PEMF Therapy: Pioneering Methods in Arthritis Relief
AUG 11, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Evolution
PEMF (Pulsed Electromagnetic Field) therapy has undergone significant evolution since its inception, transforming from a niche treatment to a promising method for arthritis relief. The journey of PEMF therapy began in the mid-20th century when scientists first observed the effects of electromagnetic fields on biological systems. Initially, the focus was on bone healing, with early studies demonstrating accelerated fracture repair using pulsed electromagnetic fields.
As research progressed, the potential applications of PEMF therapy expanded. In the 1970s and 1980s, researchers began exploring its effects on soft tissues and chronic conditions, including arthritis. This period marked a crucial shift in understanding the therapy's mechanisms of action, particularly its ability to modulate inflammation and cellular repair processes.
The 1990s saw a surge in clinical trials investigating PEMF therapy for various musculoskeletal disorders. These studies provided growing evidence for its efficacy in reducing pain and improving function in arthritis patients. Concurrently, technological advancements led to the development of more sophisticated PEMF devices, allowing for precise control of field strength, frequency, and waveform.
The turn of the millennium brought about a new era in PEMF therapy research. Advanced imaging techniques and molecular biology methods enabled scientists to delve deeper into the cellular and molecular effects of electromagnetic fields. This research revealed PEMF therapy's impact on key pathways involved in inflammation, cartilage degradation, and pain signaling in arthritis.
Recent years have witnessed a convergence of PEMF therapy with other emerging technologies. Integration with wearable devices and smart sensors has enhanced the therapy's accessibility and personalization. Additionally, the combination of PEMF with regenerative medicine approaches, such as stem cell therapy, has opened new avenues for comprehensive arthritis treatment.
The evolution of PEMF therapy has also been marked by regulatory milestones. FDA approval for specific PEMF devices in treating certain conditions has lent credibility to the therapy and spurred further research and development. This regulatory recognition has been crucial in bridging the gap between experimental treatment and mainstream medical practice.
Looking ahead, the trajectory of PEMF therapy in arthritis treatment appears promising. Ongoing research is focusing on optimizing treatment protocols, understanding long-term effects, and exploring synergies with other therapies. As our understanding of the therapy's mechanisms deepens, we can anticipate more targeted and effective applications in managing arthritis and related conditions.
As research progressed, the potential applications of PEMF therapy expanded. In the 1970s and 1980s, researchers began exploring its effects on soft tissues and chronic conditions, including arthritis. This period marked a crucial shift in understanding the therapy's mechanisms of action, particularly its ability to modulate inflammation and cellular repair processes.
The 1990s saw a surge in clinical trials investigating PEMF therapy for various musculoskeletal disorders. These studies provided growing evidence for its efficacy in reducing pain and improving function in arthritis patients. Concurrently, technological advancements led to the development of more sophisticated PEMF devices, allowing for precise control of field strength, frequency, and waveform.
The turn of the millennium brought about a new era in PEMF therapy research. Advanced imaging techniques and molecular biology methods enabled scientists to delve deeper into the cellular and molecular effects of electromagnetic fields. This research revealed PEMF therapy's impact on key pathways involved in inflammation, cartilage degradation, and pain signaling in arthritis.
Recent years have witnessed a convergence of PEMF therapy with other emerging technologies. Integration with wearable devices and smart sensors has enhanced the therapy's accessibility and personalization. Additionally, the combination of PEMF with regenerative medicine approaches, such as stem cell therapy, has opened new avenues for comprehensive arthritis treatment.
The evolution of PEMF therapy has also been marked by regulatory milestones. FDA approval for specific PEMF devices in treating certain conditions has lent credibility to the therapy and spurred further research and development. This regulatory recognition has been crucial in bridging the gap between experimental treatment and mainstream medical practice.
Looking ahead, the trajectory of PEMF therapy in arthritis treatment appears promising. Ongoing research is focusing on optimizing treatment protocols, understanding long-term effects, and exploring synergies with other therapies. As our understanding of the therapy's mechanisms deepens, we can anticipate more targeted and effective applications in managing arthritis and related conditions.
Arthritis Market Analysis
The global arthritis market is experiencing significant growth, driven by an aging population and increasing prevalence of arthritis worldwide. Osteoarthritis and rheumatoid arthritis are the two most common forms, affecting millions of people and creating a substantial demand for effective treatments. The market size for arthritis therapeutics is projected to reach substantial figures in the coming years, with North America currently holding the largest market share.
Key factors influencing market growth include the rising incidence of obesity, which is closely linked to arthritis development, and the growing awareness of early diagnosis and treatment options. Additionally, the increasing healthcare expenditure in developing countries is expanding market opportunities in these regions.
