PEMF Therapy: Latest Innovations in Device Technology
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Evolution
Pulsed Electromagnetic Field (PEMF) therapy has undergone significant evolution since its inception in the mid-20th century. Initially developed for bone healing, PEMF therapy has expanded its applications to various medical conditions, showcasing its versatility and potential in modern healthcare.
The 1950s marked the beginning of PEMF therapy with the discovery that electrical currents could stimulate bone growth. This led to the development of the first PEMF devices in the 1970s, primarily used for non-union fractures. These early devices were large, stationary, and limited in their applications.
The 1980s and 1990s saw a surge in research and development, expanding PEMF therapy's scope to include pain management, wound healing, and anti-inflammatory treatments. This period also witnessed the miniaturization of devices, making them more portable and accessible for home use.
The turn of the millennium brought about a new era for PEMF therapy. Advancements in electronics and materials science allowed for the creation of more sophisticated devices with precise control over frequency, intensity, and waveform. This precision enabled tailored treatments for specific conditions, enhancing the therapy's effectiveness.
In recent years, PEMF therapy has seen integration with other technologies. The incorporation of smart sensors and IoT capabilities has led to devices that can adapt treatments based on real-time physiological data. Additionally, the combination of PEMF with other therapies, such as light therapy or biofeedback, has opened new avenues for holistic treatment approaches.
The latest innovations in PEMF device technology focus on improving user experience and treatment efficacy. Wearable PEMF devices have emerged, allowing for continuous therapy during daily activities. These devices often feature smartphone connectivity, enabling users to track their treatment progress and adjust settings remotely.
Another significant development is the use of AI algorithms to optimize treatment protocols. These systems analyze patient data and treatment outcomes to refine and personalize PEMF therapy, potentially improving its effectiveness across various conditions.
As PEMF therapy continues to evolve, researchers are exploring its potential in new areas such as neurodegenerative disorders, mental health, and athletic performance enhancement. The ongoing miniaturization of components and advancements in battery technology promise even more compact and efficient devices in the future.
The 1950s marked the beginning of PEMF therapy with the discovery that electrical currents could stimulate bone growth. This led to the development of the first PEMF devices in the 1970s, primarily used for non-union fractures. These early devices were large, stationary, and limited in their applications.
The 1980s and 1990s saw a surge in research and development, expanding PEMF therapy's scope to include pain management, wound healing, and anti-inflammatory treatments. This period also witnessed the miniaturization of devices, making them more portable and accessible for home use.
The turn of the millennium brought about a new era for PEMF therapy. Advancements in electronics and materials science allowed for the creation of more sophisticated devices with precise control over frequency, intensity, and waveform. This precision enabled tailored treatments for specific conditions, enhancing the therapy's effectiveness.
In recent years, PEMF therapy has seen integration with other technologies. The incorporation of smart sensors and IoT capabilities has led to devices that can adapt treatments based on real-time physiological data. Additionally, the combination of PEMF with other therapies, such as light therapy or biofeedback, has opened new avenues for holistic treatment approaches.
The latest innovations in PEMF device technology focus on improving user experience and treatment efficacy. Wearable PEMF devices have emerged, allowing for continuous therapy during daily activities. These devices often feature smartphone connectivity, enabling users to track their treatment progress and adjust settings remotely.
Another significant development is the use of AI algorithms to optimize treatment protocols. These systems analyze patient data and treatment outcomes to refine and personalize PEMF therapy, potentially improving its effectiveness across various conditions.
As PEMF therapy continues to evolve, researchers are exploring its potential in new areas such as neurodegenerative disorders, mental health, and athletic performance enhancement. The ongoing miniaturization of components and advancements in battery technology promise even more compact and efficient devices in the future.
Market Demand Analysis
The market demand for PEMF (Pulsed Electromagnetic Field) therapy devices has been steadily increasing in recent years, driven by growing awareness of its potential health benefits and advancements in device technology. This non-invasive treatment method has gained traction across various medical fields, including orthopedics, neurology, and sports medicine.
The global PEMF therapy devices market is experiencing significant growth, with a projected compound annual growth rate (CAGR) of over 5% from 2021 to 2026. This expansion is primarily fueled by the rising prevalence of chronic diseases, an aging population, and increasing adoption of alternative pain management solutions.
One of the key drivers of market demand is the growing preference for non-pharmacological pain management techniques. As concerns about opioid addiction and side effects of long-term medication use continue to rise, patients and healthcare providers are increasingly turning to PEMF therapy as a safe and effective alternative for pain relief and tissue healing.
