Role of Triton X-100 in Detergent-Free Membrane Protein Purification
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background
Triton X-100, a nonionic surfactant, has been a cornerstone in membrane protein research for decades. Its chemical structure consists of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, making it an effective detergent for solubilizing membrane proteins. Developed in the 1950s, Triton X-100 quickly gained popularity in biochemistry due to its mild nature and ability to maintain protein functionality.
The surfactant's critical micelle concentration (CMC) of 0.2-0.9 mM allows for efficient membrane protein extraction at relatively low concentrations. This property, combined with its low cost and stability, has made Triton X-100 a go-to choice for many researchers in the field of membrane protein purification. Its widespread use has contributed significantly to our understanding of membrane protein structure and function.
Traditionally, Triton X-100 has been employed in a variety of applications, including cell lysis, protein extraction, and membrane solubilization. Its effectiveness in disrupting lipid-lipid and lipid-protein interactions without denaturing proteins has been crucial in maintaining the native state of membrane proteins during purification processes. This characteristic has been particularly valuable in structural biology studies and functional assays.
However, the use of Triton X-100 is not without challenges. Its non-UV-transparent nature can interfere with spectrophotometric measurements, complicating protein quantification and characterization. Additionally, its low CMC can make it difficult to remove completely from protein samples, potentially affecting downstream applications such as crystallization or functional studies.
In recent years, there has been a growing interest in developing detergent-free methods for membrane protein purification. This shift is driven by the desire to overcome limitations associated with traditional detergent-based approaches, including potential protein destabilization and interference with native lipid environments. The emergence of alternative techniques, such as styrene-maleic acid (SMA) copolymer-based extraction, has challenged the dominance of Triton X-100 in membrane protein research.
Despite these developments, Triton X-100 remains a relevant tool in the biochemist's arsenal. Its long history of use has resulted in a wealth of protocols and optimized procedures, making it a reliable choice for many applications. Furthermore, the extensive knowledge base surrounding Triton X-100 serves as a valuable reference point for comparing and evaluating newer purification methods.
As the field of membrane protein research continues to evolve, the role of Triton X-100 is being reevaluated. While it may no longer be the default choice for all applications, its unique properties and well-established protocols ensure its continued relevance in specific contexts. The ongoing exploration of detergent-free purification methods is not so much a replacement of Triton X-100 as it is an expansion of the toolbox available to researchers, offering new possibilities for studying these challenging but crucial biomolecules.
The surfactant's critical micelle concentration (CMC) of 0.2-0.9 mM allows for efficient membrane protein extraction at relatively low concentrations. This property, combined with its low cost and stability, has made Triton X-100 a go-to choice for many researchers in the field of membrane protein purification. Its widespread use has contributed significantly to our understanding of membrane protein structure and function.
Traditionally, Triton X-100 has been employed in a variety of applications, including cell lysis, protein extraction, and membrane solubilization. Its effectiveness in disrupting lipid-lipid and lipid-protein interactions without denaturing proteins has been crucial in maintaining the native state of membrane proteins during purification processes. This characteristic has been particularly valuable in structural biology studies and functional assays.
However, the use of Triton X-100 is not without challenges. Its non-UV-transparent nature can interfere with spectrophotometric measurements, complicating protein quantification and characterization. Additionally, its low CMC can make it difficult to remove completely from protein samples, potentially affecting downstream applications such as crystallization or functional studies.
In recent years, there has been a growing interest in developing detergent-free methods for membrane protein purification. This shift is driven by the desire to overcome limitations associated with traditional detergent-based approaches, including potential protein destabilization and interference with native lipid environments. The emergence of alternative techniques, such as styrene-maleic acid (SMA) copolymer-based extraction, has challenged the dominance of Triton X-100 in membrane protein research.