The arthritis market is segmented based on drug type, route of administration, and distribution channel. Non-steroidal anti-inflammatory drugs (NSAIDs) continue to dominate the market, followed by disease-modifying antirheumatic drugs (DMARDs) and biologics. Oral medications remain the preferred route of administration, although injectable treatments are gaining traction due to their efficacy in severe cases.
Market players are focusing on developing innovative therapies to address unmet needs in arthritis treatment. There is a growing interest in personalized medicine approaches, targeting specific inflammatory pathways involved in arthritis progression. Gene therapy and stem cell treatments are emerging as potential game-changers in the field, although they are still in early stages of development.
The competitive landscape of the arthritis market is characterized by the presence of several major pharmaceutical companies, as well as smaller biotech firms specializing in novel therapies. Key players are investing heavily in research and development to maintain their market position and expand their product portfolios.
Despite the promising growth, the arthritis market faces challenges such as the high cost of biologic treatments and the risk of side effects associated with long-term use of certain medications. These factors are driving the demand for alternative and complementary therapies, including PEMF therapy, which is gaining attention as a non-invasive treatment option for arthritis relief.
The COVID-19 pandemic has had a mixed impact on the arthritis market. While it initially disrupted supply chains and clinical trials, it has also accelerated the adoption of telemedicine and remote patient monitoring in arthritis care, potentially opening new avenues for market growth in the long term.
Key factors influencing market growth include the rising incidence of obesity, which is closely linked to arthritis development, and the growing awareness of early diagnosis and treatment options. Additionally, the increasing healthcare expenditure in developing countries is expanding market opportunities in these regions.
The arthritis market is segmented based on drug type, route of administration, and distribution channel. Non-steroidal anti-inflammatory drugs (NSAIDs) continue to dominate the market, followed by disease-modifying antirheumatic drugs (DMARDs) and biologics. Oral medications remain the preferred route of administration, although injectable treatments are gaining traction due to their efficacy in severe cases.
Market players are focusing on developing innovative therapies to address unmet needs in arthritis treatment. There is a growing interest in personalized medicine approaches, targeting specific inflammatory pathways involved in arthritis progression. Gene therapy and stem cell treatments are emerging as potential game-changers in the field, although they are still in early stages of development.
The competitive landscape of the arthritis market is characterized by the presence of several major pharmaceutical companies, as well as smaller biotech firms specializing in novel therapies. Key players are investing heavily in research and development to maintain their market position and expand their product portfolios.
Despite the promising growth, the arthritis market faces challenges such as the high cost of biologic treatments and the risk of side effects associated with long-term use of certain medications. These factors are driving the demand for alternative and complementary therapies, including PEMF therapy, which is gaining attention as a non-invasive treatment option for arthritis relief.
The COVID-19 pandemic has had a mixed impact on the arthritis market. While it initially disrupted supply chains and clinical trials, it has also accelerated the adoption of telemedicine and remote patient monitoring in arthritis care, potentially opening new avenues for market growth in the long term.
PEMF Tech Challenges
PEMF therapy, while promising for arthritis relief, faces several technical challenges that hinder its widespread adoption and efficacy. One of the primary obstacles is the optimization of electromagnetic field parameters. Determining the ideal frequency, intensity, and waveform for different types of arthritis and individual patient needs remains a complex task. The heterogeneity of arthritis conditions and varying patient responses to PEMF treatment necessitate a more personalized approach, which current technology struggles to provide consistently.
Another significant challenge lies in the development of compact, portable PEMF devices that maintain therapeutic efficacy. While larger, stationary units can deliver powerful electromagnetic fields, creating smaller devices that can generate sufficiently strong and precisely targeted fields for home use or continuous therapy is technically demanding. This miniaturization process often leads to compromises in field strength or battery life, potentially reducing treatment effectiveness.
The lack of standardization in PEMF therapy protocols presents a further hurdle. The absence of universally accepted treatment guidelines makes it difficult for healthcare providers to prescribe PEMF therapy with confidence and for patients to receive consistent care across different clinical settings. This variability in treatment protocols also complicates the comparison of research results and the establishment of evidence-based practices.