The sports medicine sector has emerged as a particularly promising market for PEMF therapy devices. Professional athletes and sports teams are incorporating these devices into their training and recovery regimens, recognizing their potential to accelerate healing, reduce inflammation, and improve overall performance. This trend is expected to trickle down to amateur athletes and fitness enthusiasts, further expanding the consumer base.
Another significant factor contributing to market growth is the increasing prevalence of musculoskeletal disorders and chronic pain conditions. As the global population ages, the incidence of conditions such as osteoarthritis, rheumatoid arthritis, and fibromyalgia is on the rise, creating a substantial demand for effective, non-invasive treatment options like PEMF therapy.
The home healthcare segment is also showing strong potential for PEMF therapy devices. Portable, user-friendly devices designed for home use are gaining popularity among patients seeking convenient, long-term pain management solutions. This trend has been further accelerated by the COVID-19 pandemic, which has increased the demand for at-home medical treatments and wellness solutions.
Geographically, North America currently dominates the PEMF therapy devices market, followed by Europe. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of alternative therapies, and improving access to advanced medical technologies in countries like China and India.
Despite the positive market outlook, challenges such as high device costs and limited insurance coverage in some regions may hinder widespread adoption. However, ongoing technological advancements aimed at improving device efficacy, portability, and user-friendliness are expected to address these barriers and further stimulate market growth in the coming years.
The global PEMF therapy devices market is experiencing significant growth, with a projected compound annual growth rate (CAGR) of over 5% from 2021 to 2026. This expansion is primarily fueled by the rising prevalence of chronic diseases, an aging population, and increasing adoption of alternative pain management solutions.
One of the key drivers of market demand is the growing preference for non-pharmacological pain management techniques. As concerns about opioid addiction and side effects of long-term medication use continue to rise, patients and healthcare providers are increasingly turning to PEMF therapy as a safe and effective alternative for pain relief and tissue healing.
The sports medicine sector has emerged as a particularly promising market for PEMF therapy devices. Professional athletes and sports teams are incorporating these devices into their training and recovery regimens, recognizing their potential to accelerate healing, reduce inflammation, and improve overall performance. This trend is expected to trickle down to amateur athletes and fitness enthusiasts, further expanding the consumer base.
Another significant factor contributing to market growth is the increasing prevalence of musculoskeletal disorders and chronic pain conditions. As the global population ages, the incidence of conditions such as osteoarthritis, rheumatoid arthritis, and fibromyalgia is on the rise, creating a substantial demand for effective, non-invasive treatment options like PEMF therapy.
The home healthcare segment is also showing strong potential for PEMF therapy devices. Portable, user-friendly devices designed for home use are gaining popularity among patients seeking convenient, long-term pain management solutions. This trend has been further accelerated by the COVID-19 pandemic, which has increased the demand for at-home medical treatments and wellness solutions.
Geographically, North America currently dominates the PEMF therapy devices market, followed by Europe. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of alternative therapies, and improving access to advanced medical technologies in countries like China and India.
Despite the positive market outlook, challenges such as high device costs and limited insurance coverage in some regions may hinder widespread adoption. However, ongoing technological advancements aimed at improving device efficacy, portability, and user-friendliness are expected to address these barriers and further stimulate market growth in the coming years.
Current PEMF Challenges
Despite the growing popularity of PEMF (Pulsed Electromagnetic Field) therapy, several challenges persist in the current landscape of device technology. One of the primary obstacles is the lack of standardization in treatment protocols. Different manufacturers employ varying frequencies, intensities, and waveforms, making it difficult for healthcare providers and patients to compare and evaluate the efficacy of different devices. This inconsistency also hampers the development of comprehensive clinical guidelines for PEMF therapy.
Another significant challenge is the limited understanding of the optimal dosage and treatment duration for specific conditions. While PEMF therapy has shown promise in treating various ailments, there is a dearth of large-scale, long-term clinical studies that definitively establish the most effective treatment parameters. This knowledge gap often leads to suboptimal outcomes and hinders the widespread adoption of PEMF therapy in mainstream medical practice.
The miniaturization and portability of PEMF devices present another hurdle. While there is a growing demand for compact, wearable devices that can be used in daily life, achieving the necessary power output and treatment efficacy in smaller form factors remains challenging. Engineers must balance size constraints with the need for sufficient electromagnetic field strength and battery life.
