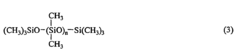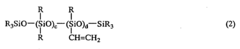Silicone Rubber Solutions for Health Safety Compliance
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicone Rubber Safety Objectives
Silicone rubber has emerged as a critical material in various industries, particularly in healthcare and medical applications, due to its unique properties and versatility. The primary safety objectives for silicone rubber in health-related contexts revolve around ensuring biocompatibility, minimizing potential health risks, and meeting stringent regulatory standards.
One of the key safety objectives is to develop silicone rubber formulations that are non-toxic and non-allergenic. This involves carefully selecting and testing raw materials, as well as optimizing the manufacturing process to eliminate potential contaminants. The goal is to create silicone rubber products that can come into direct contact with human skin, mucous membranes, or even internal organs without causing adverse reactions or long-term health effects.
Another crucial objective is to enhance the material's resistance to microbial growth and colonization. In medical settings, preventing the proliferation of harmful bacteria and fungi on silicone rubber surfaces is essential for reducing the risk of infections. This often involves incorporating antimicrobial additives or developing surface treatments that inhibit microbial adhesion without compromising the material's other desirable properties.
Durability and long-term stability are also paramount safety objectives for silicone rubber in health applications. The material must maintain its structural integrity and performance characteristics over extended periods, even when exposed to various bodily fluids, sterilization processes, and environmental stressors. This includes resistance to degradation, leaching of potentially harmful compounds, and maintaining consistent mechanical properties throughout its intended lifespan.
Achieving biocompatibility is a critical safety objective, particularly for implantable silicone rubber devices or components. This involves extensive testing to ensure that the material does not elicit undesirable biological responses, such as inflammation, immune reactions, or tissue damage. The goal is to develop silicone rubber formulations that integrate seamlessly with the human body, promoting healing and minimizing the risk of complications.
Compliance with regulatory standards and guidelines is an overarching safety objective in the development of silicone rubber solutions for health applications. This includes meeting the requirements set forth by organizations such as the FDA, ISO, and other relevant regulatory bodies. Manufacturers must demonstrate that their silicone rubber products meet or exceed these standards through rigorous testing, documentation, and quality control processes.
Lastly, an important safety objective is to improve the traceability and quality control of silicone rubber products throughout their lifecycle. This involves implementing robust manufacturing processes, establishing comprehensive documentation systems, and developing methods for identifying and tracking individual components or batches. Such measures are crucial for quickly addressing any potential safety issues that may arise and ensuring the overall reliability of silicone rubber solutions in health-related applications.
One of the key safety objectives is to develop silicone rubber formulations that are non-toxic and non-allergenic. This involves carefully selecting and testing raw materials, as well as optimizing the manufacturing process to eliminate potential contaminants. The goal is to create silicone rubber products that can come into direct contact with human skin, mucous membranes, or even internal organs without causing adverse reactions or long-term health effects.
Another crucial objective is to enhance the material's resistance to microbial growth and colonization. In medical settings, preventing the proliferation of harmful bacteria and fungi on silicone rubber surfaces is essential for reducing the risk of infections. This often involves incorporating antimicrobial additives or developing surface treatments that inhibit microbial adhesion without compromising the material's other desirable properties.
Durability and long-term stability are also paramount safety objectives for silicone rubber in health applications. The material must maintain its structural integrity and performance characteristics over extended periods, even when exposed to various bodily fluids, sterilization processes, and environmental stressors. This includes resistance to degradation, leaching of potentially harmful compounds, and maintaining consistent mechanical properties throughout its intended lifespan.
Achieving biocompatibility is a critical safety objective, particularly for implantable silicone rubber devices or components. This involves extensive testing to ensure that the material does not elicit undesirable biological responses, such as inflammation, immune reactions, or tissue damage. The goal is to develop silicone rubber formulations that integrate seamlessly with the human body, promoting healing and minimizing the risk of complications.
Compliance with regulatory standards and guidelines is an overarching safety objective in the development of silicone rubber solutions for health applications. This includes meeting the requirements set forth by organizations such as the FDA, ISO, and other relevant regulatory bodies. Manufacturers must demonstrate that their silicone rubber products meet or exceed these standards through rigorous testing, documentation, and quality control processes.