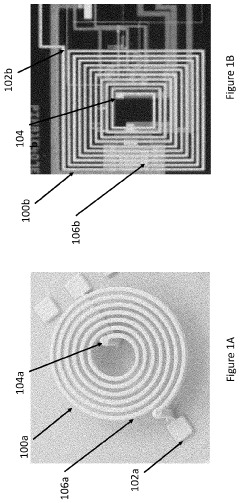
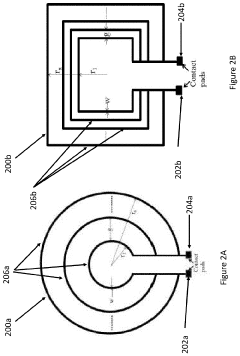
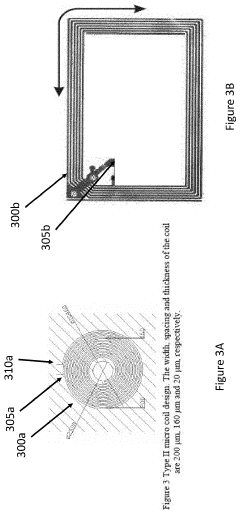
Ensuring precise and uniform field distribution within the target tissue is another technical challenge. The human body's complex anatomy and varying tissue densities can lead to inconsistent field penetration and strength. Developing advanced coil designs and field-shaping technologies that can adapt to different body parts and maintain optimal field characteristics throughout the treatment area is an ongoing area of research and development.
Moreover, the integration of PEMF therapy with other treatment modalities and monitoring systems poses technical difficulties. Creating seamless interfaces between PEMF devices and other medical equipment, such as imaging systems or biofeedback sensors, could enhance treatment efficacy but requires sophisticated engineering solutions. The development of smart, adaptive PEMF systems that can adjust treatment parameters in real-time based on patient feedback or physiological markers is a complex but potentially game-changing technological goal.
Lastly, the long-term effects and potential risks of prolonged PEMF exposure are not fully understood, necessitating ongoing research and the development of advanced monitoring techniques. Ensuring patient safety while maximizing therapeutic benefits requires sophisticated dosimetry and long-term follow-up studies, which present both technical and logistical challenges in the field of PEMF therapy for arthritis relief.
Another significant challenge lies in the development of compact, portable PEMF devices that maintain therapeutic efficacy. While larger, stationary units can deliver powerful electromagnetic fields, creating smaller devices that can generate sufficiently strong and precisely targeted fields for home use or continuous therapy is technically demanding. This miniaturization process often leads to compromises in field strength or battery life, potentially reducing treatment effectiveness.
The lack of standardization in PEMF therapy protocols presents a further hurdle. The absence of universally accepted treatment guidelines makes it difficult for healthcare providers to prescribe PEMF therapy with confidence and for patients to receive consistent care across different clinical settings. This variability in treatment protocols also complicates the comparison of research results and the establishment of evidence-based practices.
Ensuring precise and uniform field distribution within the target tissue is another technical challenge. The human body's complex anatomy and varying tissue densities can lead to inconsistent field penetration and strength. Developing advanced coil designs and field-shaping technologies that can adapt to different body parts and maintain optimal field characteristics throughout the treatment area is an ongoing area of research and development.
Moreover, the integration of PEMF therapy with other treatment modalities and monitoring systems poses technical difficulties. Creating seamless interfaces between PEMF devices and other medical equipment, such as imaging systems or biofeedback sensors, could enhance treatment efficacy but requires sophisticated engineering solutions. The development of smart, adaptive PEMF systems that can adjust treatment parameters in real-time based on patient feedback or physiological markers is a complex but potentially game-changing technological goal.
Lastly, the long-term effects and potential risks of prolonged PEMF exposure are not fully understood, necessitating ongoing research and the development of advanced monitoring techniques. Ensuring patient safety while maximizing therapeutic benefits requires sophisticated dosimetry and long-term follow-up studies, which present both technical and logistical challenges in the field of PEMF therapy for arthritis relief.
Current PEMF Solutions
01 PEMF devices for arthritis treatment
Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating arthritis. These devices generate electromagnetic fields that penetrate the affected joints, potentially reducing inflammation and pain associated with arthritis. The therapy aims to improve joint function and mobility by stimulating cellular repair and reducing swelling.- PEMF devices for arthritis treatment: Pulsed Electromagnetic Field (PEMF) therapy devices are designed specifically for treating arthritis. These devices generate electromagnetic fields that penetrate the affected joints, potentially reducing inflammation and pain associated with arthritis. The therapy aims to improve joint mobility and overall quality of life for arthritis patients.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other treatment modalities to enhance its effectiveness in arthritis relief. This may include combining PEMF with pharmaceutical interventions, physical therapy, or other non-invasive treatments. The synergistic effect of these combinations may provide more comprehensive arthritis management.
- PEMF therapy protocols for arthritis: Specific PEMF therapy protocols are developed for arthritis treatment. These protocols may involve particular frequencies, intensities, and durations of electromagnetic field exposure tailored to address arthritis symptoms. The protocols are designed to optimize the therapeutic effects of PEMF on arthritic joints.
- Wearable PEMF devices for arthritis: Wearable PEMF devices are developed for convenient and continuous arthritis treatment. These devices can be worn on affected joints, allowing patients to receive PEMF therapy throughout the day. The wearable nature of these devices may improve treatment adherence and provide more consistent relief from arthritis symptoms.