Despite these developments, Triton X-100 remains a relevant tool in the biochemist's arsenal. Its long history of use has resulted in a wealth of protocols and optimized procedures, making it a reliable choice for many applications. Furthermore, the extensive knowledge base surrounding Triton X-100 serves as a valuable reference point for comparing and evaluating newer purification methods.
As the field of membrane protein research continues to evolve, the role of Triton X-100 is being reevaluated. While it may no longer be the default choice for all applications, its unique properties and well-established protocols ensure its continued relevance in specific contexts. The ongoing exploration of detergent-free purification methods is not so much a replacement of Triton X-100 as it is an expansion of the toolbox available to researchers, offering new possibilities for studying these challenging but crucial biomolecules.
Market Analysis
The market for detergent-free membrane protein purification techniques, particularly those involving Triton X-100, has been experiencing significant growth in recent years. This surge is primarily driven by the increasing demand for high-quality, functional membrane proteins in various research and pharmaceutical applications. The global membrane protein market, which encompasses purification technologies, is projected to expand at a robust rate due to the rising focus on personalized medicine and targeted drug discovery.
Triton X-100, a non-ionic surfactant, has traditionally been used in membrane protein solubilization and purification. However, the shift towards detergent-free methods has opened new opportunities in the market. This transition is fueled by the need to overcome limitations associated with detergent-based methods, such as protein denaturation and interference with downstream applications.
The biotechnology and pharmaceutical sectors are the primary consumers of membrane protein purification technologies. These industries are investing heavily in research and development activities, particularly in areas such as drug target identification and vaccine development. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of membrane proteins in understanding viral mechanisms and developing therapeutic interventions.
Academic research institutions also contribute significantly to the market demand. Universities and research centers worldwide are increasingly focusing on structural biology and proteomics studies, which require efficient membrane protein purification techniques. This has led to a growing market for innovative, detergent-free purification methods that can maintain protein integrity and functionality.
The market for Triton X-100 alternatives in detergent-free purification is witnessing rapid expansion. Companies are developing novel technologies and reagents that can effectively replace Triton X-100 while offering improved protein stability and purity. This has resulted in a competitive landscape where both established players and startups are vying for market share through product innovation and strategic partnerships.
Geographically, North America and Europe lead the market due to their advanced research infrastructure and high R&D spending. However, the Asia-Pacific region is emerging as a fast-growing market, driven by increasing investments in life sciences research and a burgeoning biopharmaceutical industry. This regional diversification presents opportunities for market expansion and technology transfer.
The market trend indicates a shift towards more efficient, scalable, and cost-effective purification methods. End-users are seeking solutions that can be easily integrated into existing workflows and offer reproducible results. This demand is shaping the development of new products and services in the membrane protein purification space, with a particular focus on detergent-free technologies that can effectively utilize or replace Triton X-100.
Triton X-100, a non-ionic surfactant, has traditionally been used in membrane protein solubilization and purification. However, the shift towards detergent-free methods has opened new opportunities in the market. This transition is fueled by the need to overcome limitations associated with detergent-based methods, such as protein denaturation and interference with downstream applications.
The biotechnology and pharmaceutical sectors are the primary consumers of membrane protein purification technologies. These industries are investing heavily in research and development activities, particularly in areas such as drug target identification and vaccine development. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of membrane proteins in understanding viral mechanisms and developing therapeutic interventions.
Academic research institutions also contribute significantly to the market demand. Universities and research centers worldwide are increasingly focusing on structural biology and proteomics studies, which require efficient membrane protein purification techniques. This has led to a growing market for innovative, detergent-free purification methods that can maintain protein integrity and functionality.
The market for Triton X-100 alternatives in detergent-free purification is witnessing rapid expansion. Companies are developing novel technologies and reagents that can effectively replace Triton X-100 while offering improved protein stability and purity. This has resulted in a competitive landscape where both established players and startups are vying for market share through product innovation and strategic partnerships.