Additionally, the issue of electromagnetic interference (EMI) poses a significant challenge in PEMF device development. As these devices generate electromagnetic fields, they can potentially interfere with other electronic medical devices, such as pacemakers or insulin pumps. Ensuring EMI compatibility without compromising treatment efficacy is a complex engineering task that requires ongoing research and development.
The cost of PEMF devices is another barrier to widespread adoption. High-quality, FDA-approved devices often come with a substantial price tag, making them inaccessible to many potential users. Developing more cost-effective solutions without sacrificing quality and safety remains a key challenge for manufacturers.
Lastly, the regulatory landscape for PEMF devices is complex and varies significantly across different countries. Navigating the approval processes and meeting diverse regulatory requirements can be time-consuming and expensive for manufacturers, potentially slowing down innovation and market entry for new devices.
Another significant challenge is the limited understanding of the optimal dosage and treatment duration for specific conditions. While PEMF therapy has shown promise in treating various ailments, there is a dearth of large-scale, long-term clinical studies that definitively establish the most effective treatment parameters. This knowledge gap often leads to suboptimal outcomes and hinders the widespread adoption of PEMF therapy in mainstream medical practice.
The miniaturization and portability of PEMF devices present another hurdle. While there is a growing demand for compact, wearable devices that can be used in daily life, achieving the necessary power output and treatment efficacy in smaller form factors remains challenging. Engineers must balance size constraints with the need for sufficient electromagnetic field strength and battery life.
Additionally, the issue of electromagnetic interference (EMI) poses a significant challenge in PEMF device development. As these devices generate electromagnetic fields, they can potentially interfere with other electronic medical devices, such as pacemakers or insulin pumps. Ensuring EMI compatibility without compromising treatment efficacy is a complex engineering task that requires ongoing research and development.
The cost of PEMF devices is another barrier to widespread adoption. High-quality, FDA-approved devices often come with a substantial price tag, making them inaccessible to many potential users. Developing more cost-effective solutions without sacrificing quality and safety remains a key challenge for manufacturers.
Lastly, the regulatory landscape for PEMF devices is complex and varies significantly across different countries. Navigating the approval processes and meeting diverse regulatory requirements can be time-consuming and expensive for manufacturers, potentially slowing down innovation and market entry for new devices.
PEMF Device Solutions
01 Advanced PEMF device designs
Innovations in PEMF therapy devices focus on improved designs for better efficacy and user experience. These advancements include portable and wearable devices, multi-coil systems for targeted treatment, and integration with other therapeutic modalities. The designs aim to enhance treatment precision, convenience, and overall effectiveness of PEMF therapy.- Advanced PEMF device designs: Innovations in PEMF therapy devices focus on improved designs for better treatment efficacy. These advancements include portable and wearable devices, multi-coil systems for targeted therapy, and integration with other treatment modalities. The designs aim to enhance user comfort, treatment precision, and overall therapeutic outcomes.
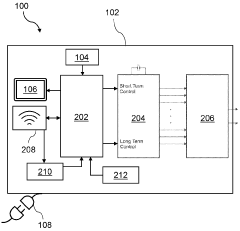
- Smart control systems for PEMF devices: Modern PEMF devices incorporate smart control systems that allow for personalized treatment protocols. These systems may include features such as programmable frequencies, intensity levels, and treatment durations. Some devices also offer connectivity options for remote monitoring and adjustment of treatment parameters.
- Integration of PEMF with other therapies: Innovative approaches combine PEMF therapy with other treatment modalities to enhance overall therapeutic effects. This may include integration with light therapy, heat therapy, or other forms of electromagnetic stimulation. Such combinations aim to provide synergistic benefits and address multiple aspects of health and wellness simultaneously.
- PEMF devices for specific medical applications: Development of specialized PEMF devices tailored for specific medical conditions or body areas. These innovations focus on optimizing treatment parameters and device designs for targeted applications such as wound healing, bone regeneration, pain management, or neurological disorders.
- Enhanced user interface and connectivity: Advancements in user interface design and connectivity features for PEMF devices. This includes the development of mobile applications for device control and treatment tracking, as well as integration with health monitoring systems. These innovations aim to improve user experience, treatment adherence, and data collection for better outcomes analysis.