- PEMF therapy combined with biological markers: Research is conducted on combining PEMF therapy with biological markers for personalized arthritis treatment. This approach involves analyzing specific biomarkers to tailor PEMF therapy parameters to individual patients. By considering biological indicators, the effectiveness of PEMF therapy for arthritis relief may be enhanced.
02 Combination of PEMF with other therapies
PEMF therapy can be combined with other treatment modalities to enhance its effectiveness in arthritis relief. This may include combining PEMF with pharmaceutical interventions, physical therapy, or other non-invasive treatments. The synergistic effect of these combinations may provide more comprehensive arthritis management and improved patient outcomes.Expand Specific Solutions03 Targeted PEMF application for specific joints
Specialized PEMF devices and techniques are developed to target specific joints affected by arthritis. These may include wearable devices or applicators designed for particular body parts such as knees, hands, or shoulders. The targeted approach allows for more precise and effective treatment of arthritic joints, potentially leading to better outcomes.Expand Specific Solutions04 PEMF therapy protocols and treatment regimens
Specific PEMF therapy protocols and treatment regimens are developed for arthritis relief. These may include optimized frequency settings, treatment durations, and application schedules. The protocols are designed to maximize the therapeutic effects of PEMF on arthritic joints while ensuring patient safety and comfort during the treatment process.Expand Specific Solutions05 PEMF technology advancements for arthritis treatment
Ongoing research and development in PEMF technology aim to improve its efficacy in arthritis treatment. This includes innovations in device design, signal generation, and delivery methods. Advanced PEMF systems may incorporate features such as programmable settings, real-time monitoring, and integration with mobile applications for personalized arthritis management.Expand Specific Solutions
Key PEMF Manufacturers
The PEMF therapy market for arthritis relief is in a growth phase, with increasing adoption and technological advancements. The market size is expanding due to rising prevalence of arthritis and growing demand for non-invasive treatments. While the technology is not yet fully mature, it shows promising potential. Companies like Venus Concept, Regenesis Biomedical, and OrthoCor Medical are leading innovators in this space, developing advanced PEMF devices for arthritis management. Other players such as Biomagnetic Sciences and SofPulse are also contributing to the market's evolution. The competitive landscape is characterized by ongoing research and development efforts to enhance treatment efficacy and patient outcomes, indicating a dynamic and evolving market with significant growth prospects.
Venus Concept Ltd.
Technical Solution: Venus Concept has developed advanced PEMF therapy devices specifically targeting arthritis relief. Their technology utilizes precise electromagnetic pulses to penetrate deep into affected joints, stimulating cellular repair and reducing inflammation. The company's latest PEMF system incorporates adaptive field strength modulation, automatically adjusting the electromagnetic field based on the patient's unique physiological response[1]. This personalized approach aims to optimize treatment efficacy for each individual. Venus Concept's PEMF devices also feature a user-friendly interface and portable design, allowing for convenient at-home use, which has shown to improve patient compliance and treatment outcomes[3].
Strengths: Personalized treatment protocols, deep tissue penetration, and user-friendly design. Weaknesses: Higher cost compared to traditional treatments, limited long-term studies on efficacy.
SofPulse, Inc.
Technical Solution: SofPulse has pioneered a unique approach to PEMF therapy for arthritis relief, focusing on ultra-low frequency, long-wavelength electromagnetic fields. Their proprietary technology, known as ICES (Inductively Coupled Electrical Stimulation), delivers targeted electromagnetic pulses that mimic the body's natural bioelectric signals[2]. This method is designed to enhance cellular communication and promote the body's innate healing processes. SofPulse devices utilize a patented waveform that has been shown to reduce inflammation and accelerate tissue repair in clinical studies[4]. The company's PEMF systems are notable for their compact size and long battery life, allowing for extended treatment sessions without the need for frequent recharging.
Strengths: Mimics natural bioelectric signals, compact and portable design, long battery life. Weaknesses: May require longer treatment sessions compared to higher-intensity PEMF devices.