Geographically, North America and Europe lead the market due to their advanced research infrastructure and high R&D spending. However, the Asia-Pacific region is emerging as a fast-growing market, driven by increasing investments in life sciences research and a burgeoning biopharmaceutical industry. This regional diversification presents opportunities for market expansion and technology transfer.
The market trend indicates a shift towards more efficient, scalable, and cost-effective purification methods. End-users are seeking solutions that can be easily integrated into existing workflows and offer reproducible results. This demand is shaping the development of new products and services in the membrane protein purification space, with a particular focus on detergent-free technologies that can effectively utilize or replace Triton X-100.
Technical Challenges
The purification of membrane proteins without detergents presents significant technical challenges due to their hydrophobic nature and tendency to aggregate in aqueous solutions. While Triton X-100 has traditionally been used as a detergent in membrane protein purification, its role in detergent-free methods is complex and multifaceted.
One of the primary challenges in detergent-free membrane protein purification is maintaining protein stability and solubility throughout the process. Membrane proteins are inherently unstable when removed from their native lipid environment, often leading to denaturation and loss of function. The absence of detergents exacerbates this issue, as there are no amphipathic molecules to mimic the lipid bilayer and shield hydrophobic regions of the protein.
Another significant hurdle is the prevention of non-specific protein aggregation. Without detergents to keep proteins dispersed, membrane proteins have a strong tendency to form large, insoluble aggregates. This not only reduces the yield of purified protein but can also lead to the co-purification of unwanted proteins, compromising the purity of the final product.
The removal of lipids and other membrane components without disrupting protein structure is also a major challenge. Traditional detergent-based methods effectively solubilize and remove these components, but detergent-free approaches must find alternative ways to achieve this without compromising protein integrity.
Furthermore, the efficiency of protein extraction from membranes is often lower in detergent-free methods. This can result in reduced yields and the need for larger starting quantities of biological material, which may not always be feasible or cost-effective.
The development of suitable alternative solubilization agents that can replace detergents while maintaining protein stability and function is an ongoing challenge. These agents must effectively solubilize membrane proteins without causing denaturation or interfering with downstream applications such as crystallization or functional assays.
Adapting existing purification techniques and developing new ones that are compatible with detergent-free conditions is another technical hurdle. Many standard chromatography methods and protein handling protocols are optimized for detergent-containing solutions and may need significant modification for detergent-free approaches.
Lastly, the characterization and quality control of membrane proteins purified without detergents can be more challenging. Traditional methods for assessing protein purity, homogeneity, and activity may need to be adapted or replaced with techniques that are compatible with detergent-free conditions.
One of the primary challenges in detergent-free membrane protein purification is maintaining protein stability and solubility throughout the process. Membrane proteins are inherently unstable when removed from their native lipid environment, often leading to denaturation and loss of function. The absence of detergents exacerbates this issue, as there are no amphipathic molecules to mimic the lipid bilayer and shield hydrophobic regions of the protein.
Another significant hurdle is the prevention of non-specific protein aggregation. Without detergents to keep proteins dispersed, membrane proteins have a strong tendency to form large, insoluble aggregates. This not only reduces the yield of purified protein but can also lead to the co-purification of unwanted proteins, compromising the purity of the final product.
The removal of lipids and other membrane components without disrupting protein structure is also a major challenge. Traditional detergent-based methods effectively solubilize and remove these components, but detergent-free approaches must find alternative ways to achieve this without compromising protein integrity.
Furthermore, the efficiency of protein extraction from membranes is often lower in detergent-free methods. This can result in reduced yields and the need for larger starting quantities of biological material, which may not always be feasible or cost-effective.
The development of suitable alternative solubilization agents that can replace detergents while maintaining protein stability and function is an ongoing challenge. These agents must effectively solubilize membrane proteins without causing denaturation or interfering with downstream applications such as crystallization or functional assays.
