with other bone healing modalities, such as growth factors, stem cell treatments, and bioengineered scaffolds. These combination therapies show promise in addressing complex bone healing challenges and may represent the future direction of orthopedic treatments.
As the body of evidence grows, meta-analyses and systematic reviews of PEMF clinical trials are becoming more prevalent. These comprehensive studies are crucial in synthesizing data from multiple trials and providing a more holistic view of PEMF therapy's effectiveness across different bone healing applications.
Safety and Regulations
The safety and regulatory landscape surrounding Pulsed Electromagnetic Field (PEMF) therapy for bone healing is a critical aspect of its widespread adoption and implementation. As with any medical treatment, ensuring patient safety is paramount, and regulatory bodies play a crucial role in overseeing the development, testing, and deployment of PEMF devices.
In the United States, the Food and Drug Administration (FDA) has classified PEMF devices for bone healing as Class III medical devices. This classification requires manufacturers to obtain premarket approval (PMA) before marketing their products. The PMA process involves rigorous clinical trials to demonstrate the safety and efficacy of the device. Several PEMF devices have successfully obtained FDA approval for specific indications, such as the treatment of non-union fractures and spinal fusion adjuncts.
The regulatory framework in Europe is governed by the Medical Device Regulation (MDR), which came into effect in May 2021. Under the MDR, PEMF devices for bone healing are typically classified as Class IIa or IIb medical devices, depending on their specific characteristics and intended use. Manufacturers must comply with the Essential Requirements outlined in the MDR and obtain CE marking before placing their devices on the European market.
Safety considerations for PEMF therapy include potential electromagnetic interference with other medical devices, particularly implanted electronic devices such as pacemakers or defibrillators. Manufacturers are required to provide clear contraindications and warnings in their product labeling and user manuals. Additionally, long-term safety studies are ongoing to assess any potential risks associated with prolonged exposure to electromagnetic fields.
The International Commission on Non-Ionizing Radiation Protection (ICNIRP) provides guidelines on exposure limits to electromagnetic fields. While PEMF therapy typically operates within these safety limits, adherence to these guidelines is essential for ensuring patient safety and regulatory compliance.
As the field of PEMF therapy continues to evolve, regulatory bodies are adapting their approaches to keep pace with technological advancements. This includes developing new standards for assessing the safety and efficacy of novel PEMF devices and treatment protocols. Collaboration between regulatory agencies, researchers, and manufacturers is crucial for establishing evidence-based guidelines and ensuring the safe and effective use of PEMF therapy in bone healing applications.
In conclusion, the safety and regulatory landscape for PEMF therapy in bone healing is complex and dynamic. Adherence to established regulations and ongoing research into long-term safety are essential for the continued development and adoption of this promising therapeutic approach.
In the United States, the Food and Drug Administration (FDA) has classified PEMF devices for bone healing as Class III medical devices. This classification requires manufacturers to obtain premarket approval (PMA) before marketing their products. The PMA process involves rigorous clinical trials to demonstrate the safety and efficacy of the device. Several PEMF devices have successfully obtained FDA approval for specific indications, such as the treatment of non-union fractures and spinal fusion adjuncts.
The regulatory framework in Europe is governed by the Medical Device Regulation (MDR), which came into effect in May 2021. Under the MDR, PEMF devices for bone healing are typically classified as Class IIa or IIb medical devices, depending on their specific characteristics and intended use. Manufacturers must comply with the Essential Requirements outlined in the MDR and obtain CE marking before placing their devices on the European market.
Safety considerations for PEMF therapy include potential electromagnetic interference with other medical devices, particularly implanted electronic devices such as pacemakers or defibrillators. Manufacturers are required to provide clear contraindications and warnings in their product labeling and user manuals. Additionally, long-term safety studies are ongoing to assess any potential risks associated with prolonged exposure to electromagnetic fields.
The International Commission on Non-Ionizing Radiation Protection (ICNIRP) provides guidelines on exposure limits to electromagnetic fields. While PEMF therapy typically operates within these safety limits, adherence to these guidelines is essential for ensuring patient safety and regulatory compliance.
As the field of PEMF therapy continues to evolve, regulatory bodies are adapting their approaches to keep pace with technological advancements. This includes developing new standards for assessing the safety and efficacy of novel PEMF devices and treatment protocols. Collaboration between regulatory agencies, researchers, and manufacturers is crucial for establishing evidence-based guidelines and ensuring the safe and effective use of PEMF therapy in bone healing applications.
In conclusion, the safety and regulatory landscape for PEMF therapy in bone healing is complex and dynamic. Adherence to established regulations and ongoing research into long-term safety are essential for the continued development and adoption of this promising therapeutic approach.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!