Lastly, an important safety objective is to improve the traceability and quality control of silicone rubber products throughout their lifecycle. This involves implementing robust manufacturing processes, establishing comprehensive documentation systems, and developing methods for identifying and tracking individual components or batches. Such measures are crucial for quickly addressing any potential safety issues that may arise and ensuring the overall reliability of silicone rubber solutions in health-related applications.
Market Analysis for Health-Safe Silicone Products
The global market for health-safe silicone products has experienced significant growth in recent years, driven by increasing awareness of health and safety concerns across various industries. This trend is particularly evident in healthcare, personal care, and food-related sectors, where the demand for non-toxic, hypoallergenic, and biocompatible materials continues to rise.
In the healthcare industry, silicone rubber solutions are widely used in medical devices, implants, and prosthetics due to their excellent biocompatibility and durability. The market for these products is expected to expand further as the aging population increases and medical technology advances. Additionally, the COVID-19 pandemic has heightened the focus on infection control, leading to a surge in demand for silicone-based personal protective equipment (PPE) and medical-grade components.
The personal care sector represents another significant market for health-safe silicone products. Consumers are increasingly seeking out products free from harmful chemicals, driving the adoption of silicone-based alternatives in cosmetics, skincare, and haircare products. This shift is supported by stringent regulations on product safety and a growing preference for natural and organic formulations.
In the food industry, silicone rubber solutions are gaining traction for their non-reactive properties and resistance to extreme temperatures. The market for food-grade silicone products, such as bakeware, utensils, and food storage containers, is expanding as consumers prioritize safety and durability in their kitchen essentials.
The Asia-Pacific region is emerging as a key growth market for health-safe silicone products, fueled by rapid industrialization, increasing healthcare expenditure, and rising consumer awareness. North America and Europe continue to be significant markets, with established regulatory frameworks and a strong focus on product innovation.
Key market players are investing heavily in research and development to enhance the performance and safety profiles of their silicone products. This includes developing new formulations that meet or exceed health safety compliance standards while maintaining the desired physical properties. The competitive landscape is characterized by strategic partnerships, mergers, and acquisitions as companies seek to expand their product portfolios and geographical presence.
In the healthcare industry, silicone rubber solutions are widely used in medical devices, implants, and prosthetics due to their excellent biocompatibility and durability. The market for these products is expected to expand further as the aging population increases and medical technology advances. Additionally, the COVID-19 pandemic has heightened the focus on infection control, leading to a surge in demand for silicone-based personal protective equipment (PPE) and medical-grade components.
The personal care sector represents another significant market for health-safe silicone products. Consumers are increasingly seeking out products free from harmful chemicals, driving the adoption of silicone-based alternatives in cosmetics, skincare, and haircare products. This shift is supported by stringent regulations on product safety and a growing preference for natural and organic formulations.
In the food industry, silicone rubber solutions are gaining traction for their non-reactive properties and resistance to extreme temperatures. The market for food-grade silicone products, such as bakeware, utensils, and food storage containers, is expanding as consumers prioritize safety and durability in their kitchen essentials.
The Asia-Pacific region is emerging as a key growth market for health-safe silicone products, fueled by rapid industrialization, increasing healthcare expenditure, and rising consumer awareness. North America and Europe continue to be significant markets, with established regulatory frameworks and a strong focus on product innovation.
Key market players are investing heavily in research and development to enhance the performance and safety profiles of their silicone products. This includes developing new formulations that meet or exceed health safety compliance standards while maintaining the desired physical properties. The competitive landscape is characterized by strategic partnerships, mergers, and acquisitions as companies seek to expand their product portfolios and geographical presence.
Current Challenges in Silicone Rubber Safety
The silicone rubber industry faces several significant challenges in ensuring health safety compliance. One of the primary concerns is the potential migration of low molecular weight siloxanes from silicone rubber products into food, beverages, or the human body. These compounds, particularly cyclic siloxanes like D4 and D5, have raised toxicological concerns and are subject to increasing regulatory scrutiny.
Another challenge lies in the variability of silicone rubber formulations. Different additives, fillers, and curing agents can significantly alter the material's properties and potential health impacts. This complexity makes it difficult to establish universal safety standards and necessitates case-by-case evaluations for different applications.
The industry also grapples with the issue of biocompatibility, especially for medical-grade silicone rubber used in implants and other medical devices. Ensuring long-term compatibility with human tissues and minimizing the risk of adverse reactions remains a critical challenge. This is particularly important given the increasing use of silicone rubber in long-term implantable devices.