Adapting existing purification techniques and developing new ones that are compatible with detergent-free conditions is another technical hurdle. Many standard chromatography methods and protein handling protocols are optimized for detergent-containing solutions and may need significant modification for detergent-free approaches.
Lastly, the characterization and quality control of membrane proteins purified without detergents can be more challenging. Traditional methods for assessing protein purity, homogeneity, and activity may need to be adapted or replaced with techniques that are compatible with detergent-free conditions.
Current Methodologies
01 Use in cell lysis and protein extraction
Triton X-100 is widely used as a detergent for cell lysis and protein extraction in biochemical research. It effectively disrupts cell membranes and solubilizes proteins, making it valuable in various experimental protocols and assays.- Use in cell lysis and protein extraction: Triton X-100 is widely used as a detergent for cell lysis and protein extraction in biochemical research. It effectively disrupts cell membranes and solubilizes proteins, making it valuable for isolating cellular components and preparing samples for various analytical techniques.
- Application in surface cleaning and decontamination: Triton X-100 is employed in cleaning solutions and decontamination processes due to its surfactant properties. It helps in removing contaminants from surfaces and equipment, making it useful in laboratory and industrial settings for maintaining cleanliness and preventing cross-contamination.
- Role in membrane protein solubilization: Triton X-100 is effective in solubilizing membrane proteins while maintaining their native structure and function. This property makes it valuable in studying membrane-associated proteins and in the preparation of protein samples for various analytical and structural biology techniques.
- Use in emulsion polymerization: Triton X-100 serves as a surfactant in emulsion polymerization processes. It helps stabilize the emulsion of monomers in water, facilitating the formation of polymer particles and controlling their size distribution. This application is important in the production of various polymer products.
- Application in electrochemical sensors: Triton X-100 is used in the development of electrochemical sensors and biosensors. It can modify electrode surfaces, enhance electron transfer, and improve the sensitivity and stability of sensors. This application is particularly relevant in analytical chemistry and biomedical diagnostics.
02 Application in surface modification and cleaning
Triton X-100 serves as a surfactant for surface modification and cleaning applications. It can alter surface properties, enhance wettability, and improve the efficiency of cleaning processes in various industrial and laboratory settings.Expand Specific Solutions03 Role in pharmaceutical formulations
Triton X-100 is utilized in pharmaceutical formulations as a solubilizing agent and emulsifier. It helps improve the stability and bioavailability of drug compounds, particularly in the development of novel drug delivery systems.Expand Specific Solutions04 Use in analytical and diagnostic techniques
Triton X-100 plays a crucial role in various analytical and diagnostic techniques. It is employed in sample preparation, enzyme-linked immunosorbent assays (ELISA), and other biomedical testing procedures to enhance sensitivity and specificity.Expand Specific Solutions05 Application in material science and nanotechnology
Triton X-100 finds applications in material science and nanotechnology. It is used in the synthesis and stabilization of nanoparticles, as well as in the preparation of various composite materials with enhanced properties.Expand Specific Solutions
Key Industry Players
The field of detergent-free membrane protein purification using Triton X-100 is in a mature stage of development, with a well-established market and proven technological approaches. The global market for membrane protein purification is substantial, driven by the pharmaceutical and biotechnology sectors. Key players like Biogen, Amgen, and Bristol Myers Squibb are at the forefront, leveraging their extensive R&D capabilities. Academic institutions such as Zhejiang Gongshang University and the Korea Research Institute of Chemical Technology contribute significantly to advancing the technology. The high level of involvement from both industry and academia indicates a robust and competitive landscape, with ongoing refinements in methodologies and applications.
Alexion Pharmaceuticals, Inc.
Technical Solution: Alexion Pharmaceuticals has developed a unique approach to detergent-free membrane protein purification utilizing Triton X-100 and lipid cubic phase (LCP) technology. Their method involves initial solubilization of membrane proteins with Triton X-100, followed by incorporation into a lipid cubic phase matrix[2]. The Triton X-100 is then gradually removed through a controlled diffusion process, allowing the proteins to reintegrate into a native-like lipid environment within the LCP[4]. This technique has shown particular promise for structurally sensitive membrane proteins, such as those involved in rare diseases. Alexion has further enhanced this method by developing a microfluidic system for high-throughput LCP formation and protein incorporation[6].