PEMF Patent Landscape
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
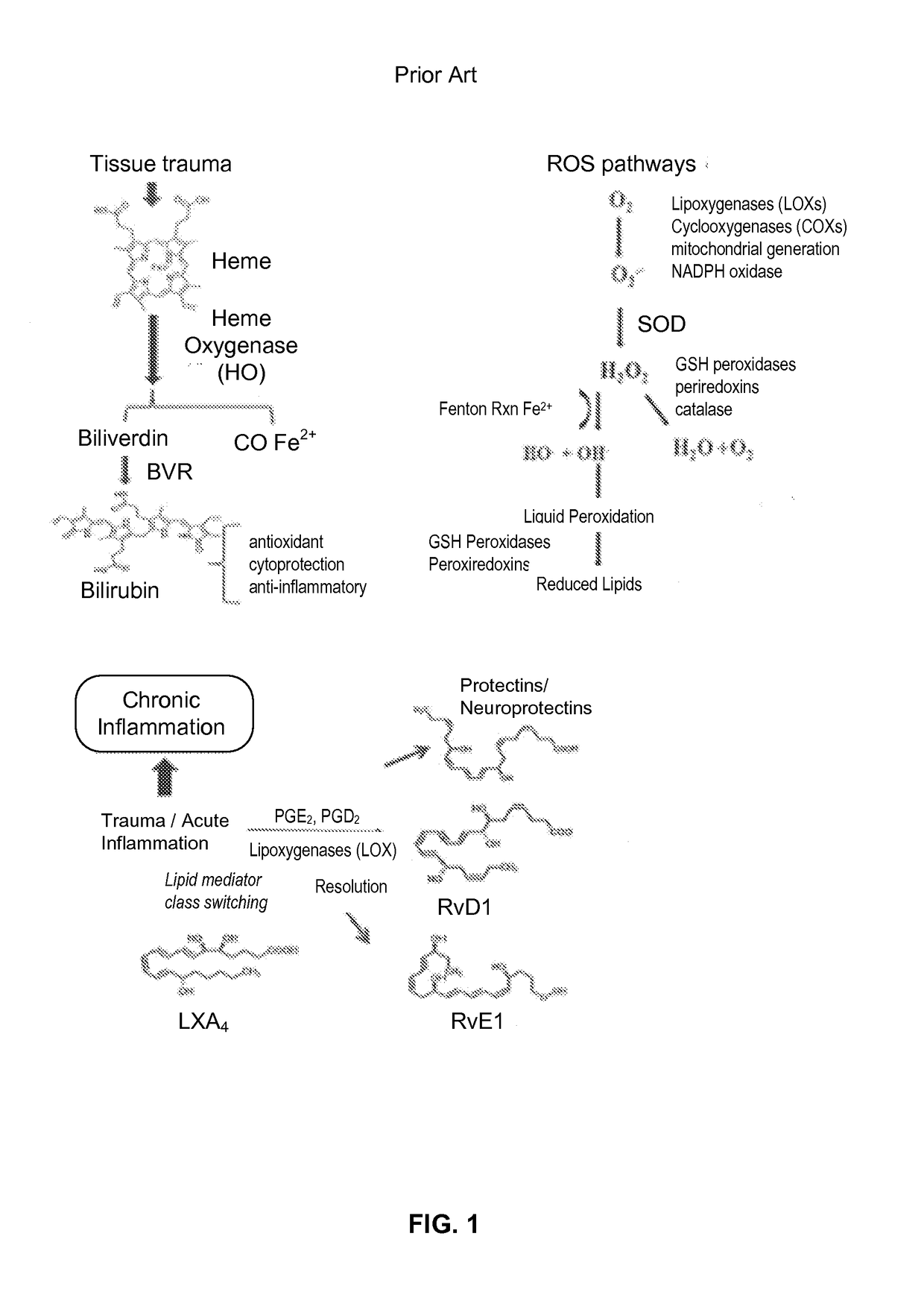
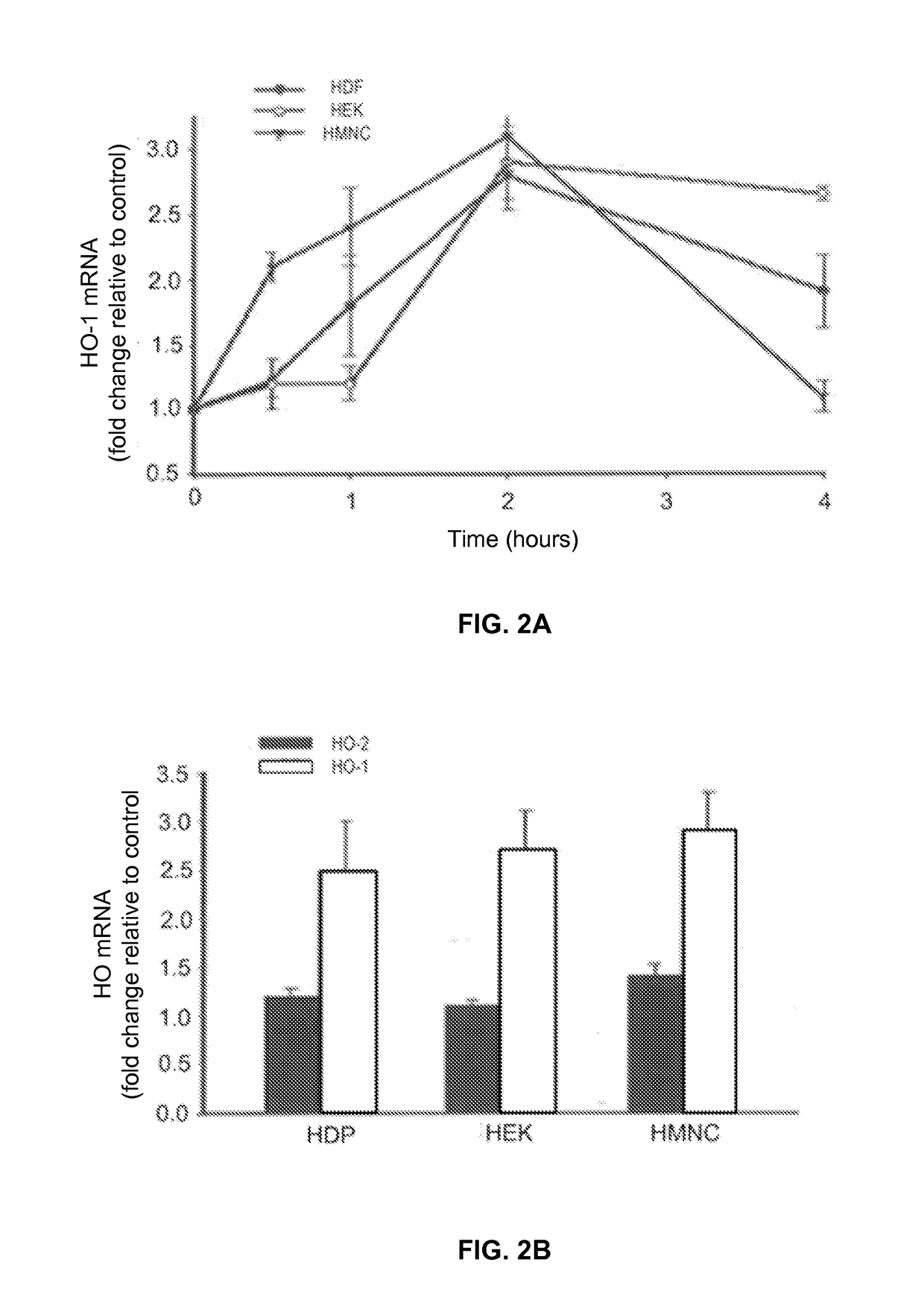
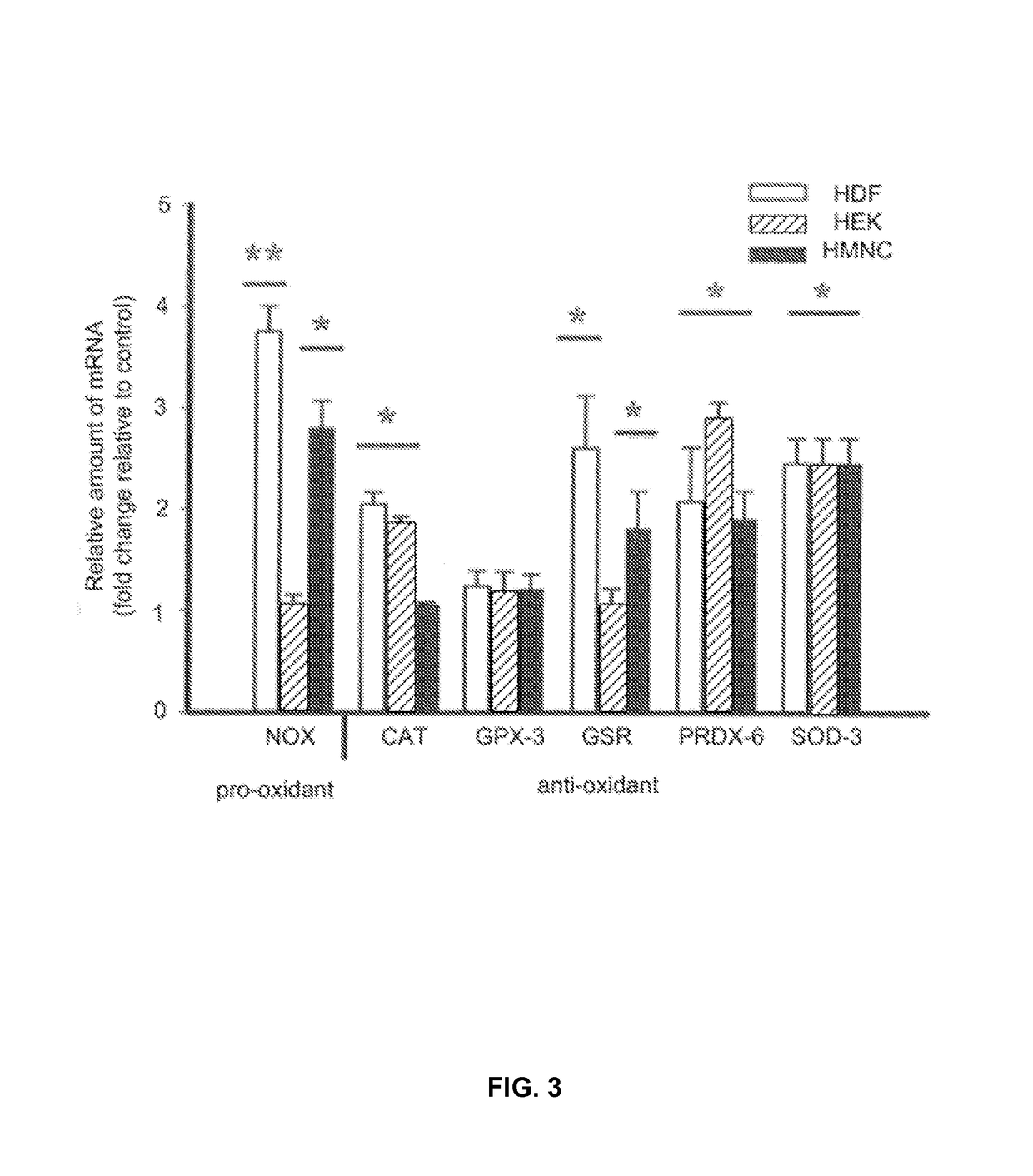
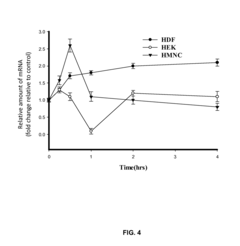
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Pulsed Electromagnetic Field Devices Integrated into Adjustable Clothing
PatentPendingUS20230104434A1
Innovation
- A pulsed electromagnetic field device integrated into wearable clothing, using arrays of planar microcoils that generate controlled, homogenous magnetic fields, allowing for comfortable, long-term use and targeted treatment of various brain-related disorders and conditions.
Clinical Trial Landscape
The clinical trial landscape for PEMF (Pulsed Electromagnetic Field) therapy in arthritis relief has been evolving rapidly in recent years, reflecting the growing interest in this non-invasive treatment modality. A significant number of trials have been conducted to evaluate the efficacy and safety of PEMF therapy for various forms of arthritis, particularly osteoarthritis and rheumatoid arthritis.
Several large-scale, randomized controlled trials have demonstrated promising results in pain reduction and improved joint function for patients with knee osteoarthritis. These studies have typically