Furthermore, the presence of catalyst residues in silicone rubber products poses potential health risks. Platinum-based catalysts, commonly used in the curing process, can leave trace amounts in the final product. While these levels are typically low, there is ongoing research to understand their long-term effects and develop safer alternatives.
Environmental concerns also intersect with health safety challenges. The persistence of silicone rubber in the environment and its potential to break down into smaller particles that may enter the food chain is an area of growing concern. This necessitates a holistic approach to safety that considers not only direct human exposure but also indirect environmental pathways.
Regulatory compliance presents another significant challenge. With evolving regulations in different regions, such as REACH in Europe and FDA guidelines in the United States, manufacturers must navigate a complex landscape of safety requirements. Keeping up with these changing regulations and ensuring global compliance is both time-consuming and resource-intensive.
Lastly, there is a growing demand for transparency in material composition and safety data. Consumers and regulatory bodies are increasingly calling for detailed information about the chemicals used in silicone rubber products. Meeting these demands while protecting proprietary formulations presents a delicate balance for manufacturers to maintain.
Another challenge lies in the variability of silicone rubber formulations. Different additives, fillers, and curing agents can significantly alter the material's properties and potential health impacts. This complexity makes it difficult to establish universal safety standards and necessitates case-by-case evaluations for different applications.
The industry also grapples with the issue of biocompatibility, especially for medical-grade silicone rubber used in implants and other medical devices. Ensuring long-term compatibility with human tissues and minimizing the risk of adverse reactions remains a critical challenge. This is particularly important given the increasing use of silicone rubber in long-term implantable devices.
Furthermore, the presence of catalyst residues in silicone rubber products poses potential health risks. Platinum-based catalysts, commonly used in the curing process, can leave trace amounts in the final product. While these levels are typically low, there is ongoing research to understand their long-term effects and develop safer alternatives.
Environmental concerns also intersect with health safety challenges. The persistence of silicone rubber in the environment and its potential to break down into smaller particles that may enter the food chain is an area of growing concern. This necessitates a holistic approach to safety that considers not only direct human exposure but also indirect environmental pathways.
Regulatory compliance presents another significant challenge. With evolving regulations in different regions, such as REACH in Europe and FDA guidelines in the United States, manufacturers must navigate a complex landscape of safety requirements. Keeping up with these changing regulations and ensuring global compliance is both time-consuming and resource-intensive.
Lastly, there is a growing demand for transparency in material composition and safety data. Consumers and regulatory bodies are increasingly calling for detailed information about the chemicals used in silicone rubber products. Meeting these demands while protecting proprietary formulations presents a delicate balance for manufacturers to maintain.
Existing Health-Safe Silicone Solutions
01 Biocompatibility and safety testing
Silicone rubber materials undergo rigorous biocompatibility and safety testing to ensure compliance with health and safety standards. This includes evaluating potential toxicity, allergic reactions, and long-term effects on human health. Various in vitro and in vivo tests are conducted to assess the material's safety for use in medical devices, food contact applications, and other consumer products.- Biocompatibility and safety testing: Silicone rubber materials undergo rigorous biocompatibility and safety testing to ensure compliance with health and safety standards. This includes evaluating potential toxicity, allergic reactions, and long-term effects on human health. Various in vitro and in vivo tests are conducted to assess the material's safety for use in medical devices, food contact applications, and other consumer products.
- Regulatory compliance and certifications: Silicone rubber products must meet specific regulatory requirements and obtain necessary certifications to ensure health safety compliance. This includes adherence to standards set by organizations such as FDA, ISO, and REACH. Manufacturers must demonstrate compliance through documentation, quality management systems, and regular audits to maintain certifications and market approval.
- Material composition and purity: The health safety of silicone rubber products is closely tied to their composition and purity. Manufacturers focus on using high-quality, medical-grade silicone materials and ensuring the absence of harmful additives or contaminants. Strict quality control measures are implemented throughout the production process to maintain material integrity and minimize potential health risks.
- Environmental impact and sustainability: Health safety compliance for silicone rubber extends to its environmental impact. Manufacturers are increasingly focusing on developing sustainable production methods, reducing waste, and improving recyclability. This includes the use of eco-friendly additives, energy-efficient manufacturing processes, and end-of-life product management to minimize potential environmental and health hazards.