02 Smart control systems for PEMF devices
Modern PEMF devices incorporate intelligent control systems that allow for customized treatment protocols, real-time monitoring, and automatic adjustments. These systems may include features such as programmable frequencies, intensity levels, and treatment durations. Some devices also offer connectivity options for data tracking and remote management.Expand Specific Solutions03 Integration of PEMF with other technologies
Innovative PEMF devices are being developed that combine PEMF therapy with other complementary technologies. This integration may include features such as biofeedback systems, light therapy, or even virtual reality interfaces. The goal is to create more comprehensive and effective treatment options for various health conditions.Expand Specific Solutions04 Novel PEMF coil designs and materials
Advancements in PEMF therapy devices include the development of new coil designs and materials. These innovations aim to improve the generation and delivery of electromagnetic fields, potentially enhancing the therapeutic effects. Some designs focus on creating more uniform field distributions or targeting specific body areas with greater precision.Expand Specific Solutions05 PEMF device management and data analysis systems
Innovations in PEMF therapy extend to the development of sophisticated management and data analysis systems. These systems may include features for tracking treatment progress, analyzing outcomes, and generating reports. Some solutions also incorporate artificial intelligence or machine learning algorithms to optimize treatment protocols based on collected data.Expand Specific Solutions
Key PEMF Manufacturers
The PEMF (Pulsed Electromagnetic Field) Therapy device market is in a growth phase, with increasing adoption across medical and wellness sectors. The global market size is expanding, driven by rising awareness of non-invasive pain management solutions. Technologically, the field is advancing rapidly, with companies like Regenesis Biomedical and Venus Concept leading innovation. Biomagnetic Sciences and SofPulse are developing specialized devices, while established players like Orthofix US LLC are diversifying their product lines. The technology's maturity varies, with some companies focusing on FDA-approved medical devices, while others target the consumer wellness market. Research institutions like the National University of Singapore and Swiss Federal Institute of Technology are contributing to the scientific advancement of PEMF technology, potentially influencing future device development and market growth.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed advanced PEMF therapy devices utilizing their proprietary Provant Therapy System. This system employs a unique pulsed radiofrequency energy (PRFE) technology that operates at 27.12 MHz, delivering non-thermal electromagnetic energy to targeted tissues[1]. The device is designed for portable use, allowing patients to receive treatment at home or in clinical settings. Regenesis has focused on optimizing the waveform and frequency of the electromagnetic pulses to enhance cellular repair and reduce inflammation. Their latest innovations include smart sensors that adjust the therapy intensity based on real-time tissue feedback, ensuring optimal treatment delivery[2].
Strengths: Proprietary PRFE technology, portable design, and smart sensor integration. Weaknesses: Limited to specific frequency range, may require more clinical studies to validate efficacy across various conditions.
Venus Concept Ltd.
Technical Solution: Venus Concept has innovated in the PEMF therapy space with their Venus Pulse technology, integrated into devices like Venus Heal. This technology combines PEMF with other modalities such as massage therapy and heat for enhanced therapeutic effects. The Venus Pulse system generates electromagnetic fields with varying frequencies and intensities, allowing for customized treatment protocols. Recent advancements include the development of multi-polar applicators that can cover larger treatment areas and provide more uniform energy distribution[3]. Venus Concept has also incorporated AI-driven treatment algorithms that adapt the PEMF parameters based on patient response and treatment progress[4].
Strengths: Multi-modal approach combining PEMF with other therapies, AI-driven treatment customization. Weaknesses: Complexity of the system may require more training for operators, potentially higher cost due to multiple technologies integrated.
PEMF Core Innovations
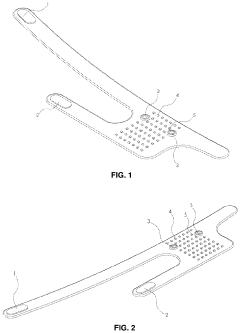
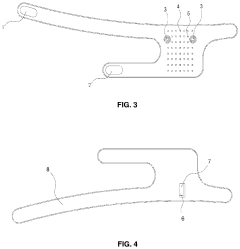
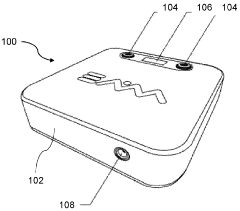
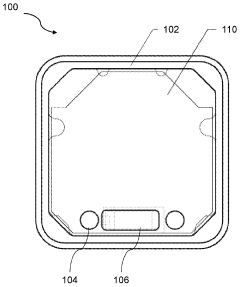
Flexible Photobiomodulation and Pulsed Electromagnetic Field Therapy Device
PatentPendingUS20230001222A1
Innovation
- A flexible wearable device that combines PEMF and PBM therapies, featuring a flexible substrate with electromagnetic coils and light-emitting diodes, controlled by a single module that can switch between pre-set frequency sequences, and is wirelessly enabled for remote control.