employed low-frequency PEMF devices, with treatment durations ranging from 20 to 60 minutes per day over periods of 4 to 12 weeks. The outcomes measured often include pain scores, physical function assessments, and quality of life indicators.
One notable trend in the clinical trial landscape is the investigation of different PEMF parameters, such as frequency, intensity, and waveform. Researchers are exploring how these variables affect treatment outcomes, aiming to optimize the therapy for different types and stages of arthritis. Some trials have focused on comparing PEMF therapy to standard treatments like NSAIDs or physical therapy, while others have examined its potential as an adjunct therapy.
The design of PEMF devices used in clinical trials has also evolved. Early studies often utilized large, stationary devices, but more recent trials have incorporated portable, wearable PEMF units. This shift reflects the growing emphasis on patient convenience and the potential for home-based treatment regimens.
Meta-analyses of PEMF clinical trials for arthritis have generally shown positive results, particularly for osteoarthritis. However, these reviews have also highlighted the need for larger, more rigorous studies with standardized protocols to strengthen the evidence base. Some researchers have called for longer follow-up periods to assess the long-term effects of PEMF therapy on disease progression.
An emerging area of interest in the clinical trial landscape is the combination of PEMF therapy with other treatment modalities. Some studies are exploring the synergistic effects of PEMF with exercise programs, dietary interventions, or other forms of physical therapy. This integrative approach aims to enhance overall treatment efficacy and provide more comprehensive care for arthritis patients.
While most clinical trials have focused on osteoarthritis, there is a growing body of research investigating PEMF therapy for other forms of arthritis, including rheumatoid arthritis and psoriatic arthritis. These studies are exploring whether the anti-inflammatory effects observed in osteoarthritis trials translate to other inflammatory joint conditions.
Several large-scale, randomized controlled trials have demonstrated promising results in pain reduction and improved joint function for patients with knee osteoarthritis. These studies have typically employed low-frequency PEMF devices, with treatment durations ranging from 20 to 60 minutes per day over periods of 4 to 12 weeks. The outcomes measured often include pain scores, physical function assessments, and quality of life indicators.