- Performance monitoring and post-market surveillance: Ensuring ongoing health safety compliance involves continuous monitoring of silicone rubber products after market release. This includes gathering data on product performance, conducting periodic safety assessments, and implementing effective recall procedures when necessary. Manufacturers maintain robust systems for tracking and addressing any emerging safety concerns or adverse events related to their products.
02 Regulatory compliance and certifications
Silicone rubber products must meet specific regulatory requirements and obtain necessary certifications to ensure health safety compliance. This includes adherence to standards set by organizations such as FDA, ISO, and REACH. Manufacturers must demonstrate compliance through documentation, quality management systems, and third-party audits to gain market approval for their silicone rubber products.Expand Specific Solutions03 Material composition and purity
The health safety of silicone rubber products is closely tied to their material composition and purity. Manufacturers must carefully control the raw materials used, ensuring they are free from harmful contaminants and meet specified purity levels. This includes monitoring for the presence of potentially hazardous substances and optimizing the formulation to minimize any risks associated with leaching or degradation of the material over time.Expand Specific Solutions04 Manufacturing process controls
Implementing strict manufacturing process controls is crucial for ensuring the health safety compliance of silicone rubber products. This involves maintaining clean production environments, utilizing validated sterilization methods, and implementing quality control measures throughout the manufacturing process. Regular monitoring and testing of production batches help maintain consistency and adherence to safety standards.Expand Specific Solutions05 Environmental and sustainability considerations
Health safety compliance for silicone rubber products also encompasses environmental and sustainability aspects. This includes assessing the environmental impact of production processes, developing eco-friendly formulations, and implementing proper disposal or recycling methods. Manufacturers are increasingly focusing on creating silicone rubber products that are not only safe for human use but also minimize negative impacts on the environment throughout their lifecycle.Expand Specific Solutions
Key Players in Silicone Industry
The research on silicone rubber solutions for health safety compliance is in a mature stage, with a competitive landscape dominated by established global players. The market size is substantial, driven by increasing demand across healthcare, medical devices, and consumer products industries. Technologically, the field is well-developed, with companies like Shin-Etsu Chemical, Wacker Chemie, and Momentive Performance Materials leading innovation. These firms, along with others such as Sumitomo Bakelite and Dow Toray, have advanced capabilities in developing specialized silicone formulations that meet stringent health and safety standards. The industry is characterized by ongoing R&D efforts to enhance product performance, biocompatibility, and regulatory compliance.
Shin-Etsu Chemical Co., Ltd.
Technical Solution: Shin-Etsu Chemical has developed advanced silicone rubber solutions for health safety compliance, focusing on biocompatibility and low toxicity. Their proprietary technology includes a range of medical-grade silicone elastomers with enhanced purity and reduced extractables. These materials are designed to meet stringent regulatory requirements, including ISO 10993 and USP Class VI standards[1]. The company has also introduced self-adhesive silicone gels for wound care applications, featuring improved breathability and gentle adhesion to sensitive skin[2]. Additionally, Shin-Etsu has developed antimicrobial silicone rubber compounds that incorporate silver-based additives, providing long-lasting protection against a broad spectrum of microorganisms in healthcare settings[3].
Strengths: Extensive experience in medical-grade silicones, strong R&D capabilities, and a wide range of specialized products. Weaknesses: Potentially higher costs due to premium quality materials and stringent manufacturing processes.
Momentive Performance Materials, Inc.
Technical Solution: Momentive has developed a range of silicone-based solutions for healthcare applications, focusing on biocompatibility and durability. Their Silopren* LSR product line includes liquid silicone rubbers specifically designed for medical devices and drug delivery systems[1]. The company has also introduced UV-curable silicone elastomers that allow for rapid prototyping and production of complex medical components[2]. Momentive's research extends to self-lubricating silicone rubbers that reduce friction in medical tubing and catheters, enhancing patient comfort and device performance[3]. Additionally, they have developed specialty silicone adhesives for transdermal drug delivery patches, offering improved skin compatibility and controlled drug release profiles[4].
Strengths: Innovative product development, strong focus on customized solutions, and global manufacturing presence. Weaknesses: May face competition from larger, more established players in certain market segments.