Strengths: Excellent for structurally sensitive proteins, provides a native-like lipid environment. Weaknesses: LCP formation can be technically challenging, limited throughput in some cases.
Amgen, Inc.
Technical Solution: Amgen has pioneered a detergent-free membrane protein purification technique utilizing Triton X-100 in combination with styrene-maleic acid (SMA) copolymers. Their approach involves initial solubilization of membrane proteins with Triton X-100, followed by the addition of SMA copolymers to form lipid-protein nanodiscs[2]. The Triton X-100 is then selectively removed using bio-beads, leaving the proteins encapsulated in SMA-lipid particles (SMALPs)[4]. This method has shown particular success with G-protein coupled receptors (GPCRs) and ion channels. Amgen has further refined this technique by developing a high-throughput screening platform to optimize SMA copolymer composition for different membrane proteins[6].
Strengths: Highly effective for GPCRs and ion channels, scalable for industrial applications. Weaknesses: SMA copolymers may interfere with some downstream applications, limited to certain pH ranges.
Innovative Approaches
Detergent and method for purifying a biotherapeutic
PatentPendingUS20240327454A1
Innovation
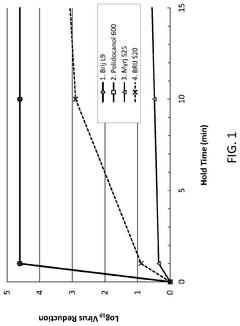
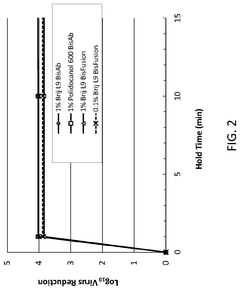
- The use of Laureth-9 as an environmentally compatible detergent for viral inactivation, cell lysis, and removal of impurities such as host cell proteins and endotoxins, which does not adversely impact product quality, is proposed. Laureth-9 is incorporated into the biotherapeutic manufacturing process for viral inactivation, cell lysis, and purification steps, demonstrating log reduction values comparable to or exceeding those of Triton X-100.
Formulations of hydrophobic proteins in an immunogenic composition having improved tolerability
PatentInactiveCN1901935A
Innovation
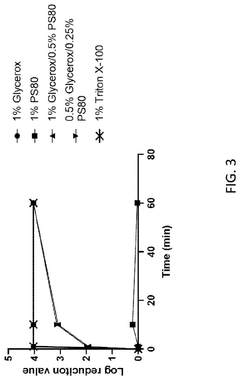
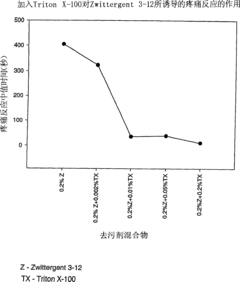
- Dilute or replace zwitterionic detergents by using a combination of lower final concentrations than zwitterionic detergents and higher final concentrations than non-ionic detergents to reduce pain responses and maintain solubility of hydrophobic proteins, forming non-ionic detergents. Pain-inducing immunogenic compositions.
Regulatory Compliance
Regulatory compliance is a critical aspect of detergent-free membrane protein purification using Triton X-100. The use of this non-ionic surfactant in biotechnology and pharmaceutical applications is subject to various regulations and guidelines set by international and national regulatory bodies.
The U.S. Food and Drug Administration (FDA) has established specific guidelines for the use of Triton X-100 in protein purification processes. These guidelines outline acceptable levels of residual Triton X-100 in final products and require manufacturers to demonstrate effective removal of the surfactant during downstream processing. Compliance with FDA regulations is essential for companies seeking approval for biopharmaceutical products purified using Triton X-100-based methods.