One notable trend in the clinical trial landscape is the investigation of different PEMF parameters, such as frequency, intensity, and waveform. Researchers are exploring how these variables affect treatment outcomes, aiming to optimize the therapy for different types and stages of arthritis. Some trials have focused on comparing PEMF therapy to standard treatments like NSAIDs or physical therapy, while others have examined its potential as an adjunct therapy.
The design of PEMF devices used in clinical trials has also evolved. Early studies often utilized large, stationary devices, but more recent trials have incorporated portable, wearable PEMF units. This shift reflects the growing emphasis on patient convenience and the potential for home-based treatment regimens.
Meta-analyses of PEMF clinical trials for arthritis have generally shown positive results, particularly for osteoarthritis. However, these reviews have also highlighted the need for larger, more rigorous studies with standardized protocols to strengthen the evidence base. Some researchers have called for longer follow-up periods to assess the long-term effects of PEMF therapy on disease progression.
An emerging area of interest in the clinical trial landscape is the combination of PEMF therapy with other treatment modalities. Some studies are exploring the synergistic effects of PEMF with exercise programs, dietary interventions, or other forms of physical therapy. This integrative approach aims to enhance overall treatment efficacy and provide more comprehensive care for arthritis patients.
While most clinical trials have focused on osteoarthritis, there is a growing body of research investigating PEMF therapy for other forms of arthritis, including rheumatoid arthritis and psoriatic arthritis. These studies are exploring whether the anti-inflammatory effects observed in osteoarthritis trials translate to other inflammatory joint conditions.
Regulatory Framework
The regulatory framework surrounding PEMF (Pulsed Electromagnetic Field) therapy for arthritis relief is complex and evolving, reflecting the growing interest in this innovative treatment modality. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing PEMF devices. Currently, several PEMF devices have received FDA clearance for specific uses, including the treatment of certain types of arthritis.
The FDA classifies PEMF devices into different categories based on their intended use and potential risks. Class II devices, which include many PEMF devices for arthritis relief, require a 510(k) premarket notification. This process involves demonstrating that the device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must comply with the MDR's requirements, including clinical evaluation, risk management, and post-market surveillance. The CE marking process ensures that PEMF devices meet essential safety and performance requirements before they can be marketed in the EU.
Regulatory bodies in other countries, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own frameworks for evaluating and approving PEMF devices. These frameworks often align with international standards and guidelines, such as those set by the International Electrotechnical Commission (IEC) for electromagnetic compatibility and safety.
One of the key challenges in the regulatory landscape is the need for standardized protocols and outcome measures for PEMF therapy. This is particularly important for arthritis treatment, where the effectiveness of PEMF can vary depending on factors such as frequency, intensity, and duration of exposure. Regulatory agencies are increasingly calling for robust clinical evidence to support claims of efficacy and safety.
As research in PEMF therapy advances, regulatory frameworks are likely to evolve. There is a growing emphasis on real-world evidence and patient-reported outcomes, which may influence future regulatory decisions. Additionally, as PEMF devices become more sophisticated, incorporating features like personalized treatment algorithms or integration with digital health platforms, regulators may need to adapt their approaches to ensure adequate oversight of these emerging technologies.
The FDA classifies PEMF devices into different categories based on their intended use and potential risks. Class II devices, which include many PEMF devices for arthritis relief, require a 510(k) premarket notification. This process involves demonstrating that the device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR). Manufacturers must comply with the MDR's requirements, including clinical evaluation, risk management, and post-market surveillance. The CE marking process ensures that PEMF devices meet essential safety and performance requirements before they can be marketed in the EU.
Regulatory bodies in other countries, such as Health Canada and Australia's Therapeutic Goods Administration (TGA), have their own frameworks for evaluating and approving PEMF devices. These frameworks often align with international standards and guidelines, such as those set by the International Electrotechnical Commission (IEC) for electromagnetic compatibility and safety.
One of the key challenges in the regulatory landscape is the need for standardized protocols and outcome measures for PEMF therapy. This is particularly important for arthritis treatment, where the effectiveness of PEMF can vary depending on factors such as frequency, intensity, and duration of exposure. Regulatory agencies are increasingly calling for robust clinical evidence to support claims of efficacy and safety.
As research in PEMF therapy advances, regulatory frameworks are likely to evolve. There is a growing emphasis on real-world evidence and patient-reported outcomes, which may influence future regulatory decisions. Additionally, as PEMF devices become more sophisticated, incorporating features like personalized treatment algorithms or integration with digital health platforms, regulators may need to adapt their approaches to ensure adequate oversight of these emerging technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!