Innovative Silicone Safety Technologies
Addition reaction-curable liquid silicone rubber compositions and process of preparing same
PatentActiveUS20050277725A1
Innovation
- An addition reaction-curable liquid silicone rubber composition comprising specific organopolysiloxanes, finely divided silica, hexamethyldisilazane, water, and an organohydrogenpolysiloxane, along with a hydrosilation catalyst, is developed, allowing for a cured product with a hardness of at least 75 and an elongation at break of at least 200%, enabling broader application beyond insulation potting.
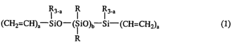
Addition reaction-curable liquid silicone rubber compositions and process of preparing same
PatentInactiveEP1607444B1
Innovation
- An addition reaction-curable liquid silicone rubber composition comprising specific organopolysiloxanes, finely divided silica, hexamethyldisilazane, water, and an organohydrogenpolysiloxane, with a hydrosilation catalyst, which is processed through a series of kneading and heat treatment steps to achieve a cured product with a hardness of at least 75 and an elongation at break of at least 200%, enabling broader application in fields like food-related and industrial uses.
Regulatory Framework for Silicone Products
The regulatory framework for silicone products is a complex and evolving landscape that plays a crucial role in ensuring the safety and compliance of silicone rubber solutions in various industries, particularly in healthcare applications. This framework encompasses a wide range of regulations, standards, and guidelines set forth by governmental bodies, international organizations, and industry associations.
At the forefront of silicone product regulation is the Food and Drug Administration (FDA) in the United States. The FDA has established specific guidelines for silicone materials used in medical devices, food contact applications, and cosmetics. These regulations outline requirements for material composition, manufacturing processes, and performance characteristics to ensure product safety and efficacy.
In the European Union, the regulatory landscape is governed by the European Chemicals Agency (ECHA) and the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH mandates that manufacturers and importers of silicone products register their substances and provide detailed information on their properties, potential risks, and safe use guidelines.
The International Organization for Standardization (ISO) has developed several standards specific to silicone materials, such as ISO 14949 for silicone rubber in healthcare applications. These standards provide guidelines for material properties, testing methods, and quality control procedures, ensuring consistency and reliability across the industry.
In addition to these overarching regulations, specific industries have their own sets of standards and requirements. For instance, the automotive industry follows the International Material Data System (IMDS) for reporting material composition, while the aerospace sector adheres to strict specifications set by organizations like the SAE International.
Compliance with these regulatory frameworks often requires extensive testing and documentation. Manufacturers must conduct biocompatibility tests, chemical analyses, and performance evaluations to demonstrate the safety and suitability of their silicone products for intended applications. This process can be time-consuming and resource-intensive, but it is essential for market access and consumer trust.
As environmental concerns gain prominence, regulatory bodies are increasingly focusing on the sustainability aspects of silicone products. This includes regulations on recyclability, biodegradability, and the overall environmental impact of silicone materials throughout their lifecycle. Manufacturers are now required to consider these factors in their product development and marketing strategies.
The global nature of the silicone industry necessitates a harmonized approach to regulation. Efforts are underway to align standards and requirements across different regions, facilitating international trade and ensuring consistent safety measures worldwide. However, challenges remain in reconciling disparate regulatory approaches and keeping pace with rapid technological advancements in silicone materials and applications.
At the forefront of silicone product regulation is the Food and Drug Administration (FDA) in the United States. The FDA has established specific guidelines for silicone materials used in medical devices, food contact applications, and cosmetics. These regulations outline requirements for material composition, manufacturing processes, and performance characteristics to ensure product safety and efficacy.
In the European Union, the regulatory landscape is governed by the European Chemicals Agency (ECHA) and the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation. REACH mandates that manufacturers and importers of silicone products register their substances and provide detailed information on their properties, potential risks, and safe use guidelines.
The International Organization for Standardization (ISO) has developed several standards specific to silicone materials, such as ISO 14949 for silicone rubber in healthcare applications. These standards provide guidelines for material properties, testing methods, and quality control procedures, ensuring consistency and reliability across the industry.
In addition to these overarching regulations, specific industries have their own sets of standards and requirements. For instance, the automotive industry follows the International Material Data System (IMDS) for reporting material composition, while the aerospace sector adheres to strict specifications set by organizations like the SAE International.