In the European Union, the European Medicines Agency (EMA) has similar requirements for the use of Triton X-100 in membrane protein purification. The EMA's guidelines emphasize the need for validated analytical methods to detect and quantify Triton X-100 residues in purified protein samples. Manufacturers must provide comprehensive data on the removal efficiency of Triton X-100 during the purification process to ensure product safety and quality.
Environmental regulations also play a significant role in the use of Triton X-100 for protein purification. The surfactant is known to have potential environmental impacts, particularly on aquatic ecosystems. As a result, many countries have implemented strict regulations on the disposal of Triton X-100-containing waste. Companies must adhere to local and national environmental protection laws when handling and disposing of Triton X-100 and related waste products.
Good Manufacturing Practice (GMP) guidelines, which are essential for pharmaceutical and biotechnology industries, include specific provisions for the use of Triton X-100 in protein purification processes. These guidelines require thorough documentation of all steps involving Triton X-100, including its sourcing, handling, and removal. Companies must establish and maintain robust quality control systems to ensure consistent compliance with GMP standards throughout the purification process.
Occupational health and safety regulations also apply to the use of Triton X-100 in laboratory and industrial settings. These regulations mandate proper handling procedures, personal protective equipment, and safety measures to protect workers from potential exposure to the surfactant. Companies must provide adequate training and implement safety protocols to comply with these occupational health standards.
As the field of membrane protein purification continues to evolve, regulatory bodies are likely to update their guidelines and requirements. Companies and researchers working with Triton X-100 in detergent-free purification methods must stay informed about these regulatory changes and adapt their processes accordingly to maintain compliance and ensure the safety and efficacy of their products.
The U.S. Food and Drug Administration (FDA) has established specific guidelines for the use of Triton X-100 in protein purification processes. These guidelines outline acceptable levels of residual Triton X-100 in final products and require manufacturers to demonstrate effective removal of the surfactant during downstream processing. Compliance with FDA regulations is essential for companies seeking approval for biopharmaceutical products purified using Triton X-100-based methods.
In the European Union, the European Medicines Agency (EMA) has similar requirements for the use of Triton X-100 in membrane protein purification. The EMA's guidelines emphasize the need for validated analytical methods to detect and quantify Triton X-100 residues in purified protein samples. Manufacturers must provide comprehensive data on the removal efficiency of Triton X-100 during the purification process to ensure product safety and quality.
Environmental regulations also play a significant role in the use of Triton X-100 for protein purification. The surfactant is known to have potential environmental impacts, particularly on aquatic ecosystems. As a result, many countries have implemented strict regulations on the disposal of Triton X-100-containing waste. Companies must adhere to local and national environmental protection laws when handling and disposing of Triton X-100 and related waste products.
Good Manufacturing Practice (GMP) guidelines, which are essential for pharmaceutical and biotechnology industries, include specific provisions for the use of Triton X-100 in protein purification processes. These guidelines require thorough documentation of all steps involving Triton X-100, including its sourcing, handling, and removal. Companies must establish and maintain robust quality control systems to ensure consistent compliance with GMP standards throughout the purification process.
Occupational health and safety regulations also apply to the use of Triton X-100 in laboratory and industrial settings. These regulations mandate proper handling procedures, personal protective equipment, and safety measures to protect workers from potential exposure to the surfactant. Companies must provide adequate training and implement safety protocols to comply with these occupational health standards.
As the field of membrane protein purification continues to evolve, regulatory bodies are likely to update their guidelines and requirements. Companies and researchers working with Triton X-100 in detergent-free purification methods must stay informed about these regulatory changes and adapt their processes accordingly to maintain compliance and ensure the safety and efficacy of their products.