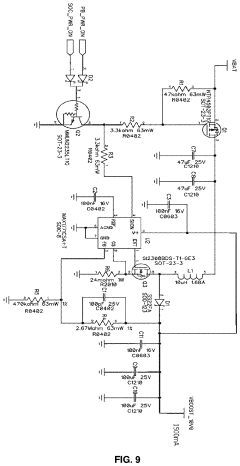
A pulsed electromagnetic field apparatus and method for generating frequencies
PatentWO2024127242A1
Innovation
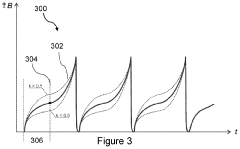
- A PEMF apparatus with a pulse generator and electromagnetic field generation means that uses modified sawtooth waveforms with pre-stress and relaxation periods, and quasi-sine signals with pulse width modulation, along with a feedback circuit for frequency stability and precision, and a bifilar antenna for scalar wave generation.
Regulatory Framework
The regulatory framework surrounding PEMF (Pulsed Electromagnetic Field) therapy devices plays a crucial role in ensuring safety, efficacy, and market access for these innovative medical technologies. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device.
The FDA's regulatory approach for PEMF devices focuses on several key aspects. These include the device's intended use, technical specifications, electromagnetic output parameters, and potential risks associated with its use. Manufacturers must provide comprehensive data on the device's safety and effectiveness, including clinical studies where applicable. Additionally, they must adhere to Good Manufacturing Practices (GMP) to ensure consistent quality and performance of the devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR has introduced more stringent requirements for medical device manufacturers, including enhanced clinical evaluation processes and post-market surveillance. PEMF devices are typically classified as Class IIa under the MDR, requiring a conformity assessment by a Notified Body before obtaining CE marking for market access.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. The approval process in Japan often requires local clinical data, which can be more demanding compared to other markets. This regulatory landscape has led some PEMF device manufacturers to pursue strategic partnerships with Japanese companies to navigate the market entry process more effectively.
Emerging markets, such as China and India, are developing their regulatory frameworks for PEMF devices. China's National Medical Products Administration (NMPA) has been refining its approach to medical device regulation, with PEMF devices typically falling under Class II classification. In India, the Central Drugs Standard Control Organization (CDSCO) oversees medical device regulation, with recent efforts to align more closely with global standards.
As PEMF therapy continues to evolve, regulatory bodies worldwide are adapting their frameworks to keep pace with technological advancements. This includes addressing potential cybersecurity risks in connected PEMF devices and evaluating the integration of artificial intelligence in treatment protocols. The ongoing harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes globally, potentially accelerating market access for innovative PEMF technologies across different regions.
The FDA's regulatory approach for PEMF devices focuses on several key aspects. These include the device's intended use, technical specifications, electromagnetic output parameters, and potential risks associated with its use. Manufacturers must provide comprehensive data on the device's safety and effectiveness, including clinical studies where applicable. Additionally, they must adhere to Good Manufacturing Practices (GMP) to ensure consistent quality and performance of the devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR has introduced more stringent requirements for medical device manufacturers, including enhanced clinical evaluation processes and post-market surveillance. PEMF devices are typically classified as Class IIa under the MDR, requiring a conformity assessment by a Notified Body before obtaining CE marking for market access.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) regulates PEMF devices under the Pharmaceutical and Medical Device Act. The approval process in Japan often requires local clinical data, which can be more demanding compared to other markets. This regulatory landscape has led some PEMF device manufacturers to pursue strategic partnerships with Japanese companies to navigate the market entry process more effectively.
Emerging markets, such as China and India, are developing their regulatory frameworks for PEMF devices. China's National Medical Products Administration (NMPA) has been refining its approach to medical device regulation, with PEMF devices typically falling under Class II classification. In India, the Central Drugs Standard Control Organization (CDSCO) oversees medical device regulation, with recent efforts to align more closely with global standards.