Compliance with these regulatory frameworks often requires extensive testing and documentation. Manufacturers must conduct biocompatibility tests, chemical analyses, and performance evaluations to demonstrate the safety and suitability of their silicone products for intended applications. This process can be time-consuming and resource-intensive, but it is essential for market access and consumer trust.
As environmental concerns gain prominence, regulatory bodies are increasingly focusing on the sustainability aspects of silicone products. This includes regulations on recyclability, biodegradability, and the overall environmental impact of silicone materials throughout their lifecycle. Manufacturers are now required to consider these factors in their product development and marketing strategies.
The global nature of the silicone industry necessitates a harmonized approach to regulation. Efforts are underway to align standards and requirements across different regions, facilitating international trade and ensuring consistent safety measures worldwide. However, challenges remain in reconciling disparate regulatory approaches and keeping pace with rapid technological advancements in silicone materials and applications.
Environmental Impact of Silicone Rubber Production
The production of silicone rubber has significant environmental implications that warrant careful consideration in the context of health safety compliance. The manufacturing process involves several stages, each with potential environmental impacts. The primary raw materials for silicone rubber production are silica and methyl chloride, which are combined to form silicone polymers. This initial step requires substantial energy input and may result in emissions of volatile organic compounds (VOCs) if not properly controlled.
During the polymerization and curing processes, additional chemicals such as catalysts and crosslinking agents are introduced. These substances, if released into the environment, can contribute to air and water pollution. Proper handling and disposal of these chemicals are crucial to minimize environmental contamination and ensure worker safety. Furthermore, the curing process often involves heat treatment, which contributes to the overall energy consumption and carbon footprint of silicone rubber production.
Water usage is another significant environmental factor in silicone rubber manufacturing. Large volumes of water are typically required for cooling and cleaning processes. Wastewater from these operations may contain trace amounts of silicone compounds and other chemicals, necessitating appropriate treatment before discharge to prevent aquatic ecosystem disruption.
The disposal and end-of-life management of silicone rubber products also present environmental challenges. While silicone rubber is generally inert and non-toxic, it is not biodegradable. Improper disposal can lead to long-term accumulation in landfills or marine environments. However, recent advancements in recycling technologies offer promising solutions for reducing the environmental impact of silicone rubber waste.
On a positive note, the durability and long lifespan of silicone rubber products can contribute to resource conservation by reducing the need for frequent replacements. Additionally, some silicone rubber formulations are being developed with bio-based ingredients, potentially lowering the reliance on petrochemical feedstocks and reducing the overall carbon footprint of production.
As health safety compliance becomes increasingly intertwined with environmental considerations, manufacturers are exploring cleaner production methods. These include closed-loop systems to minimize waste, energy-efficient curing processes, and the use of renewable energy sources in production facilities. Such innovations not only address environmental concerns but also align with broader sustainability goals and regulatory requirements in the healthcare sector.
During the polymerization and curing processes, additional chemicals such as catalysts and crosslinking agents are introduced. These substances, if released into the environment, can contribute to air and water pollution. Proper handling and disposal of these chemicals are crucial to minimize environmental contamination and ensure worker safety. Furthermore, the curing process often involves heat treatment, which contributes to the overall energy consumption and carbon footprint of silicone rubber production.
Water usage is another significant environmental factor in silicone rubber manufacturing. Large volumes of water are typically required for cooling and cleaning processes. Wastewater from these operations may contain trace amounts of silicone compounds and other chemicals, necessitating appropriate treatment before discharge to prevent aquatic ecosystem disruption.
The disposal and end-of-life management of silicone rubber products also present environmental challenges. While silicone rubber is generally inert and non-toxic, it is not biodegradable. Improper disposal can lead to long-term accumulation in landfills or marine environments. However, recent advancements in recycling technologies offer promising solutions for reducing the environmental impact of silicone rubber waste.
On a positive note, the durability and long lifespan of silicone rubber products can contribute to resource conservation by reducing the need for frequent replacements. Additionally, some silicone rubber formulations are being developed with bio-based ingredients, potentially lowering the reliance on petrochemical feedstocks and reducing the overall carbon footprint of production.
As health safety compliance becomes increasingly intertwined with environmental considerations, manufacturers are exploring cleaner production methods. These include closed-loop systems to minimize waste, energy-efficient curing processes, and the use of renewable energy sources in production facilities. Such innovations not only address environmental concerns but also align with broader sustainability goals and regulatory requirements in the healthcare sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!