Environmental Impact
The use of Triton X-100 in detergent-free membrane protein purification methods has raised concerns about its potential environmental impact. As a non-ionic surfactant, Triton X-100 is known for its effectiveness in solubilizing membrane proteins, but its persistence in the environment has become a significant issue.
Triton X-100 is classified as an alkylphenol ethoxylate (APE), a group of chemicals that have been identified as endocrine disruptors. These compounds can mimic natural hormones, potentially interfering with the reproductive systems of aquatic organisms. Studies have shown that even at low concentrations, Triton X-100 can affect the development and reproduction of fish, amphibians, and other aquatic life.
The biodegradation of Triton X-100 in the environment is relatively slow, leading to its accumulation in water bodies and sediments. This persistence increases the likelihood of long-term exposure for aquatic ecosystems. Furthermore, the breakdown products of Triton X-100, particularly octylphenol, have been found to be more toxic and persistent than the parent compound.
In wastewater treatment plants, Triton X-100 is not completely removed, allowing it to enter natural water systems. This has led to detectable levels of the surfactant and its metabolites in rivers, lakes, and even coastal waters. The widespread use of Triton X-100 in various industries, including biotechnology and pharmaceuticals, contributes to its environmental ubiquity.
The bioaccumulation potential of Triton X-100 and its metabolites in the food chain is another area of concern. Studies have detected these compounds in the tissues of aquatic organisms, raising questions about potential impacts on higher trophic levels, including humans who consume seafood.
Regulatory bodies in several countries have recognized the environmental risks associated with APEs, including Triton X-100. The European Union, for instance, has placed restrictions on the use of nonylphenol ethoxylates, a closely related group of compounds. These regulatory actions have spurred research into alternative, more environmentally friendly surfactants for use in various applications, including membrane protein purification.
The development of detergent-free methods for membrane protein purification is partly driven by these environmental concerns. Techniques such as styrene-maleic acid (SMA) copolymer-based extraction and amphipol-mediated purification offer promising alternatives that could reduce reliance on traditional detergents like Triton X-100. These methods not only address environmental issues but also often provide benefits in terms of protein stability and functionality.
Triton X-100 is classified as an alkylphenol ethoxylate (APE), a group of chemicals that have been identified as endocrine disruptors. These compounds can mimic natural hormones, potentially interfering with the reproductive systems of aquatic organisms. Studies have shown that even at low concentrations, Triton X-100 can affect the development and reproduction of fish, amphibians, and other aquatic life.
The biodegradation of Triton X-100 in the environment is relatively slow, leading to its accumulation in water bodies and sediments. This persistence increases the likelihood of long-term exposure for aquatic ecosystems. Furthermore, the breakdown products of Triton X-100, particularly octylphenol, have been found to be more toxic and persistent than the parent compound.
In wastewater treatment plants, Triton X-100 is not completely removed, allowing it to enter natural water systems. This has led to detectable levels of the surfactant and its metabolites in rivers, lakes, and even coastal waters. The widespread use of Triton X-100 in various industries, including biotechnology and pharmaceuticals, contributes to its environmental ubiquity.
The bioaccumulation potential of Triton X-100 and its metabolites in the food chain is another area of concern. Studies have detected these compounds in the tissues of aquatic organisms, raising questions about potential impacts on higher trophic levels, including humans who consume seafood.
Regulatory bodies in several countries have recognized the environmental risks associated with APEs, including Triton X-100. The European Union, for instance, has placed restrictions on the use of nonylphenol ethoxylates, a closely related group of compounds. These regulatory actions have spurred research into alternative, more environmentally friendly surfactants for use in various applications, including membrane protein purification.
The development of detergent-free methods for membrane protein purification is partly driven by these environmental concerns. Techniques such as styrene-maleic acid (SMA) copolymer-based extraction and amphipol-mediated purification offer promising alternatives that could reduce reliance on traditional detergents like Triton X-100. These methods not only address environmental issues but also often provide benefits in terms of protein stability and functionality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!