As PEMF therapy continues to evolve, regulatory bodies worldwide are adapting their frameworks to keep pace with technological advancements. This includes addressing potential cybersecurity risks in connected PEMF devices and evaluating the integration of artificial intelligence in treatment protocols. The ongoing harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), aim to streamline regulatory processes globally, potentially accelerating market access for innovative PEMF technologies across different regions.
Safety Considerations
Safety considerations are paramount in the development and application of PEMF (Pulsed Electromagnetic Field) therapy devices. As these technologies continue to advance, manufacturers and healthcare providers must prioritize patient safety and adhere to stringent regulatory standards. One of the primary safety concerns is the potential for electromagnetic interference with other medical devices, particularly implanted electronic devices such as pacemakers or defibrillators. To mitigate this risk, PEMF devices are designed with shielding mechanisms and undergo rigorous testing to ensure compatibility with a wide range of medical equipment.
The intensity and frequency of electromagnetic fields generated by PEMF devices are carefully calibrated to remain within safe limits for human exposure. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established guidelines for maximum permissible exposure levels, which manufacturers must comply with. These guidelines are based on extensive research into the biological effects of electromagnetic fields and are regularly updated as new scientific evidence emerges.
Thermal effects are another safety consideration in PEMF therapy. While the electromagnetic fields used in these devices are generally non-thermal, prolonged exposure to high-intensity fields could potentially cause localized heating of tissues. To address this, modern PEMF devices incorporate temperature monitoring systems and automatic shut-off features to prevent overheating.
User safety is enhanced through the implementation of intuitive control interfaces and clear operating instructions. Manufacturers are increasingly focusing on user-friendly designs that minimize the risk of improper use or accidental exposure to excessive field strengths. Additionally, many devices now include built-in safety protocols that limit treatment duration and intensity based on pre-programmed therapeutic profiles.
Long-term safety studies are ongoing to assess the potential cumulative effects of PEMF therapy. While current evidence suggests that PEMF therapy is generally safe when used as directed, researchers continue to investigate any possible long-term impacts on various physiological systems. This ongoing research informs the development of future safety guidelines and device improvements.
Manufacturers are also addressing the safety of materials used in PEMF devices, ensuring that all components are biocompatible and free from toxic substances. This includes the selection of hypoallergenic materials for parts that come into direct contact with the skin, as well as the use of environmentally friendly and recyclable materials in device construction.
As PEMF therapy devices become more portable and accessible for home use, manufacturers are implementing additional safety features such as child-lock mechanisms and automatic power-off functions to prevent misuse or accidents. Furthermore, the integration of smart technology allows for remote monitoring and control of devices, enabling healthcare providers to oversee treatment protocols and adjust settings as needed to ensure patient safety.
The intensity and frequency of electromagnetic fields generated by PEMF devices are carefully calibrated to remain within safe limits for human exposure. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established guidelines for maximum permissible exposure levels, which manufacturers must comply with. These guidelines are based on extensive research into the biological effects of electromagnetic fields and are regularly updated as new scientific evidence emerges.
Thermal effects are another safety consideration in PEMF therapy. While the electromagnetic fields used in these devices are generally non-thermal, prolonged exposure to high-intensity fields could potentially cause localized heating of tissues. To address this, modern PEMF devices incorporate temperature monitoring systems and automatic shut-off features to prevent overheating.
User safety is enhanced through the implementation of intuitive control interfaces and clear operating instructions. Manufacturers are increasingly focusing on user-friendly designs that minimize the risk of improper use or accidental exposure to excessive field strengths. Additionally, many devices now include built-in safety protocols that limit treatment duration and intensity based on pre-programmed therapeutic profiles.
Long-term safety studies are ongoing to assess the potential cumulative effects of PEMF therapy. While current evidence suggests that PEMF therapy is generally safe when used as directed, researchers continue to investigate any possible long-term impacts on various physiological systems. This ongoing research informs the development of future safety guidelines and device improvements.
Manufacturers are also addressing the safety of materials used in PEMF devices, ensuring that all components are biocompatible and free from toxic substances. This includes the selection of hypoallergenic materials for parts that come into direct contact with the skin, as well as the use of environmentally friendly and recyclable materials in device construction.
As PEMF therapy devices become more portable and accessible for home use, manufacturers are implementing additional safety features such as child-lock mechanisms and automatic power-off functions to prevent misuse or accidents. Furthermore, the integration of smart technology allows for remote monitoring and control of devices, enabling healthcare providers to oversee treatment protocols and adjust settings as needed to ensure patient safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!